Photocatalytic Decomposition of Amoxicillin Using Zinc Ferrite Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Materials/Reagents
2.2. Oxidized Graphene (GO) Obtained via Hummer’s Method [20]
2.3. Reduced and Exfoliated Graphite (RGO) Obtained Using Ultrasounds
2.4. Synthesis of Oxides Mixtures of Nanoparticles
2.4.1. By Coprecipitation
- Oxides mixtures, enriched in ZFO
- Nanoparticles mixed with reduced graphene (RGO) (Copr-ZFO-RGO)
2.4.2. Hydrothermal Treatments—Xia et al. [19]
2.5. Characterization Techniques
2.6. Photocatalytic Tests
3. Results
3.1. Zn and Fe Containing Powders
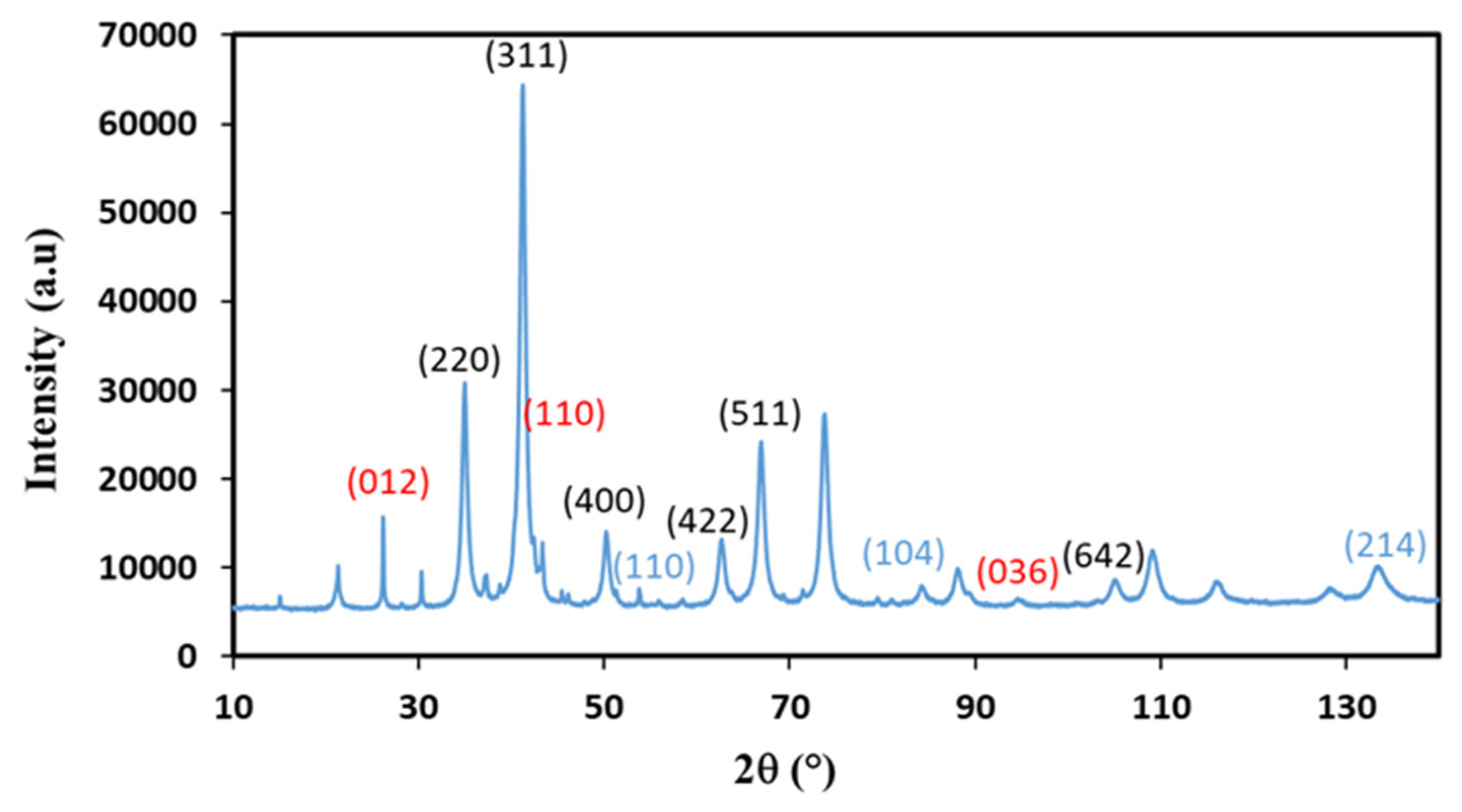
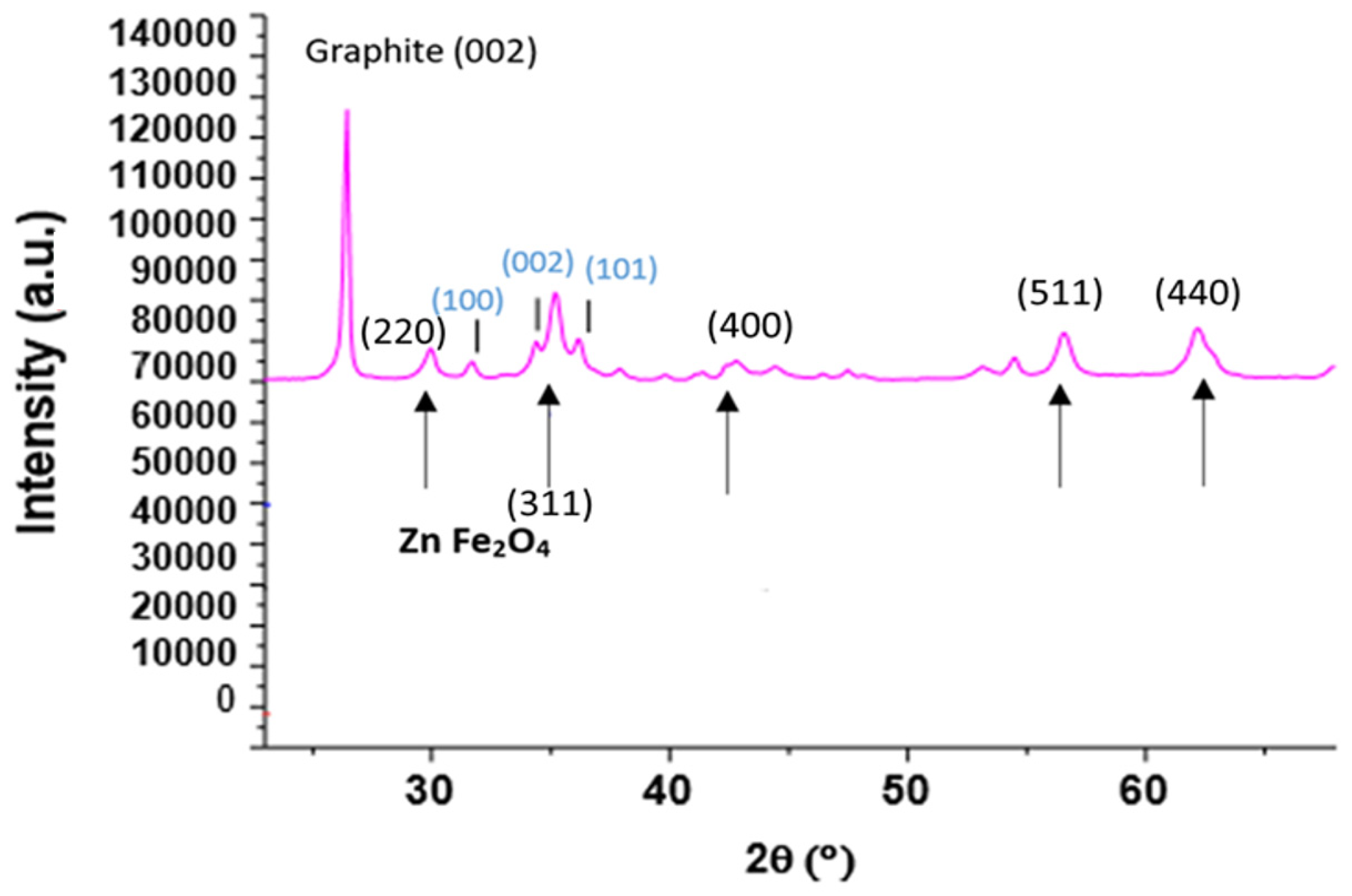
3.2. More Information Provided by XRD
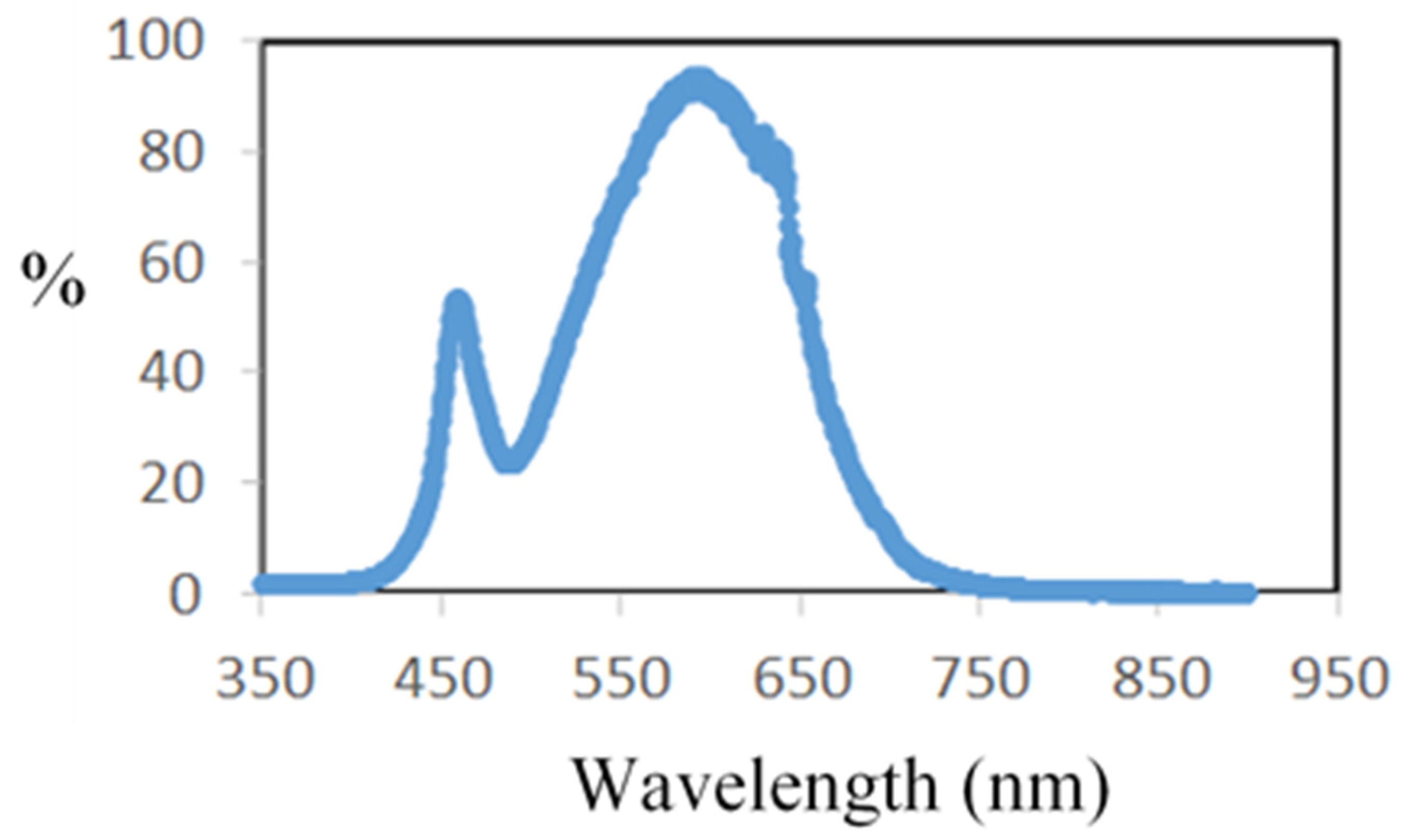
3.3. Photocatalytic Results
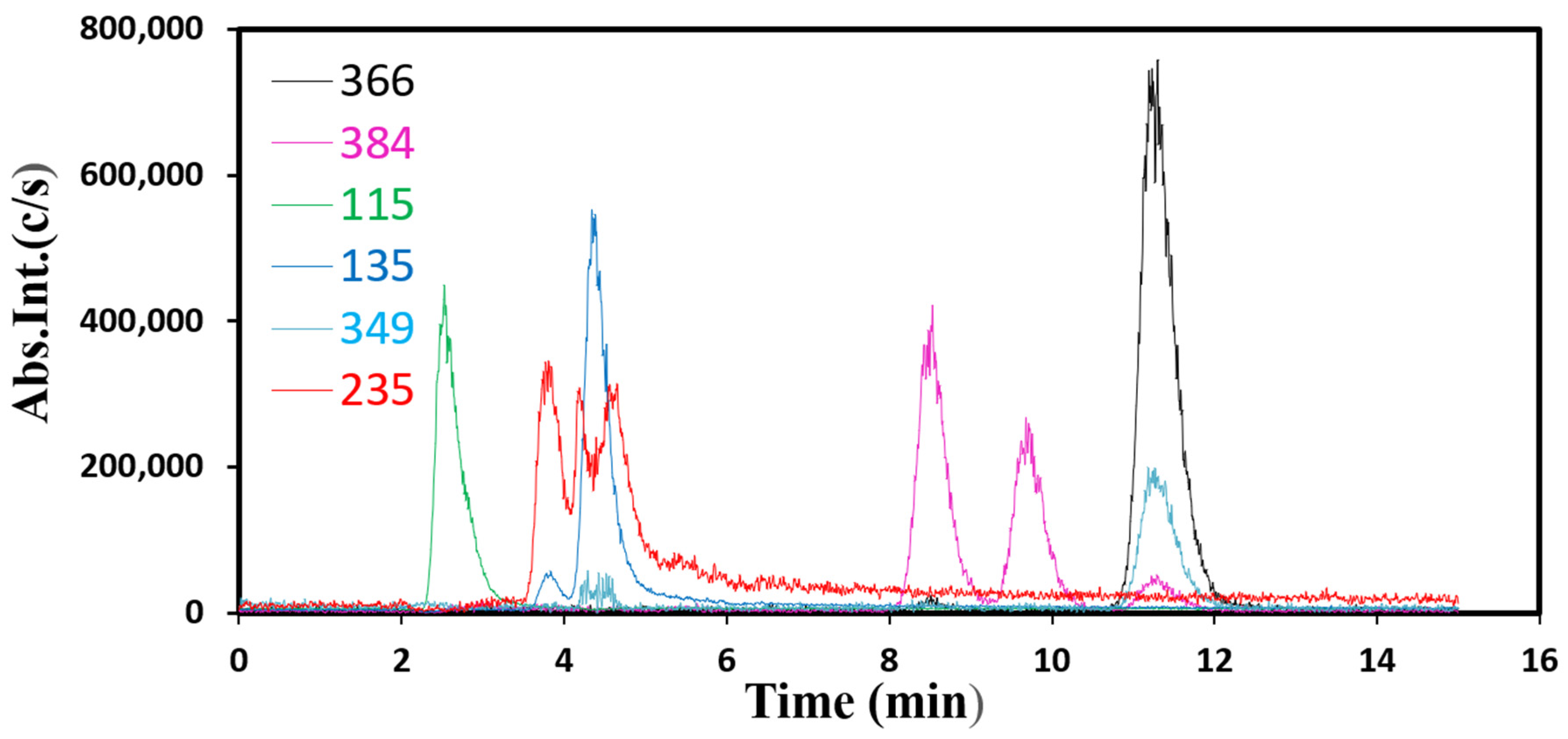
- -
- At 11.3 min of elution, m/z = 366
- -
- At 8.5 and 9.7 min of elution, same m/z = 384
- -
- At 2.5 min of elution m/z = 115
- -
- At 4.4 min m/z = 135, 235 and 349
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Canterino, M.; Marotta, R.; Paxeus, N. Antibiotic removal from wastewaters: The ozonation of amoxicillin. J. Hazard. Mater. 2005, 122, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Al-Madanat, O.; AlSalka, Y.; Curti, M.; Dillert, R.; Bahnemann, D.W. Mechanistic insights into hydrogen evolution by photocatalytic reforming of naphthalene. ACS Catal. 2020, 10, 7398–7412. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium dioxide as the most used photocatalyst for water purification: An overview. Catalysts 2022, 13, 26. [Google Scholar] [CrossRef]
- AlSalka, Y.; Al-Madanat, O.; Hakki, A. TiO2-based photocatalytic hydrogen production: How to transfer it to an applicable approach? Appl. Catal. A Gen. 2023, 662, 119287. [Google Scholar] [CrossRef]
- Narayan, H.; Alemu, H.; Macheli, L.; Sekota, M.; Thakurdesai, M.; Rao, G. Role of particle size in visible light photocatalysis of Congo Red using. Bull. Mater. Sci. 2009, 32, 499–506. [Google Scholar] [CrossRef]
- Sun, S.; Yang, X.; Zhang, Y.; Zhang, F.; Ding, J.; Bao, J.; Gao, C. Enhanced photocatalytic activity of sponge-like ZnFe2O4 synthesized by solution combustion method. Prog. Nat. Sci. Mater. Int. 2012, 22, 639–643. [Google Scholar] [CrossRef]
- Tabaja, N. Nanoparticules D’oxydes de fer et de Ferrites Obtenues par Nano-Réplication: Réactivité Chimique et Application en Dépollution des Eaux. Chimie Analytique; Université Pierre et Marie Curie—Paris VI; Université Libanaise, Faculté des Sciences: Beyrouth, Liban, 2015; Available online: https://theses.hal.science/tel-01255306 (accessed on 1 February 2024).
- Tabaja, N.; Brouri, D.; Casale, S.; Zein, S.; Jaafar, M.; Selmane, M.; Toufaily, J.; Davidson, A.; Hamieh, T. Use of SBA-15 Silica Grains for Engineering Mixtures of Oxides CoFe and NiFe for Advanced Oxidation Reactions under Visible and NIR. Appl. Catal. B Environ. 2019, 253, 369–378. [Google Scholar] [CrossRef]
- Ferroudj, N.; Nzimoto, J.; Davidson, A.; Talbot, D.; Briot, E.; Dupuis, V.; Bée, A.; Medjram, M.S.; Abramson, S. Maghemite nanoparticles and maghemite/silica nanocomposite microspheres as magnetic Fenton catalysts for the removal of water pollutants. Appl. Catal. B Environ. 2013, 136–137, 9–18. [Google Scholar] [CrossRef]
- Naseri, M.G.; Saion, E.B.; Hashim, M.; Shaari, A.H.; Ahangar, H.A. Synthesis and characterization of zinc ferrite nanoparticles by a thermal treatment method. Solid. State Commun. 2011, 151, 1031–1035. [Google Scholar] [CrossRef]
- Pérez-Parada, A.; Agüera, A.; Gómez-Ramos, M.d.M.; García-Reyes, J.F.; Heinzen, H.; Fernández-Alba, A.R. Behavior of amoxicillin in wastewater and river water: Identification of its main transformation products by liquid chromatography/electrospray quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass. Spectrom. 2011, 25, 731–742. [Google Scholar] [CrossRef]
- Yao, C.; Zeng, Q.; Goya, G.F.; Torres, T.; Liu, J.; Wu, H.; Ge, M.; Zeng, Y.; Wang, Y.; Jiang, J.Z. ZnFe2O4 Nanocrystals: Synthesis and Magnetic Properties. J. Phys. Chem. C 2007, 111, 12274–12278. [Google Scholar] [CrossRef]
- Chen, X.-J.; Dai, Y.-Z.; Wang, X.-Y.; Guo, J.; Liu, T.-H.; Li, F.-F. Synthesis and characterization of Ag3PO4 immobilized with graphene oxide (GO) for enhanced photocatalytic activity and stability over 2,4-dichlorophenol under visible light irradiation. J. Hazard. Mater. 2015, 292, 9–18. [Google Scholar] [CrossRef]
- Shi, X.F.; Xia, X.Y.; Cui, G.W.; Deng, N.; Zhao, Y.Q.; Zhuo, L.H.; Tang, B. Multiple exciton generation application of PbS quantum dots in ZnO@ PbS/graphene oxide for enhanced photocatalytic activity. Appl. Catal. B Environ. 2015, 163, 123–128. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, P.; Liu, M. Graphene Oxide Enwrapped Ag/AgX (X = Br, Cl) Nanocomposite as a Highly Efficient Visible-Light Plasmonic Photocatalyst. ACS Nano 2011, 5, 4529–4536. [Google Scholar] [CrossRef] [PubMed]
- Mata, J.P. Concentration, temperature, and salt-induced micellization of a triblock copolymer Pluronic L64 in aqueous media. J. Colloid Interface Sci. 2005, 292, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Qian, Y.; Fu, Y.; Wang, X. Graphene anchored with ZnFe2O4 nanoparticles as a high-capacity anode material for lithium-ion batteries. Solid. State Sci. 2013, 17, 67–71. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C (RSC) 2020, 8, 1198. [Google Scholar] [CrossRef]
- Park Ruoff Park, S.; Ruoff, R.S. Chemical Methods for the Production of Graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Merino, F. Vitamin C Is an Ideal Substitute for Hydrazine in the Reduction of Graphene Oxide Suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Sinthiya, M.M.; Ramamurthi, K.; Mathuri, S.; Manimozhi, T.; Kumaresan, N.; Margoni, M.M.; Karthika, P.C. Synthesis of zinc ferrite (ZnFe2O4) nanoparticles with different capping agents. Int. J. Chem. Tech. Res. 2015, 7, 2144–2149. [Google Scholar]
- Zia, H.; Shalchian, N.; Borhanian, F. Kinetics of amoxicillin degradation in aqueous solutions. Can. J. Pharm. Sci. 2008, 12, 80–83. [Google Scholar]
- Carvaval, R. Fullprof: A Program for Rietveld Refinement and Pattern Matching Analysis, Abstracts of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr. In Proceedings of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr, Toulouse, France, 16–19 July 1990; p. 127. [Google Scholar]
- Zhao, X.; Yang, Q.; Cui, J. XPS study of surface absorbed oxygen of ABO3 mixed oxides. J. Rare Earths 2008, 26, 511–514. [Google Scholar] [CrossRef]
- Levy, D.; Pavese, A.; Hanfland, M. Synthetic MgAl2O4 (spinel) at high-pressure conditions (0.0001–30 GPa): A synchrotron X-ray powder diffraction study. Am. Mineral. 2003, 88, 93–98. [Google Scholar] [CrossRef]
- Yang, C.; You, X.; Cheng, J.; Zheng, H.; Chen, Y. A novel visible-light-driven In-based MOF/graphene oxide composite photocatalyst with enhanced photocatalytic activity toward the degradation of amoxicillin. Appl. Catal. B Environ. 2017, 200, 673–680. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Yilmaz, M.; Ramírez-Coronel, A.A.; Al-Awsi, G.R.L.; Alwaily, E.R.; Asghari, A.; Balarak, D. Degradation of amoxicillin under a UV or visible light photocatalytic treatment process using Fe2O3/bentonite/TiO2: Performance, kinetic, degradation pathway, energy consumption, and toxicology studies. Optik 2023, 272, 170230. [Google Scholar] [CrossRef]
- Fakhri, A.; Khakpour, R. Synthesis and characterization of carbon or/and boron-doped CdS nanoparticles and investigation of optical and photoluminescence properties. J. Lumin. 2015, 160, 233–237. [Google Scholar] [CrossRef]
- Reyns, T.; Cherlet, M.; De Baere, S.; De Backer, P.; Croubels, S. Rapid method for the quantification of amoxicillin and its major metabolites in pig tissues by liquid chromatography-tandem mass spectrometry with emphasis on stability issues. J. Chromatogr. B 2008, 861, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Trovó, A.G.; Pupo Nogueira, R.F.; Agüera, A.; Fernandez-Alba, A.R.; Malato, S. Degradation of the antibiotic amoxicillin by Photo-Fenton process—Chemical and toxicological assessment. Water Res. 2011, 45, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Frański, R.; Czerniel, J.; Kowalska, M.; Frańska, M. Electrospray ionization collision-induced dissociation tandem mass spectrometry of amoxicillin and ampicillin and their degradation products: ESI-CID-MS/MS of amoxicillin and ampicillin and degradation products. Rapid Commun. Mass. Spectrom. 2014, 28, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Arsand, J.B.; Hoff, R.B.; Jank, L.; Meirelles, L.N.; Díaz-Cruz, M.S.; Pizzolato, T.M.; Barceló, D. Transformation products of amoxicillin and ampicillin after photolysis in aqueous matrices: Identification and kinetics. Sci. Total Environ. 2018, 642, 954–967. [Google Scholar] [CrossRef]






| Preparation Method | Specific Surface Area (m2·g−1) |
|---|---|
| Commercial Graphite GP | 14 |
| Chemical Method GO | 85 |
| Physical Method (RGO) Ultrasounds (pulses of 15 s, stop 15 s, overall time 2 h) | 16 |
| HT-ZFO | 54 |
| HT-ZFO-GO | 100 |
| Copr-ZFO | 35 |
| Copr-ZFO-L64 = 3.6 g | 51 |
| Copr-ZFO-L64 = 1.8 g | 47 |
| Copr-ZFO-RGO | 17 |
| Sample | Composition Fullprof Qualitative Quantification of Identified Phases in Weight (%) | Size of ZFO Nanoparticles (nm) |
|---|---|---|
| HT-ZFO | 86% ZFO 4% Fe-oxide (hematite variety) (α-Fe2O3, hematite variety) 10% Zn-oxide (hexagonal, wurtzite) | 6.0 |
| HT-ZFO-GO | 95% ZFO 5% Fe-oxide (α-Fe2O3, hematite) | 7.0 |
| Copr-ZFO-L64 = 3.6 g | 91% ZFO 9% Zn-oxide (hexagonal, zimcite) | 9.0 |
| Copr-ZFO-L64 = 1.8 g | 97% ZFO 2% Zn-oxide 1% (α-Fe2O3, hematite variety) | 17.0 |
| Copr-ZFO-RGO | 12% ZnFe2O4 8% ZnO (wurtzite) 81% Graphite | 11.0 |
| A. Sample | Peak | Attribution | Position (eV) | Relative Surface % | By Element Atomic Percentage |
|---|---|---|---|---|---|
| Commercial Sigma Aldrich | C 1s | C sp2 | 284.530 | 58.38 | 99.4 |
| C sp3 | 285.006 | 14.40 | |||
| C-OH, C-O-C | 286.690 | 7.48 | |||
| C=O | 288.300 | 5.20 | |||
| COOH | 290.015 | 4.33 | |||
| π-π* | 291.875 | 9.72 | |||
| O 1s | O with two negative charges | 532.561 | 18.12 | 0.6 | |
| -OH | 534.038 | 76.42 | |||
| O with one negative charge | 536.41 | 5.46 | |||
| Washed graphite | C 1s | C sp2 | 284.534 | 62.91 | 97.2 |
| C sp3 | 285.000 | 10.34 | |||
| C-OH, C-O-C | 286.415 | 6.69 | |||
| C=O | 287.729 | 4.45 | |||
| COOH | 289.103 | 3.23 | |||
| π-π* | 291.345 | 12.37 | |||
| O 1s | C-OH | 531.824 | 70.74 | 2.8 | |
| C=O | 533.303 | 23.36 | |||
| COOH | 535.208 | 5.90 | |||
| GO | C 1s | C sp2 | 284.118 | 29.94 | 63.0 |
| C sp3 | 283.353 | 14.47 | |||
| C-OH C-O-C | 285.506 | 13.81 | |||
| C=O | 286.713 | 32.31 | |||
| COOH | 288.483 | 5.66 | |||
| π-π* | 290.522 | 3.82 | |||
| O 1s | O with two negative charges; C=O | 530.91 | 4.75 | 30.0 | |
| C-OH or C-O | 532.36 | 47.09 | |||
| O with one negative charge C-O-C | 533.95 | 48.17 | |||
| B1 | Attribution | Position | Area | Atomic % | |
| HT-ZFO | C sp2 C=C | 284.4 | 57.5 | 2.2 | |
| C sp3 C-C | 284.9 | 488.1 | 18.8 | ||
| C-OH, C-O-C | 286.3 | 96.3 | 3.7 | ||
| C=O | 287.8 | 20.4 | 0.8 | ||
| O-C=O | 288.6 | 102.1 | 3.9 | ||
| π -> π* | 290.5 | 35.7 | 1.4 | ||
| N 1s | 399.9 | 17.9 | 0.4 | ||
| O 1s (O with two negative charges, O2−) | 529.8 | 2000.8 | 26.3 | ||
| O 1s (−OH, hydroxide) | 531.3 | 1448.1 | 19.1 | ||
| O 1s (O with one negative charge O−) | 532.7 | 67.2 | 0.9 | ||
| Zn 2p | 1021.4 | 2879.8 | 6.5 | ||
| Fe 2p | 710.8 | 4815.4 | 12.5 | ||
| Cl 2p | 198.6 | 40.3 | 0.8 | ||
| Si 2p | 100.7 | 48.6 | 2.7 | ||
| B2 | Attribution | Position | Area | Atomic % | |
| HT-ZFO-GO | C sp2 | 284.6 | 378.0 | 14.5 | |
| C sp3 | 285.1 | 344.9 | 13.4 | ||
| C-OH, C-O-C | 285.5 | 122.4 | 4.7 | ||
| C=O | 287.9 | 35.6 | 1.5 | ||
| π -> π* | 291.1 | 77.2 | 2.9 | ||
| N 1s | 400.1 | 39.4 | 0.8 | ||
| O 1s (O with two negative charges) | 530.2 | 1601.8 | 21.0 | ||
| O 1s (-OH, Hydroxide) | 531.8 | 1232.5 | 16.2 | ||
| O 1s (O with one negative charge) | 533.2 | 182.53 | 2.4 | ||
| Zn 2p | 1021.9 | 2109.5 | 4.8 | ||
| Fe 2p | 711.2 | 3170.7 | 9.2 | ||
| Cl 2p | 198.8 | 29.5 | 0.6 | ||
| Si 2p | 103.4 | 3.7 | 3.7 | ||
| Sample | Removal of AMX after 165 or 225 min (in Concentration %) |
|---|---|
| Copr-ZFO | Weak |
| Copr-ZFO-RGO | 45% of AMX removal after 225 min but never 100% |
| Copr-ZFO-L64 = 1.8 g | Strong, 79% of AMX removal after 225 min but never 100% |
| Copr-ZFO-L64 = 3.6 g | Strong, 73% of AMX removal after 225 min but never 100% |
| HT-ZFO-GO | Very strong, 100% AMX removal after 100 min |
| HT-ZFO | Very strong, 100% AMX removal in less than 100 min if light is on. |
| Article | Optimum Conditions | Efficiency (%) |
|---|---|---|
| This study | 1 g/L HT-ZnFe2O4, 10 mg/L AMX Under visible LED light pH = 5 | −100% in less than 100 min |
| Tarek Al Musawi et al. (2023) [31] | 0.75 mg/L Fe2O3/bentite/TiO2,25 mg/L AMX Under visible LED light pH = 5 | −98.8% after 90 min |
| Yang et al. (2017) [30] | 0.6 g/L MOF/graphene oxide, 20 mg/L AMX Under visible light pH = 5 | −93% after 120 min |
| Fakhri et al. (2015) [32] | 0.1 g/L C-B-CdS nanoparticles, 10 mg/L AMX Under visible light pH = 5 | −40% after 120 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jezzini, A.; Chen, Y.; Davidson, A.; Wallez, G.; Hamieh, T.; Toufaily, J. Photocatalytic Decomposition of Amoxicillin Using Zinc Ferrite Nanoparticles. Crystals 2024, 14, 291. https://doi.org/10.3390/cryst14030291
Jezzini A, Chen Y, Davidson A, Wallez G, Hamieh T, Toufaily J. Photocatalytic Decomposition of Amoxicillin Using Zinc Ferrite Nanoparticles. Crystals. 2024; 14(3):291. https://doi.org/10.3390/cryst14030291
Chicago/Turabian StyleJezzini, Aya, Yujin Chen, Anne Davidson, Gilles Wallez, Tayssir Hamieh, and Joumana Toufaily. 2024. "Photocatalytic Decomposition of Amoxicillin Using Zinc Ferrite Nanoparticles" Crystals 14, no. 3: 291. https://doi.org/10.3390/cryst14030291







