Analysis and Modification of a Colorimetric Nanosensor for Rapid Detection of Escherichia coli in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. E. coli Culturing
2.2. E. coli Sensing Agents
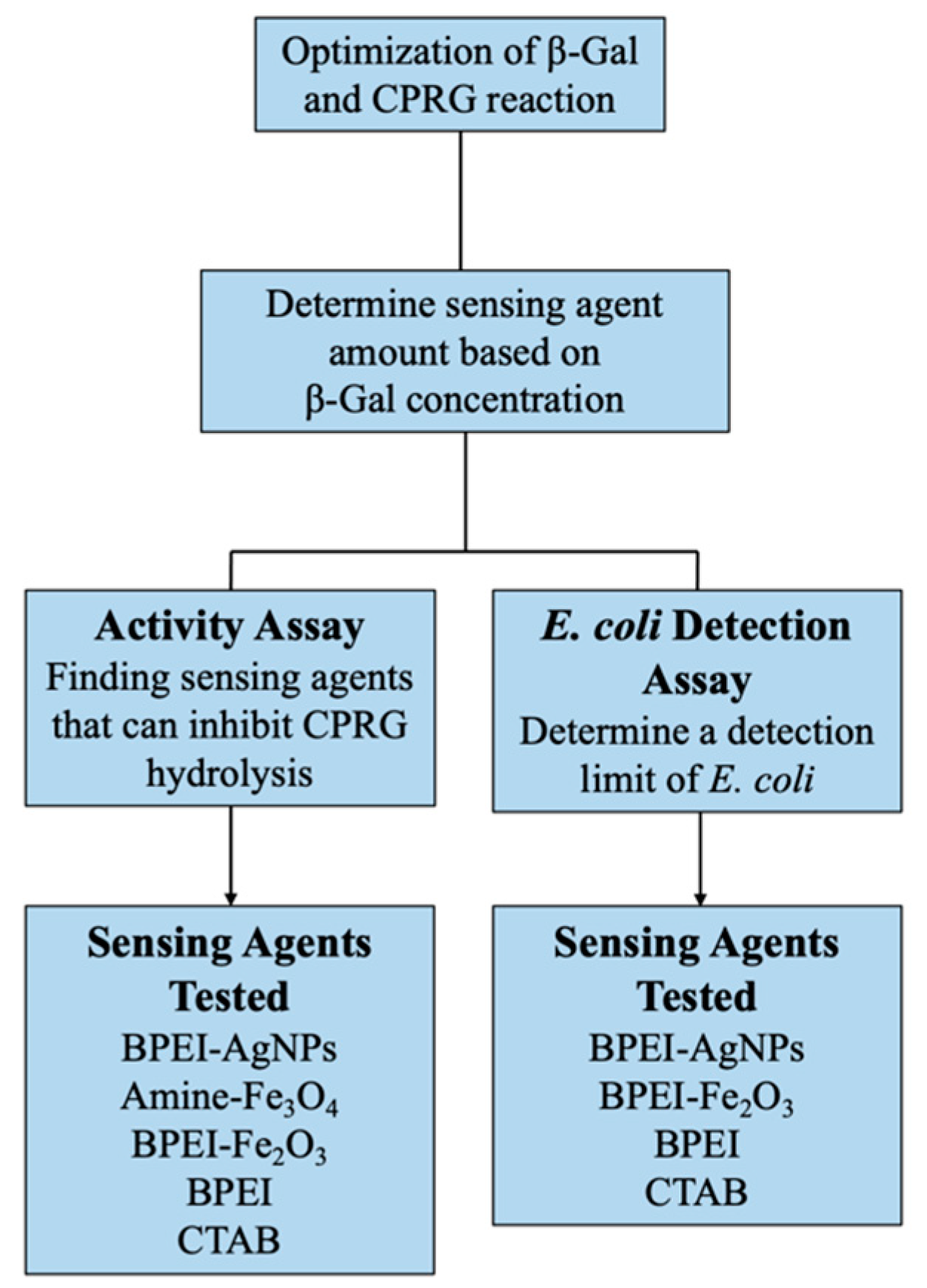
2.3. The Experimental Testing Program
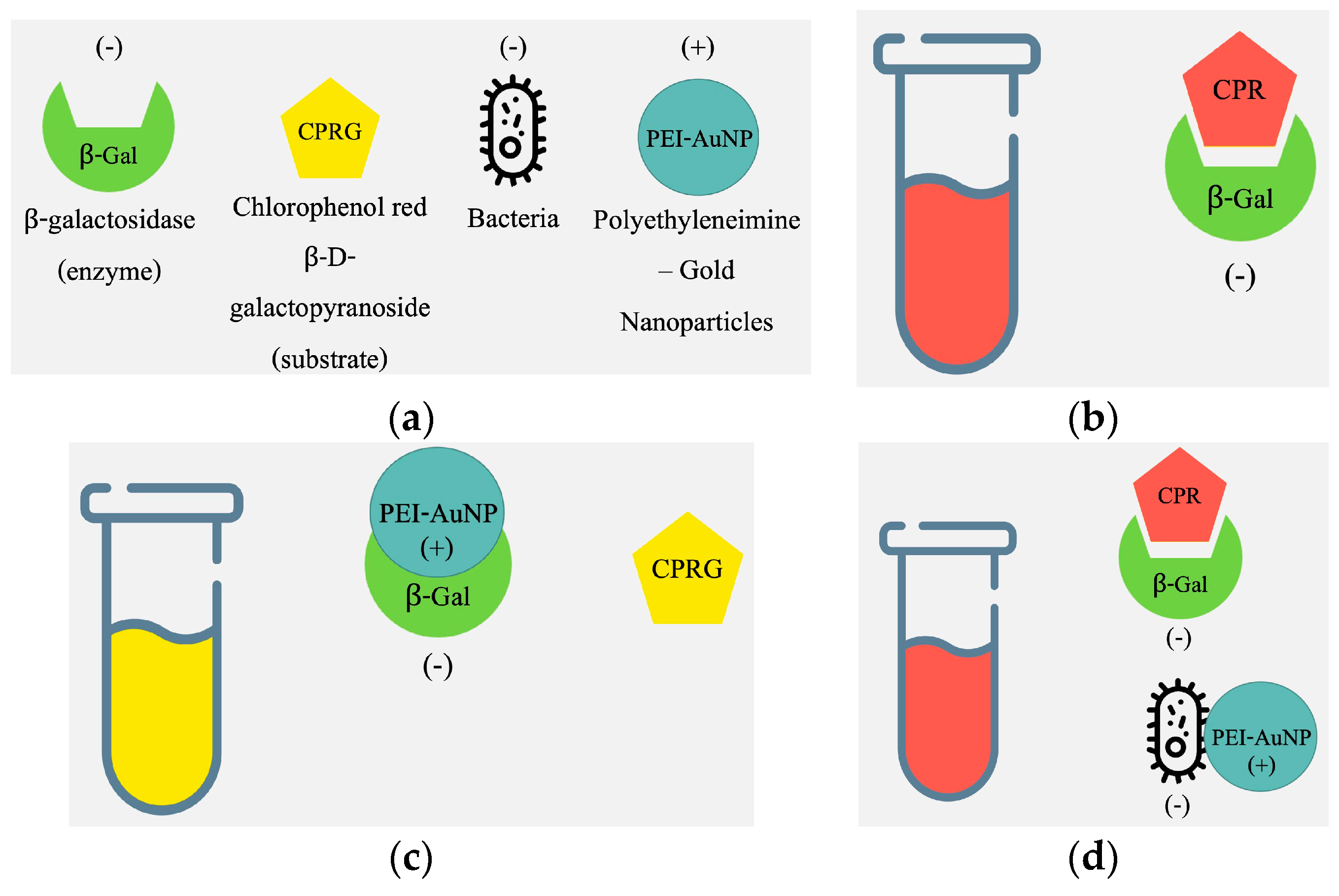
2.3.1. Optimization of the Colorimetric Assay
2.3.2. Activity Assays
2.3.3. E. coli Detection Assays
3. Results and Discussion
3.1. E. coli Stock Concentration
3.2. Optimization of the Colorimetric Assay
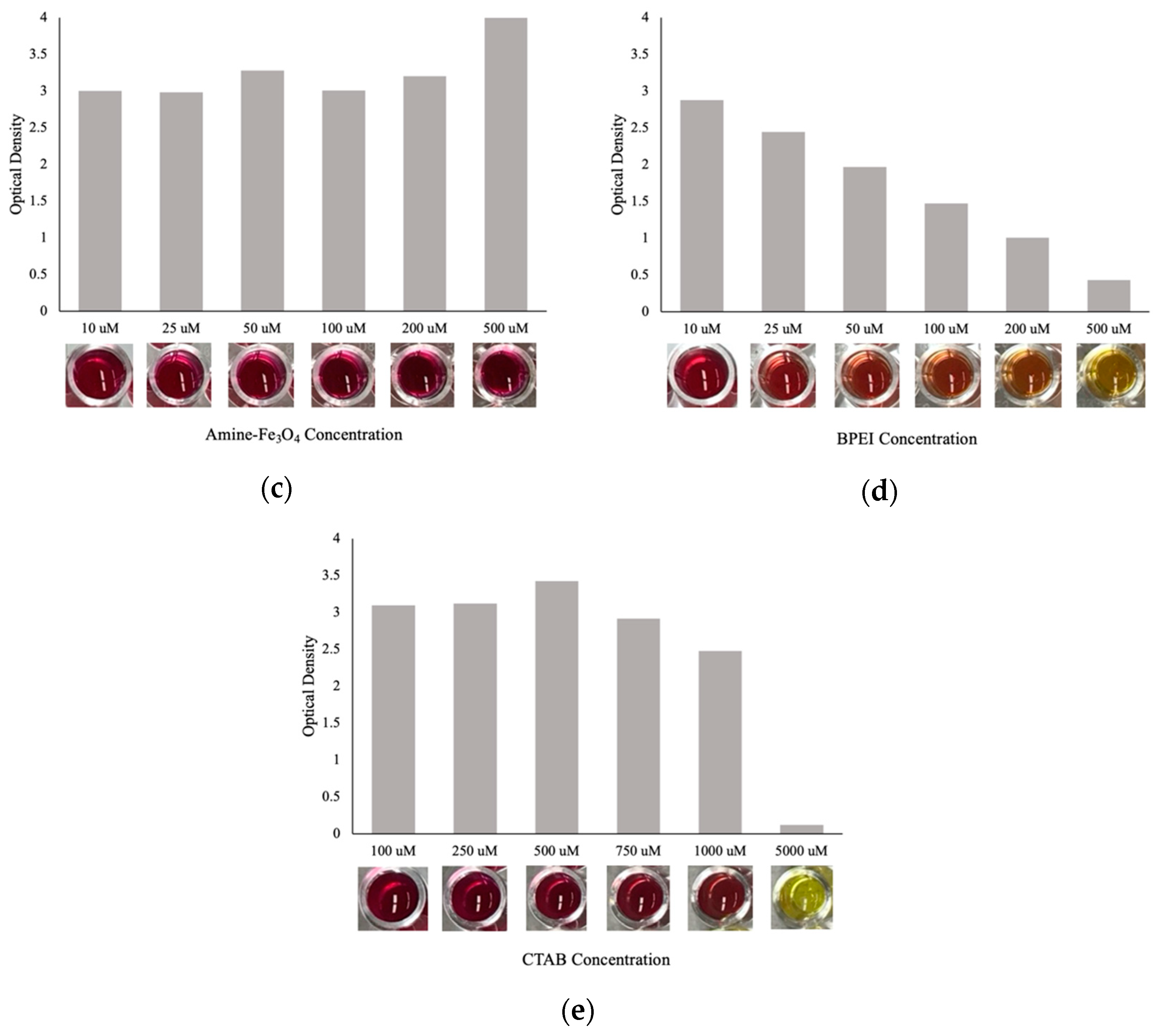
3.3. Activity Assay Results
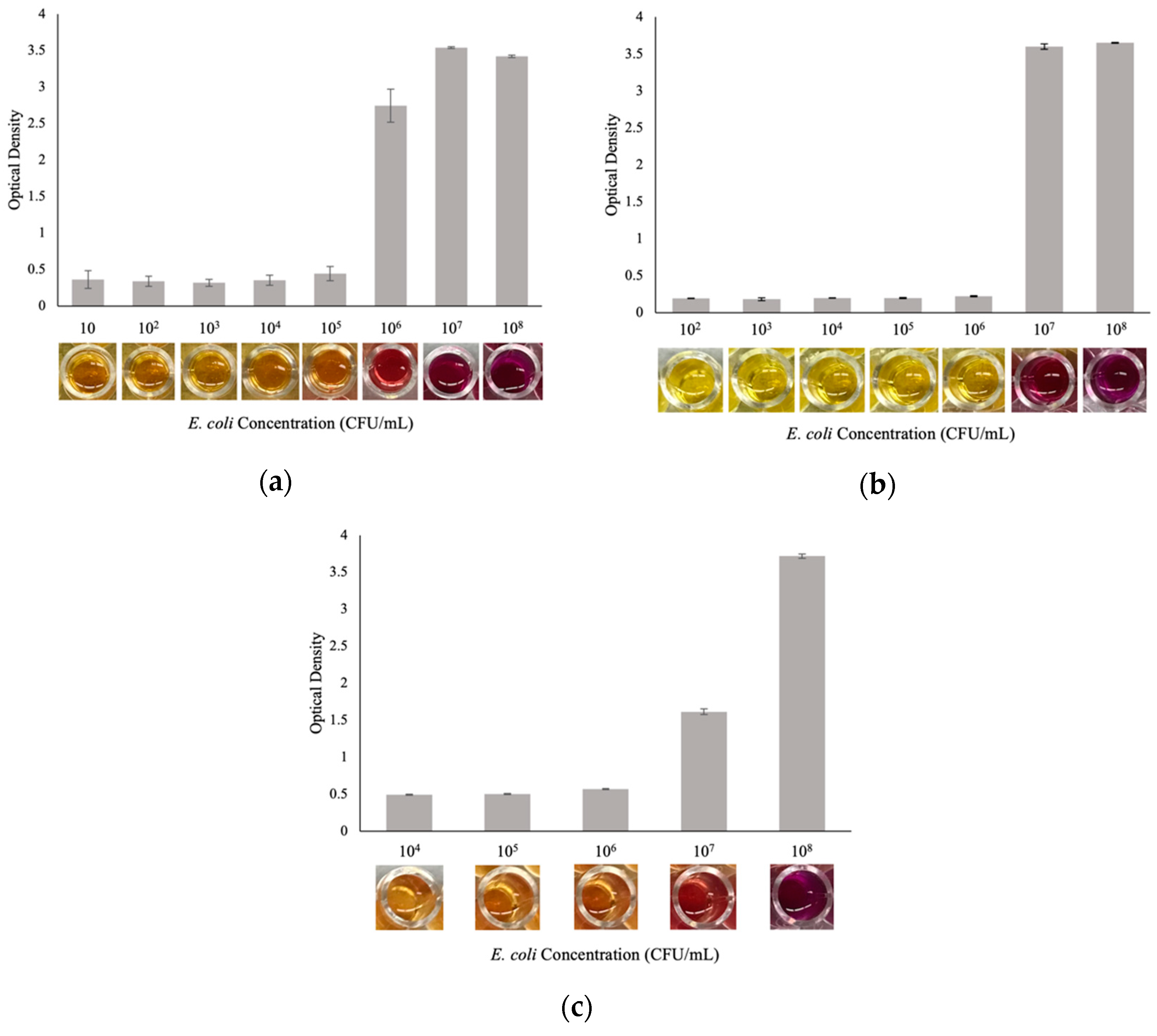
3.4. E. coli Detection Assay Results
3.5. Significance
3.5.1. Material Cost Analysis
3.5.2. Recyclability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Drinking-Water. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 25 May 2021).
- Cabral, J.P. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Castillo, F.Y.; Loera-Muro, A.; Jacques, M.; Garneau, P.; Avelar-González, F.J.; Harel, J.; Guerrero-Barrera, A.L. Waterborne pathogens: Detection methods and challenges. Pathogens 2015, 4, 307–334. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Method 1103.1: Escherichia coil (E. coli) in Water by Membrane Filtration Using Membrane-Thermotolerant Escherichia coli (E. coli) Agar (mTEC); Report EPA-821-R-10-002; U.S. Environmental Protection Agency: Washington, DC, USA, 2010.
- Spatola Rossi, C.; Coulon, F.; Ma, S.; Zhang, Y.S.; Yang, Z. Microfluidics for rapid detection of live pathogens. Adv. Funct. Mater. 2023, 33, 2212081. [Google Scholar] [CrossRef]
- Tambi, A.; Brighu, U.; Gupta, A.B. Methods for detection and enumeration of coliforms in drinking water: A review. Water Supply 2023, 23, 4047–4058. [Google Scholar] [CrossRef]
- Pebdeni, A.B.; Al-Baiati, M.N.; Hosseini, M. New application of bimetallic Ag/Pt nanoplates in a colorimetric biosensor for specific detection of E. coli in water. Beilstein J. Nanotechnol. 2024, 15, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ertaş, T.; Dinç, B.; Üstünsoy, R.; Eraslan, H.; Ergenç, A.F.; Bektaş, M. Novel electrochemical biosensor for Escherichia coli using gold-coated tungsten wires and antibody functionalized short multiwalled carbon nanotubes. Instrum. Sci. Technol. 2024, 52, 109–124. [Google Scholar] [CrossRef]
- Tian, C.; Zhao, L.; Qi, G.; Zhang, S. Trace detection of E. coli O157: H7 cells by an Au nanoparticle-based SERS aptasensor. ACS Appl. Nano Mater. 2023, 6, 1386–1394. [Google Scholar] [CrossRef]
- Dimitrievska, I.; Paunovic, P.; Grozdanov, A. Recent advancements in nano sensors for air and water pollution control. Mater. Sci. Eng. 2023, 7, 113–128. [Google Scholar]
- Ahangari, A.; Mahmoodi, P.; Mohammadzadeh, A. Advanced nano biosensors for rapid detection of zoonotic bacteria. Biotechnol. Bioeng. 2023, 120, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Hu, Y.; Yang, K.; Dong, N.; Yu, M.; Jiang, N. A novel SERS nanoprobe based on the use of core-shell nanoparticles with embedded reporter molecule to detect E. coli O157: H7 with high sensitivity. Microchim. Acta 2018, 185, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Ji, R.Y.; Lang, W.W.; Qin, K.X.; Bai, F.Y.; Xi, H.Y.; Zheng, Y.; Xia, B.X.; Dong, L.Y.; Wang, X.H. Integrating PEGylated peptide-oriented bacteria-imprinted matrix and PdPt bimetallic-doped imidazolium zeolite framework-8 for sensitive detection of Escherichia coli with smartphone readout system. Sens. Actuators B Chem. 2024, 411, 135749. [Google Scholar] [CrossRef]
- Choi, Y.; Hwang, J.H.; Lee, S.Y. Recent trends in nanomaterials-based colorimetric detection of pathogenic bacteria and viruses. Small Methods 2018, 2, 1700351. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Kanwal, T.; Ahmad, N.; Fatima, B.; Najam-ul-Haq, M.; Hussain, D. Advances and challenges in portable optical biosensors for onsite detection and point-of-care diagnostics. TrAC Trends Anal. Chem. 2024, 173, 117640. [Google Scholar] [CrossRef]
- Yang, L.; Xu, X.; Song, Y.; Huang, J.; Xu, H. Research progress of nanozymes in colorimetric biosensing: Classification, activity and application. Chem. Eng. J. 2024, 487, 150612. [Google Scholar] [CrossRef]
- John, G.S.; Johnson, A.M.; Suresh, P.A.; Unnikrishnan, N.V.; Kumar, K.A. Recent Advances in Carbon Nanotube-Based Electrochemical and Optical Biosensors. In Carbon Nanotubes for Biomedical Applications and Healthcare; Apple Academic Press: New York, NY, USA, 2024; pp. 147–166. [Google Scholar]
- Thiramanas, R.; Laocharoensuk, R. Competitive binding of polyethyleneimine-coated gold nanoparticles to enzymes and bacteria: A key mechanism for low-level colorimetric detection of gram-positive and gram-negative bacteria. Microchim. Acta 2016, 183, 389–396. [Google Scholar] [CrossRef]
- Miranda, O.R.; Li, X.; Garcia-Gonzalez, L.; Zhu, Z.J.; Yan, B.; Bunz, U.H.; Rotello, V.M. Colorimetric bacteria sensing using a supramolecular enzyme–nanoparticle biosensor. J. Am. Chem. Soc. 2011, 133, 9650–9653. [Google Scholar] [CrossRef] [PubMed]
- nanoComposix. Branched Polyethylenimine (BPEI) Surface. nanoComposix. Available online: https://nanocomposix.com/pages/branched-polyethylenimine-bpei-surface#applications (accessed on 26 April 2021).
- nanoComposix. BioPure Silver Nanospheres—BPEI. nanoComposix. Available online: https://nanocomposix.com/collections/material-silver/products/biopure-silver-nanospheres-bpei?variant=15906741551193 (accessed on 26 April 2021).
- Millipore Sigma. Iron Oxide (II, III), Magnetic Nanoparticles Solution. Sigma Aldrich. Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/747300?lang=en®ion=US (accessed on 26 April 2021).
- Millipore Sigma. Cerium Doped Iron Oxide. Sigma Aldrich. Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/909203?lang=en®ion=US (accessed on 26 April 2021).
- Park, J.Y.; Park, K.; Ok, G.; Chang, H.J.; Park, T.J.; Choi, S.W.; Lim, M.C. Detection of Escherichia coli O157: H7 using automated immunomagnetic separation and enzyme-based colorimetric assay. Sensors 2020, 20, 1395. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kumar, A.; Kumar, S.; Pinnaka, A.K.; Singhal, N.K. Naked eye colorimetric detection of Escherichia coli using aptamer conjugated graphene oxide enclosed Gold nanoparticles. Sens. Actuators B Chem. 2021, 329, 129100. [Google Scholar] [CrossRef]
- Hach. Presence/Absence Test, pk/12. Hach. Available online: https://www.hach.com/presence-absencetest-pk-12/product?id=7640249610 (accessed on 27 May 2021).
- Analytics Shop. IDEXX Quanti-Tray/2000, 97-Well, Sterile, 100pc/PAK, Shelf Life: 18 Months from Date of Manufacture. Analytics Shop. Available online: https://www.analytics-shop.com/gb/ix982167500-gb.html (accessed on 27 May 2021).




| Hypotheses Tested | Sensing Agent Investigated | Acronym |
|---|---|---|
| Nanoparticle type (i.e., Ag or Fe2O3) is not the governing factor for the sensing mechanism. Therefore, other nanoparticle types can replace gold as a nanosensor for detection of E. coli. | Silver nanoparticles coated with branched polyethyleneimine | BPEI-AgNPs |
| Cerium (Ce3/4+) doped iron oxide nanoparticles coated with branched polyethyleneimine | BPEI-Fe2O3 | |
| Amine functionalized iron oxide nanoparticles | Amine-Fe3O4 | |
| The surface charge, not the type of coating, is the governing factor for the sensing mechanism in the competitive binding approach. Therefore, other positively charged polymers (e.g., CTAB) can replace the PEI-AuNPs sensing agent. In addition, a carrier nanoparticle is not needed for the positively charged polymer to detect the microbes. | (1-Hexadecyl) trimethylammonium bromide | CTAB |
| Branched polyethyleneimine | BPEI |
| Nanoparticles | Concentration | Diameter | Zeta Potential | Reference |
|---|---|---|---|---|
| BPEI-AgNPs | 1 mg/mL | 40 nm | +30 to +95 mV | [21] |
| Amine-Fe3O4 | 1 mg/mL Fe | 10 nm | Not Provided | [22] |
| BPEI-Fe2O3 | 1.3 mg/mL | 7–15 nm | +29.7 mV | [23] |
| Sensing Agent | M) |
|---|---|
| BPEI-AgNPs | 10, 25, 50, 100, 200, and 500 |
| BPEI-Fe2O3 | |
| Amine-Fe3O4 | |
| BPEI | |
| CTAB | 100, 250, 500, 750, 1000, and 5000 |
| Sensing Agent | M) | E. coli Concentrations Tested (CFU/mL) |
|---|---|---|
| BPEI-AgNPs | 200 | 1–102 |
| 200 | 1–108 | |
| 300 | ||
| 400 | ||
| 800 | ||
| BPEI-Fe2O3 | 50 | 1–102 |
| 100 | 1–108 | |
| 200 | ||
| 200 | ||
| 400 | ||
| 600 | ||
| 800 | ||
| BPEI | 500 | 1 and 102 |
| 500 | 106–108 | |
| 800 | 1–108 | |
| CTAB | 5000 | 1 and 102 |
| 5000 | 106–108 |
| Cost of Individual Components of Sensing Agents | |||
|---|---|---|---|
| Individual Components | Cost per Sample ($) | Quantity Used | |
| CPRG | 0.05 | L of 0.75 mM | |
| -Gal | 0.02 | M | |
| BPEI | 0.00002 | M | |
| BPEI-AgNPs | 0.15 | M | |
| BPEI-Fe2O3 | 0.35 | M | |
| Overall Material Cost of the Sensor | |||
| Sensor (including all components) | Cost per Analysis ($) | ||
| BPEI | 0.07 | ||
| BPEI-AgNPs | 0.22 | ||
| BPEI-Fe2O3 | 0.42 | ||
| Commercial E. coli Detection Methods | |||
| Method | Cost per Test ($) | ||
| Hach P/A Test [26] | 5.04 | ||
| IDEXX Quanti-Tray 2000 [27] | 7.40 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabler, S.; Lang, R.A.; El Badawy, A.; Yeung, M.; Lee, J. Analysis and Modification of a Colorimetric Nanosensor for Rapid Detection of Escherichia coli in Water. Crystals 2024, 14, 386. https://doi.org/10.3390/cryst14040386
Stabler S, Lang RA, El Badawy A, Yeung M, Lee J. Analysis and Modification of a Colorimetric Nanosensor for Rapid Detection of Escherichia coli in Water. Crystals. 2024; 14(4):386. https://doi.org/10.3390/cryst14040386
Chicago/Turabian StyleStabler, Sarah, Ruby Anne Lang, Amro El Badawy, Marie Yeung, and Jean Lee. 2024. "Analysis and Modification of a Colorimetric Nanosensor for Rapid Detection of Escherichia coli in Water" Crystals 14, no. 4: 386. https://doi.org/10.3390/cryst14040386





