Energetic Features of H-Bonded and π-Stacked Assemblies in Pyrazole-Based Coordination Compounds of Mn(II) and Cu(II): Experimental and Theoretical Studies
Abstract
:1. Introduction
2. Materials and Methods
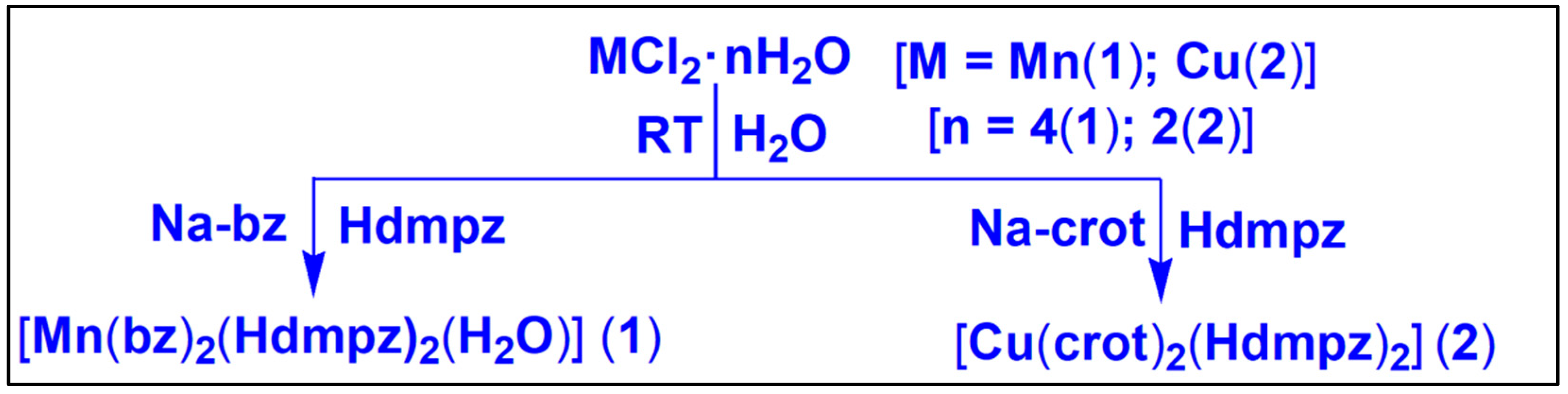
2.1. Synthesis
2.1.1. Synthesis of [Mn(bz)2(Hdmpz)2(H2O)] (1)
2.1.2. Synthesis of [Cu(crot)2(Hdmpz)2] (2)
2.2. Crystallographic Data Collection and Refinement
2.3. Computational Methods
3. Results and Discussion
3.1. Syntheses and General Aspects
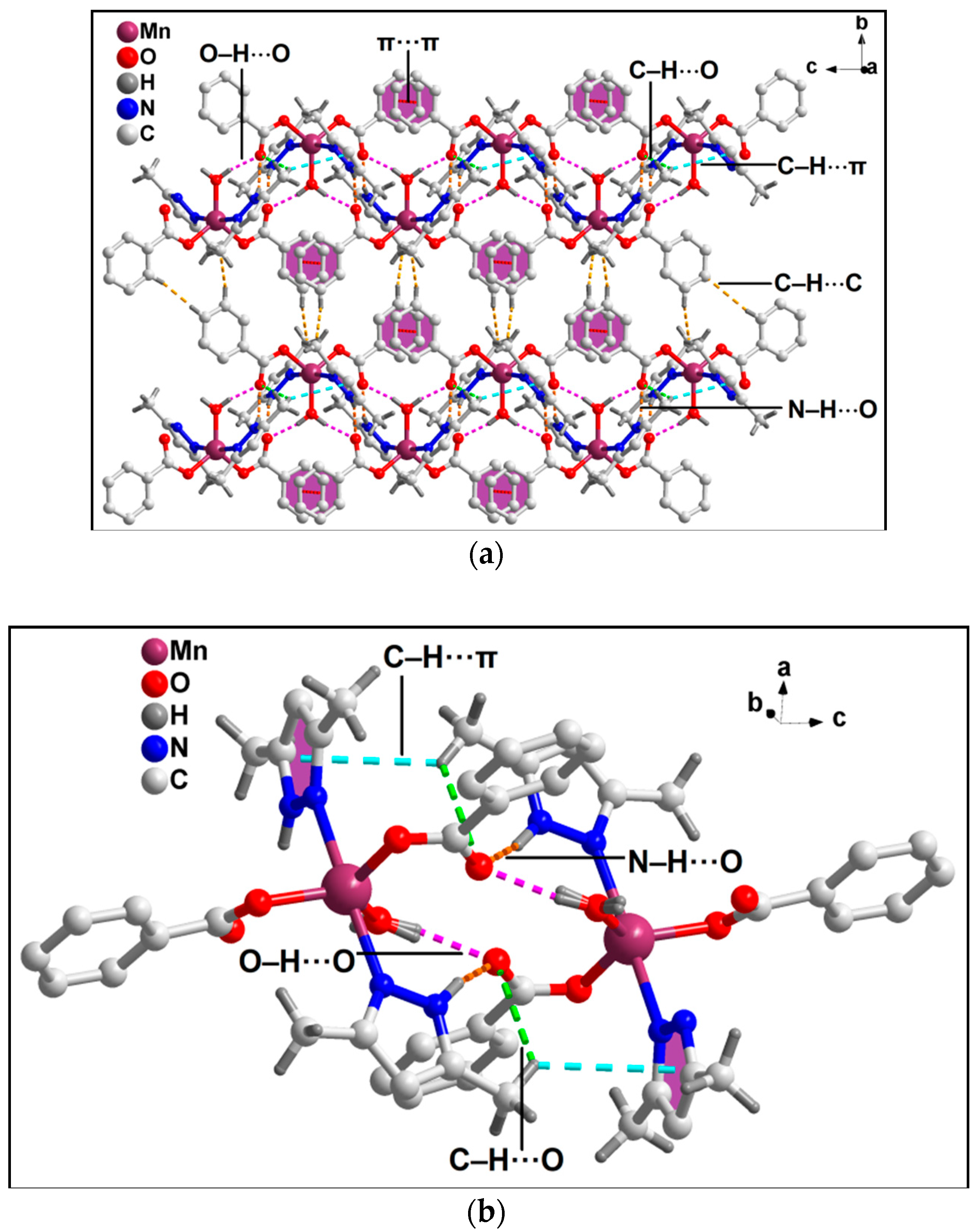
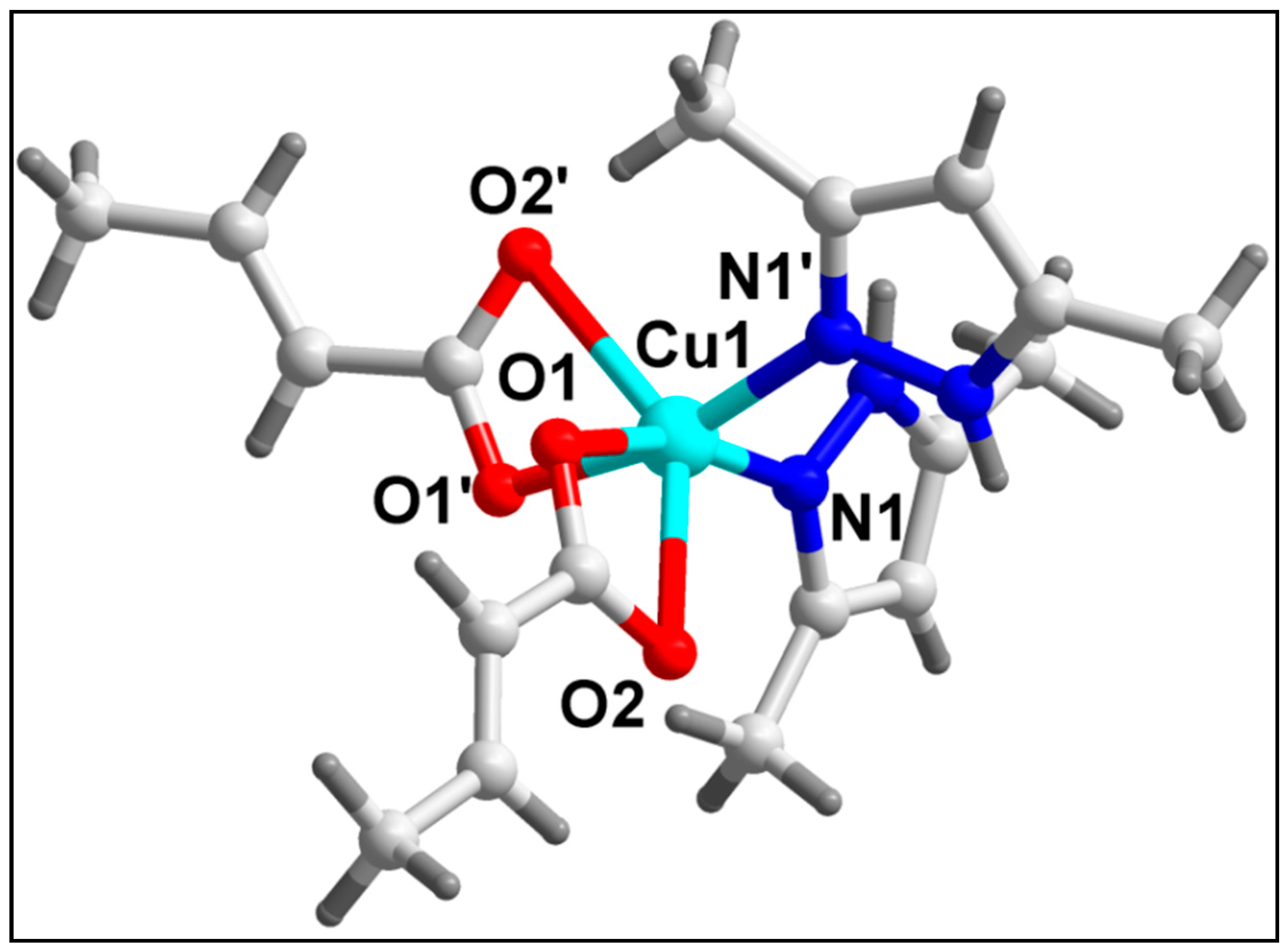
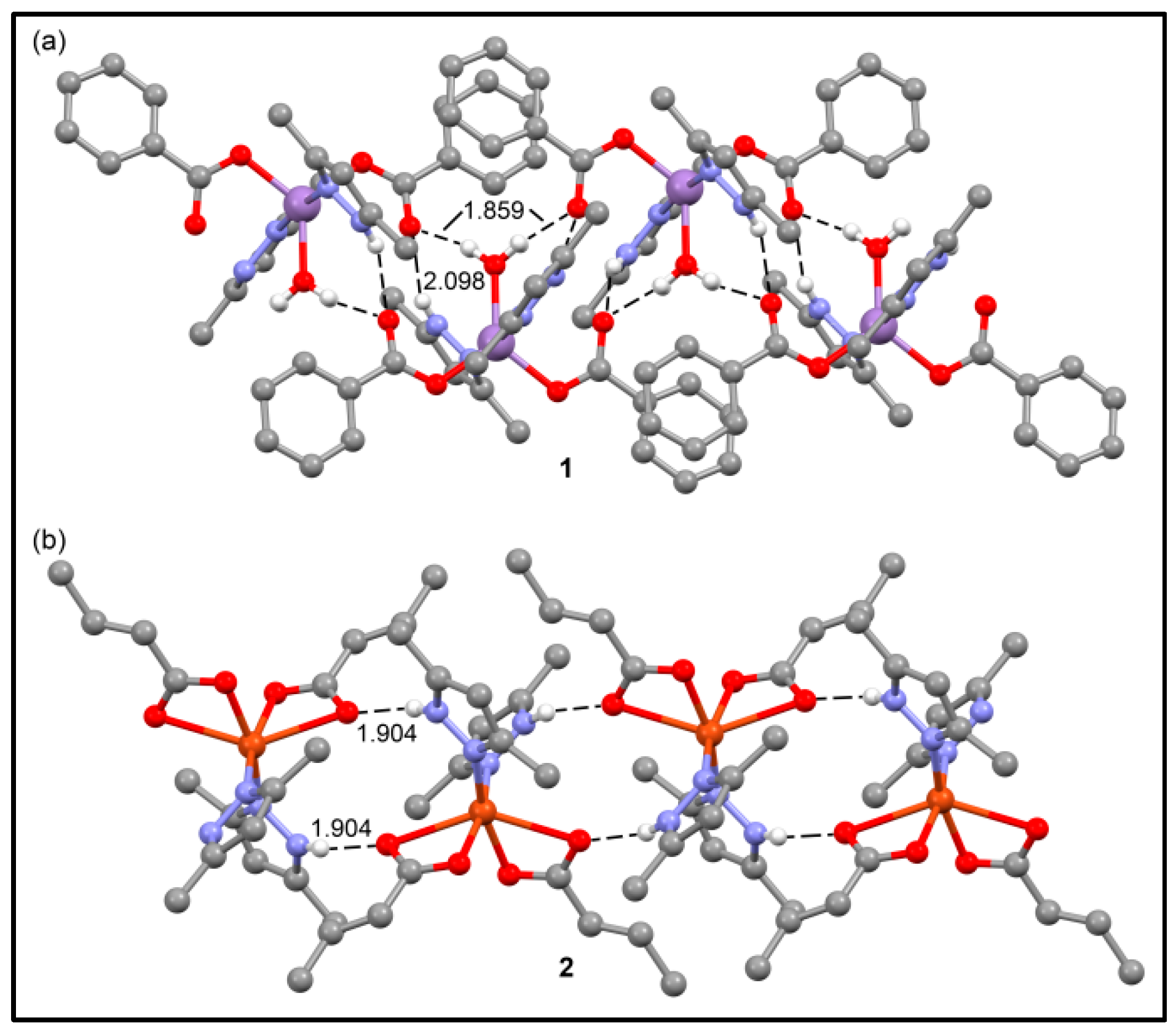
3.2. Crystal Structure Analysis
3.3. Spectral Studies
3.3.1. FT-IR Spectroscopy
3.3.2. Electronic Spectroscopy
3.4. Thermogravimetric Analysis
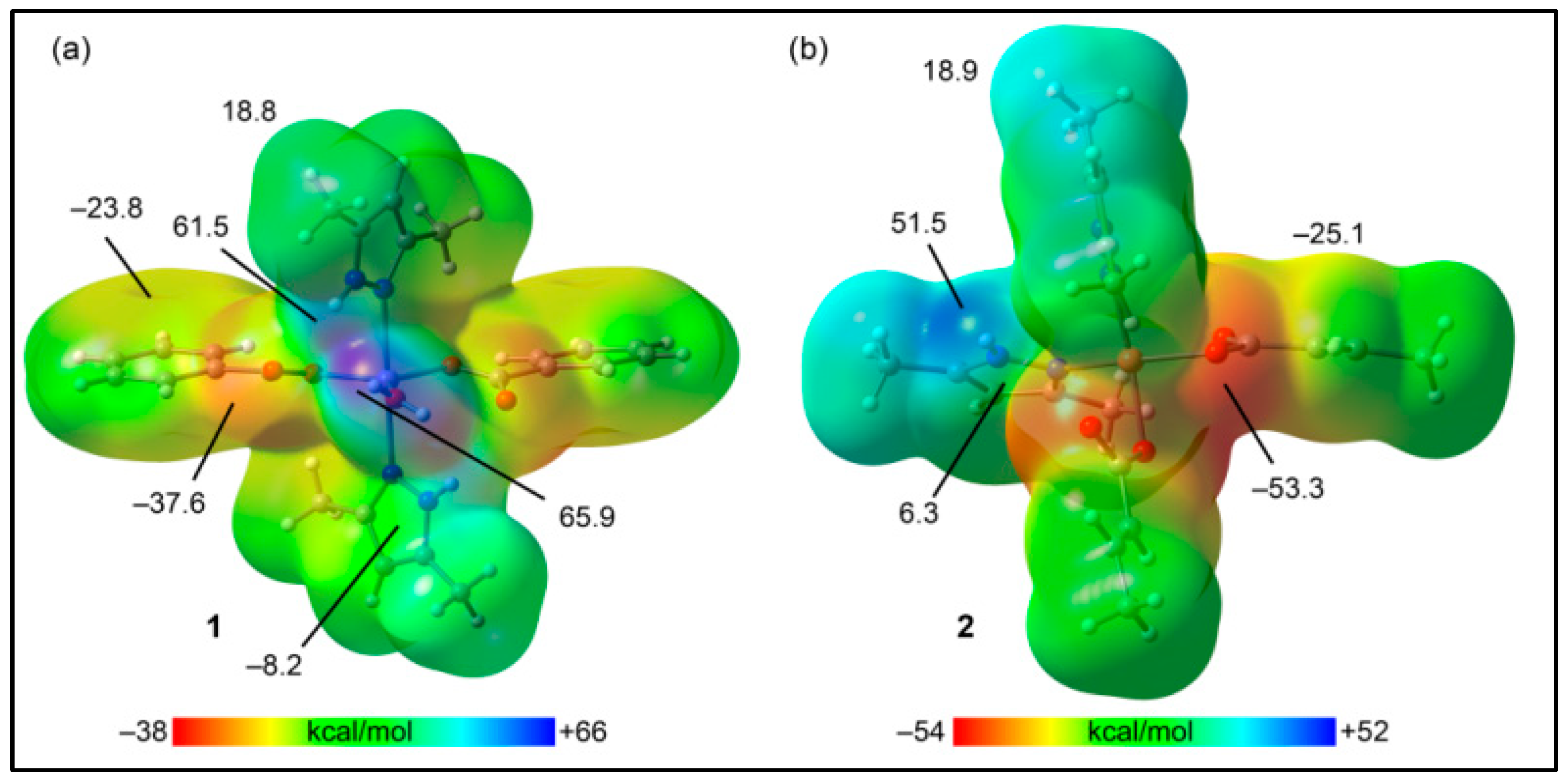
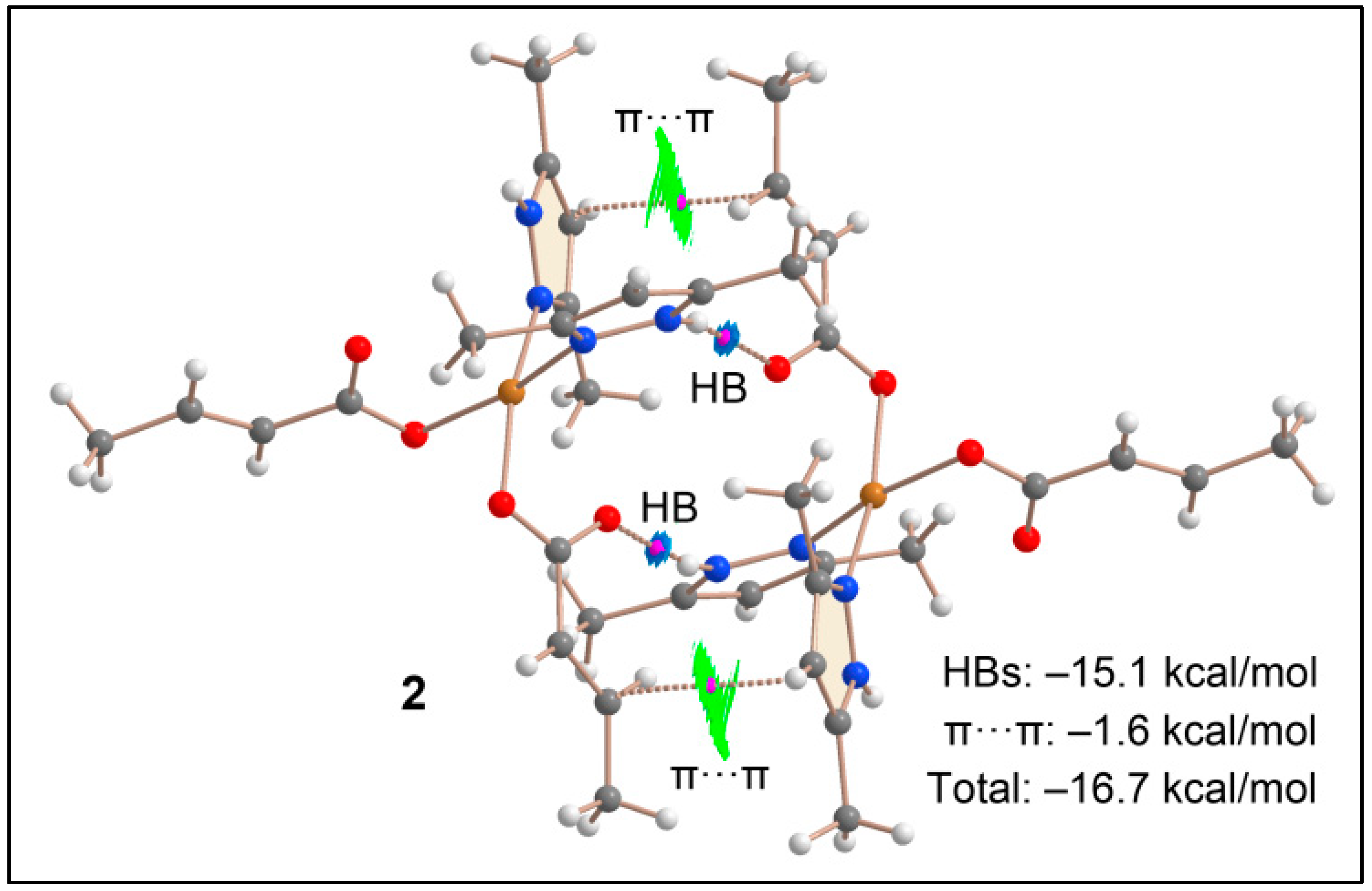
3.5. Theoretical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional Metal–Organic Frameworks as Effective Sensors of Gases and Volatile Compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ho, W.; Qin, X.; Leung, C.-F.; Au, V.K.-M.; Lee, S. Metal–Organic Frameworks for NOx Adsorption and Their Applications in Separation, Sensing, Catalysis, and Biology. Small 2022, 18, 2105484–2105498. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.S.; Skorupskii, G.; Dincă, M. Electrically Conductive Metal–Organic Frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef] [PubMed]
- Hambley, T.W. Developing new metal-based therapeutics: Challenges and opportunities. Dalton Trans. 2007, 43, 4929–4937. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.H.; Orvig, C. Metal complexes in medicinal chemistry: New vistas and challenges in drug design. Dalton Trans. 2006, 6, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hamanaka, S.; Nishimoto, Y.; Irle, S.; Yokoyama, T.; Yoshikawa, H.; Awaga, K. In Operando X-ray Absorption Fine Structure Studies of Polyoxometalate Molecular Cluster Batteries: Polyoxometalates as Electron Sponges. J. Am. Chem. Soc. 2012, 134, 4918–4928. [Google Scholar] [CrossRef] [PubMed]
- Swieger, G.F.; Malefetse, T.J. New Self-Assembled Structural Motifs in Coordination Chemistry. Chem. Rev. 2000, 100, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Koohpayeh, S.M. Single Crystal Growth by the Traveling Solvent Technique: A Review. Prog. Cryst. Growth Charact. Mater. 2016, 62, 22–34. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Champness, N.R.; Janiak, C. Recent advances in crystal engineering. CrystEngComm 2012, 12, 22–40. [Google Scholar] [CrossRef]
- Calvin, J.J.; Brewer, A.S.; Alivisatos, A.P. The Role of Organic Ligand Shell Structures in Colloidal Nanocrystal Synthesis. Nat Synth 2022, 1, 127–137. [Google Scholar] [CrossRef]
- Schiebi, J.; Schulmeister, J.; Doppiu, A.; Worner, E.; Rudolph, M.; Karch, R.; Hashmi, A.S.K. An Industrial Perspective on Counter Anions in Gold Catalysis: On Alternative Counter Anions. Adv. Synth. Catal. 2018, 360, 3949–3965. [Google Scholar]
- Khavasi, H.R.; Sadegh, B.M.M. Temperature-Dependent Supramolecular Motif in Coordination Compounds. Inorg. Chem. 2010, 49, 5356–5370. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, W.; Jiang, Z.W.; Zhao, T.T.; Li, Y.; Li, C.M.; Huang, C.Z.; Li, Y.F. Novel solvent-triggered transformation of Cu-based metal-organic gels to highly monodisperse metal-organic frameworks with controllable shapes. Chem. Eng. J. 2019, 374, 1231. [Google Scholar] [CrossRef]
- Chen, H.; Fraser Stoddart, J. From Molecular to Supramolecular Electronics. Nat. Rev. Mater 2021, 6, 804–828. [Google Scholar] [CrossRef]
- Hobza, P.; Zahradník, R.; Müller-Dethlefs, K. The World of Non-Covalent Interactions: 2006. Collect. Czech. Chem. Commun. 2006, 71, 443–531. [Google Scholar] [CrossRef]
- Lackinger, M.; Heckl, W.M. Langmuir. Carboxylic Acids: Versatile Building Blocks and Mediators for Two-Dimensional Supramolecular Self-Assembly. Langmuir 2009, 25, 11307–11321. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar]
- Hong, M.C.; Chen, L. Design and Construction of Coordination Polymers; John Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11190. [Google Scholar] [CrossRef] [PubMed]
- Bauza, A.; Mooibroek, T.J.; Frontera, A. Tetrel-Bonding Interaction: Rediscovered Supramolecular Force? Angew. Chem. Int. Ed. 2013, 52, 12317–12329. [Google Scholar] [CrossRef] [PubMed]
- Egli, M.; Sarkhel, S. Lone Pair−Aromatic Interactions: To Stabilize or Not to Stabilize. Acc. Chem. Res. 2007, 40, 197. [Google Scholar] [CrossRef]
- Mooibroek, T.J.; Gamez, P.; Reedijk, J. Lone pair–π interactions: A new supramolecular bond? CrystEngComm 2008, 10, 1501–1520. [Google Scholar] [CrossRef]
- Kumar, P.; Banerjee, S.; Radha, A.; Firdoos, T.; Sahoo, S.C.; Pandey, S.K. Role of Non-Covalent Interactions in the Supramolecular Architectures of Mercury(II) Diphenyldithiophosphates: An Experimental and Theoretical Investigation. New J. Chem. 2021, 45, 2249–2263. [Google Scholar] [CrossRef]
- Reeda, V.J.; Sakthivel, S.; Divya, P.; Javed, S.; Jothy, V.B. Conformational Stability, Quantum Computational (DFT), Vibrational, Electronic and Non-Covalent Interactions (QTAIM, RDG and IGM) of Antibacterial Compound N-(1-Naphthyl)Ethylenediamine Dihydrochloride. J. Mol. Struct. 2024, 1298, 137043–137060. [Google Scholar] [CrossRef]
- Wheeler, S.E.; McNeil, A.J.; Muller, P.; Swager, T.M.; Houk, K.N. Probing Substituent Effects in Aryl-Aryl Interactions Using Stereoselective Diels-Alder Cycloadditions. J. Am. Chem. Soc. 2010, 132, 3304–3311. [Google Scholar] [CrossRef] [PubMed]
- Verevkin, S.P.; Kondratev, S.O.; Zaitsau, D.H.; Zherikova, K.V.; Ludwig, R. Quantification and Understanding of Non-Covalent Interactions in Molecular and Ionic Systems: Dispersion Interactions and Hydrogen Bonding Analysed by Thermodynamic Methods. J. Mol. Liq. 2021, 343, 117547–117560. [Google Scholar] [CrossRef]
- Park, T.; Zimmerman, S.C.; Nakashima, S. A Highly Stable Quadruply Hydrogen-Bonded Heterocomplex Useful for Supramolecular Polymer Blends. J. Am. Chem. Soc. 2005, 127, 6520–6536. [Google Scholar] [CrossRef]
- Pu, L.-M.; An, X.-X.; Liu, C.; Long, H.-T.; Zhao, L. Insights into Crystal Structures, Supramolecular Architectures and Antioxidant Activities of Self-Assembled Fluorescent Hetero-Multinuclear [Cu (II)-Ln (III)] (Ln = La, Ce, Pr and Nd) Salamo-like Complexes. Appl. Organomet. Chem. 2020, 34, 5980–5995. [Google Scholar] [CrossRef]
- Banerjee, A.; Mukherjee, P.S. Chapter Nine—Self-Assembled Discrete Coordination Architectures toward Biological Applications. Adv. Inorg. Chem. 2023, 81, 345–387. [Google Scholar]
- Cai, H.-Q.; Liu, C.-H.; Xin, Y.; Wang, C.; Bai, F.-Y.; Sun, L.-X.; Xing, Y.-H. Construction of Poly-Iodine Aromatic Carboxylate Mn/Co Frameworks and Iodine Adsorption Behavior. Transit. Met. Chem. 2021, 46, 633–644. [Google Scholar] [CrossRef]
- Phukan, N.; Baruah, J.B. Hydrolysis of 4-(4-oxopentan-2-ylideneamino) benzoic acid and in-situ formation of nickel (II), zinc (II) and cadmium (II) complexes of 4-aminobenzoic acid. Inorg. Chim. Acta 2013, 396, 430–435. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Sherazi, S.K.A. Synthesis, Characterization and Role of Anions (Nitrate, Sulphate, Oxalate and Acetate) in the Biological Activity of Hydrazine Derived Compounds and Their Metal Chelates. Met-Based Drugs 1997, 4, 327–332. [Google Scholar] [CrossRef]
- Wu, H.; Wang, F.; Shi, F.; Yang, Z.; Zhang, H.; Peng, H. Three-and Four-Coordinate Ag(I) Complexes of Crotonate andBis(Benzimidazole)-2-OxapropaneLigands: Syntheses, Crystal Structures, DNA-Binding Studies and Antioxidant Activities. Transit. Met. Chem. 2015, 40, 555–564. [Google Scholar] [CrossRef]
- Xu, Z.-H.; Huang, Z.-Q.; Liu, X.-H.; Zhao, Y.; Lu, Y.; Sun, W.-Y. Luminescent Silver(I) Complexes with Pyrazole-Tetraphenylethene Ligands: Turn-on Fluorescence Due to the Coordination-Driven Rigidification and Solvent-Oriented Structural Transformation. Dalton Trans. 2021, 50, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Lunagariya, M.V.; Thakor, K.P.; Varma, R.R.; Waghela, B.N.; Pathak, C.; Patel, M.N. Synthesis, characterization and biological application of 5-quinoline 1,3,5-trisubstituted pyrazole based platinum(ii) complexes. Med Chem Comm 2018, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.M.; Junior, H.C.S.; Costa, S.C.; Cardoso, C.M.; Cruz, A.G.B.; Santos, C.E.R.; Candela, D.R.S.; Soriano, S.; Marques, M.M.; Ferreira, G.B.; et al. Mononuclear Coordination Compounds Containing a Pyrazole-Based Ligand: Syntheses, Magnetism and Acetylcholinesterase Inhibition Assays. J. Mol. Struct. 2020, 1205, 127564–127579. [Google Scholar] [CrossRef]
- Bouroumane, N.; El Kodadi, M.; Touzani, R.; El Boutaybi, M.; Oussaid, A.; Hammouti, B.; Nandiyanto, A.B.D. New Pyrazole-Based Ligands: Synthesis, Characterization, and Catalytic Activity of Their Copper Complexes. Arab. J. Sci. Eng. 2022, 47, 269–279. [Google Scholar] [CrossRef]
- Draoui, Y.; Radi, S.; Tanan, A.; Oulmidi, A.; Miras, H.N.; Benabbes, R.; Ouahhoudo, S.; Mamri, S.; Rotaru, A.; Garcia, Y. NovelFamily of Bis-Pyrazole Coordination Complexesas Potent Antibacterialand Antifungal Agents. RSC Adv. 2022, 12, 17755–17764. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, S.; Ghadermazi, M.; Fattahi, A.; Shokraii, S.; Alimoradi, M.; Shahbazi, B.; Azar, A.R.J. Synthesis, crystallographic and spectroscopic studies, evaluation as antimicrobial and cytotoxic agents of a novel mixed-ligand nickel(II) complex. J. Coord. Chem. 2017, 70, 1406–1422. [Google Scholar] [CrossRef]
- Azam, M.; Mohammad Wabaidur, S.; Alam, M.; Trzesowska-Kruszynska, A.; Kruszynski, R.; Al-Resayes, S.I.; Fahhad Alqahtani, F.; Rizwan Khan, M. Rajendra Design, Structural Investigations and Antimicrobial Activity of Pyrazole Nucleating Copper and Zinc Complexes. Polyhedron 2021, 195, 114991–115004. [Google Scholar] [CrossRef]
- Alam, M.J.; Alam, O.; Naim, M.J.; Nawaz, F.; Manaithiya, A.; Imran, M.; Thabet, H.K.; Alshehri, S.; Ghoneim, M.M.; Alam, P.; et al. Recent Advancement in Drug Design and Discovery of Pyrazole Biomolecules as Cancer and Inflammation Therapeutics. Molecules 2022, 27, 8708. [Google Scholar] [CrossRef]
- Harit, T.; Malek, F.; El Bali, B.; Khan, A.; Dalvandi, K.; Marasini, B.P.; Noreen, S.; Malik, R.; Khan, S.; Choudhary, M.I. Synthesis and enzyme inhibitory activities of some new pyrazole-based heterocyclic compounds. Med. Chem. Res. 2012, 21, 2772–2778. [Google Scholar] [CrossRef]
- Li, G.; Cheng, Y.; Han, C.; Song, C.; Huang, N.; Du, Y. Pyrazole-Containing Pharmaceuticals: Target, Pharmacological Activity, and Their SAR Studies. RSC Med. Chem. 2022, 13, 1300–1321. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Sharma, P.K.; Kaushik, N. Pyrazole: A Versatile Moiety. Int. J. Chem. Technol. Res. 2011, 3, 11–32. [Google Scholar]
- Shagufta; Ahmad, I. Transition Metal Complexes as Proteasome Inhibitors for Cancer Treatment. Inorganica Chim. Acta 2020, 506, 119521–119535. [Google Scholar] [CrossRef]
- Li, J.; Chen, T. Transition Metal Complexes as Photosensitizers for Integrated Cancer Theranostic Applications. Coordination Chem. Rev. 2020, 418, 213355–213370. [Google Scholar] [CrossRef]
- Imberti, C.; Zhang, P.; Huang, H.; Sadler, P.J. New Designs for Phototherapeutic Transition Metal Complexes. Angew. Chem. Int. Ed. 2020, 59, 61–73. [Google Scholar] [CrossRef]
- Permyakov, E.A. Metal Binding Proteins. Encyclopedia 2021, 1, 261–292. [Google Scholar] [CrossRef]
- Salih, B.D.; Dalaf, A.H.; Alheety, M.A.; Rashed, W.M.; Abdullah, I.Q. Biological Activity and Laser Efficacy of New Co (II), Ni (II), Cu (II), Mn (II) and Zn (II) Complexes with Phthalic Anhydride. Mater. Today Proc. 2021, 43, 869–874. [Google Scholar] [CrossRef]
- Bouzerafa, B.; Ourari, A.; Aggoun, D.; Ruiz-Rosas, R.; Ouennoughi, Y.; Morallon, E. Novel nickel(II) and manganese(III) complexes with bidentate Schiff-base ligand: Synthesis, spectral, thermogravimetry, electrochemical and electrocatalytical properties. Res. Chem. Intermed. 2016, 42, 4839–4858. [Google Scholar] [CrossRef]
- Osypiuk, D.; Cristóvão, B.; Bartyzel, A. New Coordination Compounds of CuII with Schiff Base Ligands—Crystal Structure, Thermal, and Spectral Investigations. Crystals 2020, 10, 1004. [Google Scholar] [CrossRef]
- Barma, A.; Bhattacharjee, A.; Roy, P. Dinuclear Copper(II) Complexes with N,O Donor Ligands: Partial Ligand Hydrolysis and Alcohol Oxidation Catalysis. Eur. J. Inorg. Chem. 2021, 2021, 2284–2292. [Google Scholar] [CrossRef]
- Czylkowska, A.; Rogalewicz, B.; Raducka, A.; Błaszczyk, N.; Maniecki, T.; Wieczorek, K.; Mierczyński, P. Synthesis, Spectroscopic, Thermal and Catalytic Properties of Four New Metal (II) Complexes with Selected N- and O-Donor Ligands. Materials 2020, 13, 3217. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Baruah, J.B. Stable Host–Guest Complexes of Bis-2,6-Pyridinedicarboxylate Iron(III) with Dihydroxybenzenes. Polyhedron 2017, 138, 103–108. [Google Scholar] [CrossRef]
- Moriyasu, R.; John, S.G.; Bian, X.; Yang, S.-C.; Moffett, J.W. Cu Exists Predominantly as Kinetically Inert Complexes Throughout the Interior of the Equatorial and North Pacific Ocean. Glob. Biogeochem. Cycles 2023, 37, e2022GB007521. [Google Scholar] [CrossRef]
- SADABS, V2.05; Bruker AXS: Madison, WI, USA, 1999.
- Sheldrick, G.M. A Short History of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX Suite for Small-Molecule Single-Crystal Crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond 3.1f; Crystal Impact GbR: Bonn, Germany, 2008. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Keith, T.A. AIMAll (Version 19.10.12); TK Gristmill Software: Overland Park, KS, USA, 2019. [Google Scholar]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Orojloo, M.; Zolgharnein, P.; Solimannejad, M.; Amani, S. Synthesis and characterization of cobalt (II), nickel (II), copper (II) and zinc (II) complexes derived from two Schiff base ligands: Spectroscopic, thermal, magnetic moment, electrochemical and antimicrobial studies. Inorganica Chim. Acta. 2017, 467, 227–237. [Google Scholar] [CrossRef]
- Ammar, R.A.; Alaghaz, A.M.A.; Zayed, M.E.; Bedair, L.A.A. Synthesis, spectroscopic, molecular structure, antioxidant, antimicrobial and antitumor behavior of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes of O2N type tridentate chromone-2-carboxaldehyde Schiff’s base ligand. J. Mol. Struct. 2017, 1141, 368–381. [Google Scholar] [CrossRef]
- Psomas, G. Copper(II) and zinc(II) coordination compounds of non-steroidal anti-inflammatory drugs: Structural features and antioxidant activity. Coord. Chem. Rev. 2020, 412, 213259–213271. [Google Scholar] [CrossRef]
- Singh, U.P.; Sharma, A.K.; Tyagi, P.; Upreti, S.; Singh, R.K. Mononuclear Manganese Carboxylate Complexes: Synthesis and Structural Studies. Polyhedron 2006, 25, 3628–3638. [Google Scholar] [CrossRef]
- Uvarova, M.A.; Ageshina, A.A.; Golubnichaya, M.A.; Nefedov, S.E. Manganese(II) Carboxylates Containing Coordinated 3,5-Dimethylpyrazole. Russ. J. Inorg. Chem. 2015, 60, 934–941. [Google Scholar] [CrossRef]
- Gogoi, A.; Das, A.; Frontera, A.; Verma, A.K.; Bhattacharyya, M.K. Energetically significant unconventional π-π contacts involving fumarate in a novel coordination polymer of Zn(II): In-vitro anticancer evaluation and theoretical studies. Inorganica Chim. Acta. 2019, 493, 1–13. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Kokina, T.E.; Glinskaya, L.A.; Tkachev, A.V.; Plyusnin, V.F.; Tsoy, Y.V.; Bagryanskaya, I.Y.; Vasilyev, E.S.; Piryazev, D.A.; Sheludyakova, L.A.; Larionov, S.V. Chiral zinc(II) and cadmium(II) complexes with a dihydrophenanthroline ligand bearing (–)-α-pinene fragments: Synthesis, crystal structures and photophysical properties. Polyhedron 2016, 117, 437–444. [Google Scholar] [CrossRef]
- Das, S.; Bharadwaj, P.K. Self-Assembly of a Luminescent Zinc(II) Complex: a Supramolecular Host−Guest Fluorescence Signaling System for Selective Nitrobenzene Inclusion. Inorg. Chem. 2006, 45, 5257–5259. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Platts, J.A.; Das, B.K. Novel C–H⋯C contacts involving 3,5-dimethylpyrazoleligands in a tetracoordinate Co(ii) complex. Dalton Trans. 2011, 40, 11605–11612. [Google Scholar] [CrossRef]
- Khan, E.; Gul, Z.; Shahzad, A.; Jan, M.S.; Ullah, F.; Tahir, M.N.; Noor, A. Coordination Compounds of 4,5,6,7-Tetrahydro-1H-Indazole with Cu(II), Co(II) and Ag(I): Structural, Antimicrobial, Antioxidant and Enzyme Inhibition Studies. J. Coord. Chem. 2017, 70, 4054–4069. [Google Scholar] [CrossRef]
- Saha, U.; Dutta, D.; Nath, H.; Franconetti, A.; Frontera, A.; Bhattacharyya, M.K. Supramolecular Association in Cu(II) Coordination Complexes Involving Energetically Significant NO⋯NO π–Hole Interaction and Cooperative π-Stacked Ternary Assembly: Experimental and Theoretical Studies. Inorganica Chim. Acta 2019, 488, 159–169. [Google Scholar] [CrossRef]
- Manna, S.C.; Mistri, S.; Jana, A.D. A rare supramolecular assembly involving ion pairs of coordination complexes with a host-guest relationship: Synthesis, crystal structure, photoluminescence and thermal study. CrystEngComm 2012, 14, 7415–7422. [Google Scholar] [CrossRef]
- Islam, S.M.N.; Dutta, D.; Guha, A.K.; Bhattacharyya, M.K. An unusual werner type clathrate of Mn(II) benzoate involving energetically significant weak C-H⋯C contacts: A combined experimental and theoretical study. J. Mol. Struct. 2019, 1175, 130–138. [Google Scholar] [CrossRef]
- Nath, H.; Dutta, D.; Sharma, P.; Frontera, A.; Verma, A.K.; Barceló-Oliver, M.; Devi, M.; Bhattacharyya, M.K. Adipatobridgednovel hexanuclear Cu(ii) and polymeric Co(ii) coordination compounds involving cooperative supramolecular assemblies and encapsulated guest water clusters in a square grid host: Antiproliferative evaluation and theoretical studies. Dalton Trans. 2020, 49, 9863–9881. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, H.H.; Hecke, K.V.; Cui, G.H. Cobalt(II) and Copper(II) Complexes Constructed from Bis(benzimidazole) and 2,6-Pyridinedicarboxylate Co-ligands: Synthesis, Crystal Structures, and Catalytic Properties. Z. Anorg. Allg. Chem. 2015, 641, 642–649. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared Spectra of Inorganic and Cordination Compounds Part B, 5th ed.; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Ay, B.; Yildiz, E.; Kani, I. Novel heteroleptic lanthanide organic frameworks containing pyridine-2,5-dicarboxylic acid and in situ generated piperazine-2,5-dicarboxylic acid from piperazine: Hydrothermal synthesis and luminescent properties. J. Solid State Chem. 2016, 233, 44–51. [Google Scholar] [CrossRef]
- Yang, L.; Liu, L.; Wu, L.; Zhang, H.; Song, S. A series of 3D isomorphous lanthanide coordination polymers based on flexible dicarboxylate ligand: Synthesis, structure, characterization, and properties. Dyes Pigm. 2014, 105, 180–191. [Google Scholar] [CrossRef]
- Łyszczek, R.; Mazur, L. Polynuclear complexes constructed by lanthanides and pyridine-3,5-dicarboxylate ligand: Structures, thermal and luminescent properties. Polyhedron 2012, 41, 7–19. [Google Scholar] [CrossRef]
- Li, J.; Xing, Y.H.; Zhao, H.Y.; Li, Z.P.; Wang, C.G.; Zeng, X.Q.; Ge, M.F.; Niu, S.Y. Constructions of a set of hydrogen-bonded supramolecules from reactions of transition metals with 3,5-dimethylpyrazole and different dicarboxylate ligands. Inorganica Chim. Acta. 2009, 362, 2788–2795. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, W.; Hu, K.; Jin, S.; Sun, L.; Liu, B.; Wang, D. Synthesis and structural characterizations of nine non-covalent-bonded Zn2+, and Cd2+ supramolecules based on 3,5-dimethylpyrazole and carboxylates. Polyhedron 2019, 159, 408–425. [Google Scholar] [CrossRef]
- Titi, A.; Shiga, T.; Oshio, H.; Touzani, R.; Hammouti, B.; Mouslim, M.; Warad, I. Synthesis of novel Cl2Co4L6 clusterusing 1-hydroxymethyl-3,5-dimethylpyrazole (LH) ligand: Crystal structure, spectral, thermal, Hirschfeld surface analysis and catalytic oxidation evaluation. J. Mol. Struct. 2020, 1199, 126995–127009. [Google Scholar] [CrossRef]
- Direm, A.; Tursun, M.; Parlak, C.; Cherif, N.B. Trans-dichlorotetrakis(1H-pyrazole-κN2)copper(II): Synthesis, crystal structure, hydrogen bonding graph-sets, vibrational and DFT studies. J. Mol. Struct. 2015, 1093, 208–218. [Google Scholar] [CrossRef]
- Baishya, T.; Sharma, P.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Bhattacharyya, M.K. Fumarato and Phthalato Bridged Dinuclear Metal–Organic Cu(II) and Mn(II) Compounds Involving Infinite Fumarate–Water Assemblies and Unusual Structure-Guiding H-Bonded Synthons: Antiproliferative Evaluation and Theoretical Studies. New J. Chem. 2022, 46, 17817–17833. [Google Scholar] [CrossRef]
- Lever, A.B.P. Inorganic Electronic Spectroscopy. Stud. Phys. Theor. Chem. 1984, 33, XVI-863. [Google Scholar] [CrossRef]
- Paul, P.; Roy, S.; Sarkar, S.; Chowdhury, S.; Purkayastha, R.N.D.; Raghavaiah, P.; McArdle, P.; Deb, L.; Devi, S.I. Synthesis, Structure and Some Properties of a Manganese(II) Benzoate Containing Diimine. J. Mol. Struct. 2015, 1102, 153–160. [Google Scholar] [CrossRef]
- Da Silva, P.B.; Terra, P.H.; Frem, R.C.G.; Netto, A.V.G.; Mauro, A.E. Synthesis, Characterization, and Investigation of the Thermal Behavior of Cu(II) Pyrazolyl Complexes. J. Therm. Anal. Calorim. 2011, 106, 495–499. [Google Scholar] [CrossRef]
- Giles, I.D.; DePriest, J.C.; Deschamps, J.R. Effect of Substitution and the Counterion on the Structural and Spectroscopic Properties of CuII Complexes of Methylated Pyrazoles. J. Coord. Chem. 2015, 68, 3611–3635. [Google Scholar] [CrossRef]
- Ghosh, M.; Majee, A.; Nethaji, M.; Chattopadhyay, T. Syntheses and characterization of trans-[NiL2 (NCS) 2][L= 2-(aminomethyl) pyridine], trans-[NiL2′(NSC) 2][L′= 2-(2-aminoethyl) pyridine] and trans-[NiL2 ″(NSC) 2][L ″= 2-(2-methylaminoethyl) pyridine] complexes: X-ray single crystal structure of trans-[NiL2′(NSC) 2][L′= 2-(2-aminoethyl) pyridine]. Inorg. Chim. Acta. 2009, 362, 2052–2055. [Google Scholar]
- Kavitha, N.; Anantha Lakshmi, P.V. Synthesis, Characterization and Thermogravimetric Analysis of Co(II), Ni(II), Cu(II) and Zn(II) Complexes Supported by ONNO Tetradentate Schiff Base Ligand Derived from Hydrazino Benzoxazine. J. Saudi Chem. Soc. 2017, 21, 457–466. [Google Scholar] [CrossRef]
- Masoud, M.S.; Ali, A.E.; Elfatah, A.S.A.; Amer, G.E. Synthesis, Molecular Spectroscopy, Computational, Thermal Analysis and Biological Activity of Some Orotic Acid Complexes. Open J. Inorg. Chem. 2021, 11, 1–22. [Google Scholar] [CrossRef]
- Sharma, P.; Dutta, D.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Bhattacharyya, M.K. Benzoato Bridged Dinuclear Mn(II) and Cu(II) Compounds Involving Guest Chlorobenzoates and Dimeric Paddle Wheel Supramolecular Assemblies: Antiproliferative Evaluation and Theoretical Studies. Polyhedron 2021, 208, 115409–115422. [Google Scholar] [CrossRef]
- Aycan, T. Synthesis, Crystal Structure, Spectroscopic (FT-IR, UV–Vis, EPR) and Hirshfeld Surface Analysis Studies of Zn(II)-Benzoate Coordination Dimer. J. Mol. Struct. 2021, 1223, 128943–128955. [Google Scholar] [CrossRef]
- Barra, C.V.; Rocha, F.V.; Netto, A.V.G.; Shimura, B.; Frem, R.C.G.; Mauro, A.E.; Carlos, I.Z.; Ananias, S.R.; Quilles, M.B. New Palladium(II) Complexes with Pyrazole Ligands: Part I. Synthesis, Spectral and Thermal Studies, and Antitumor Evaluation. J. Therm. Anal. Calorim. 2011, 106, 483–488. [Google Scholar] [CrossRef]
- Allan, J.R.; Bonner, J.G.; Bowley, H.J.; Gerrard, D.L.; Hoey, S. Thermal Studies on Fumaric Acid and Crotonic Acid Compounds of Cobalt(II) and Nickel(II). Thermochim. Acta 1989, 141, 227–233. [Google Scholar] [CrossRef]
- Sarma, P.; Sharma, P.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Barthakur, T.; Bhattacharyya, M.K. Unconventional π-Hole and Semi-Coordination Regium Bonding Interactions Directed Supramolecular Assemblies in Pyridinedicarboxylato Bridged Polymeric Cu(II) Compounds: Antiproliferative Evaluation and Theoretical Studies. Inorganica Chim. Acta 2021, 525, 12046–12060. [Google Scholar] [CrossRef]
- Dutta, D.; Sharma, P.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Baishya, T.; Bhattacharyya, M.K. Supramolecular Assemblies Involving Unconventional Non-Covalent Contacts in Pyrazole-Based Coordination Compounds of Co(II) and Cu(II) Pyridinedicarboxylates: Antiproliferative Evaluation and Theoretical Studies. Polyhedron 2022, 224, 116025–116040. [Google Scholar] [CrossRef]
- Bhattacharyya, M.K.; Saha, U.; Dutta, D.; Das, A.; Verma, A.K.; Frontera, A. Solvent-Driven Structural Topology Involving Energetically Significant Intra- and Intermolecular Chelate Ring Contacts and Anticancer Activities of Cu(Ii) Phenanthroline Complexes Involving Benzoates: Experimental and Theoretical Studies. RSC Adv. 2019, 9, 16339–16356. [Google Scholar] [CrossRef]
- Lin, W.-S.; Kuwata, S. Recent Developments in Reactions and Catalysis of Protic Pyrazole Complexes. Molecules 2023, 28, 3529. [Google Scholar] [CrossRef] [PubMed]






| Crystal Parameters | 1 | 2 |
|---|---|---|
| Empirical formula | C24H28MnN4O5 | C18H26CuN4O4 |
| Formula weight | 507.44 | 425.97 |
| Temperature (K) | 100.0 | 100.0 |
| Wavelength (Å) | 1.54178 | 1.54178 |
| Crystal system | Orthorhombic | Orthorhombic |
| Space group | Pbcn | Pbcn |
| a/Å | 19.2401(1) | 15.6899(5) |
| b/Å | 12.1579(7) | 10.7161(3) |
| c/Å | 10.1312(6) | 11.3062(4) |
| α ° | 90 | 90 |
| β ° | 90 | 90 |
| γ ° | 90 | 90 |
| Volume (Å3) | 2369.9(2) | 1900.96(1) |
| Z | 4 | 4 |
| Calculated density (g/cm3) | 1.422 | 1.488 |
| Absorption coefficient (mm−1) | 4.893 | 1.897 |
| F(000) | 1060 | 892 |
| Crystal size (mm3) | 0.35 × 0.21 × 0.18 | 0.31 × 0.22 × 0.12 |
| θ range for data collection (°) | 10.76 to 136.33 | 1.604 to 23.973 |
| Index ranges | −23 ≤ h ≤ 23, −14 ≤ k ≤ 14, −11 ≤ l ≤ 12 | −18 ≤ h ≤ 18, −12 ≤ k ≤ 12, −13 ≤ l ≤ 13 |
| Reflections collected | 19,422 | 28,974 |
| Unique data (Rint) | 2972 | 1721 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2136/1/161 | 1721/0/126 |
| Goodness-of-fit on F2 | 1.086 | 1.143 |
| Final Rindices[I > 2σ (I)] R1/wR2 | R1 = 0.0414, wR2 = ‘0.1110 | R1 = 0.0475, wR2 = 0.1395 |
| Rindices (all data) R1/wR2 | R1 = 0.0434, wR2 = 0.1133 | R1 = 0.0482, wR2 = 0.1402 |
| Largest diff. peak and hole (e.Å−3) | 0.45/−0.49 | 0.37/−0.63 |
| Bond Lengths of 1 (Å) | Bond Angles of 1 (°) | ||
| Mn1–O2 | 2.0854(1) | O2–Mn1–O2´ | 106.18(8) |
| Mn1–O2´ | 2.0854(1) | O2–Mn1–O1 | 126.91(4) |
| Mn1–O1 | 2.132(2) | O2´–Mn1–O1 | 126.91(4) |
| Mn1–N1 | 2.3173(2) | O2–Mn1–N1´ | 92.39(5) |
| Mn1–N1´ | 2.3173(2) | O2–Mn1–N1 | 91.72(6) |
| O1–Mn1–N1 | 86.57(4) | ||
| O1–Mn1–N1´ | 86.57(4) | ||
| O2´–Mn1–N1´ | 91.72(6) | ||
| O2´–Mn1–N1 | 92.40(5) | ||
| N1–Mn1–N1´ | 173.15(8) | ||
| Bond Lengths of 2 (Å) | Bond Angles of 2 (°) | ||
| Cu1–N1 | 1.989(3) | N1–Cu1–N1 | ´93.24(1) |
| Cu1–N1´ | 1.989(3) | N1–Cu1–O1 | 165.45(9) |
| Cu1–O1´ | 2.002(2) | N1–Cu1–O1´ | 91.47(9) |
| Cu1–O1 | 2.002(2) | N1´–Cu1–O1 | 91.47(9) |
| Cu1–O2 | 2.492(2) | N1´–Cu1–O1´ | 165.45(9) |
| Cu1–O2´ | 2.492(2) | O1–Cu1–O1´ | 87.34(1) |
| D–H⋯A | d(D–H) | d(D⋯A) | d(H⋯A) | <(DHA) |
|---|---|---|---|---|
| 1 | ||||
| C12–H12A⋯O3 | 0.98 | 3.64 | 2.95 | 128.6 |
| N2–H2⋯O3 | 0.88 | 2.91 | 2.10 | 153.7 |
| O1–H1A⋯O3 | 0.87 | 2.70 | 1.86 | 164.1 |
| 2 | ||||
| N2–H2⋯O2 | 0.88 | 2.74 | 1.90 | 157.2 |
| C9–H9A⋯O1 | 0.98 | 3.57 | 3.07 | 113.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boro, M.; Baishya, T.; Frontera, A.; Barceló-Oliver, M.; Bhattacharyya, M.K. Energetic Features of H-Bonded and π-Stacked Assemblies in Pyrazole-Based Coordination Compounds of Mn(II) and Cu(II): Experimental and Theoretical Studies. Crystals 2024, 14, 318. https://doi.org/10.3390/cryst14040318
Boro M, Baishya T, Frontera A, Barceló-Oliver M, Bhattacharyya MK. Energetic Features of H-Bonded and π-Stacked Assemblies in Pyrazole-Based Coordination Compounds of Mn(II) and Cu(II): Experimental and Theoretical Studies. Crystals. 2024; 14(4):318. https://doi.org/10.3390/cryst14040318
Chicago/Turabian StyleBoro, Mridul, Trishnajyoti Baishya, Antonio Frontera, Miquel Barceló-Oliver, and Manjit K. Bhattacharyya. 2024. "Energetic Features of H-Bonded and π-Stacked Assemblies in Pyrazole-Based Coordination Compounds of Mn(II) and Cu(II): Experimental and Theoretical Studies" Crystals 14, no. 4: 318. https://doi.org/10.3390/cryst14040318






