From Batch to Continuous Small-Scale Production of Particles: Mixer Design Methodology for Robust Operation
Abstract
:1. Introduction
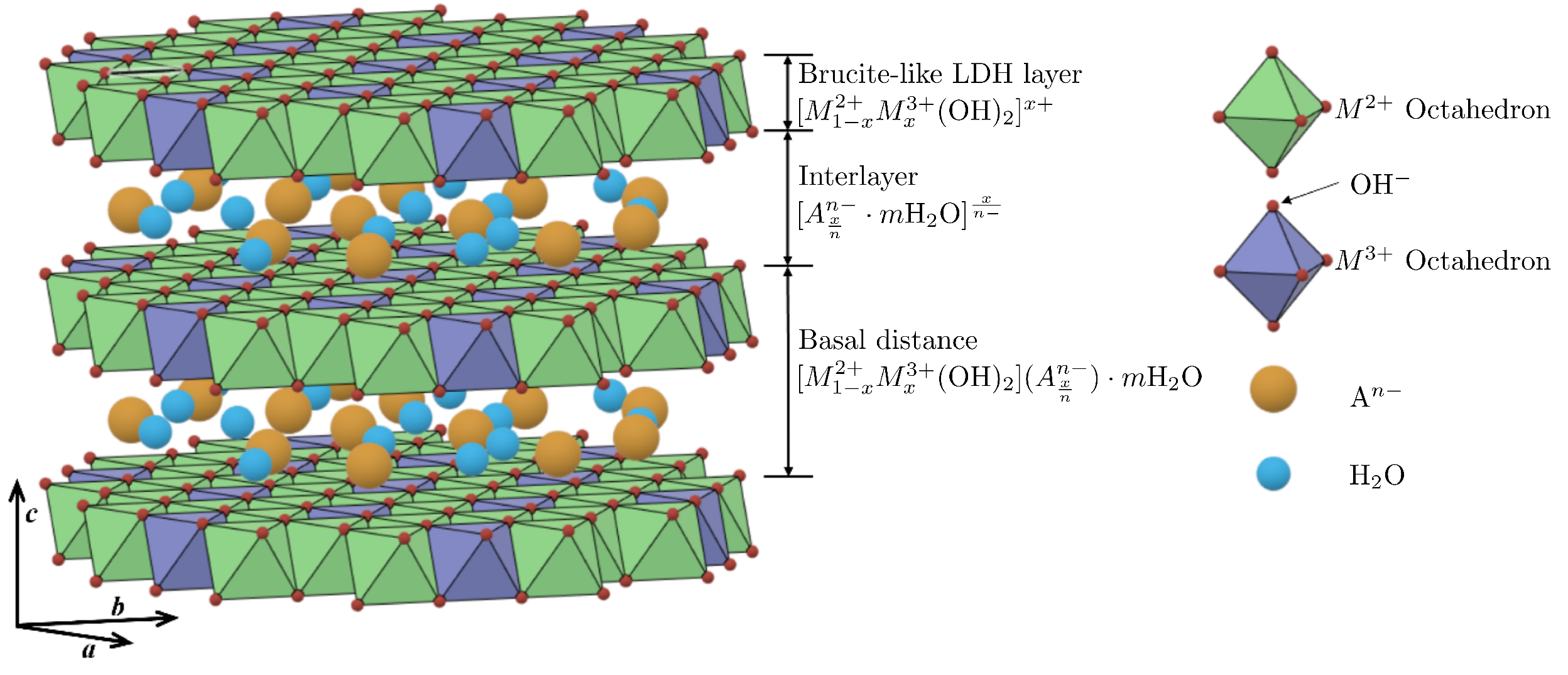
1.1. Layered Double Hydroxides
1.2. Microreactors
1.3. Continuous Production of Solids in Microreactors
2. Materials and Methods
2.1. Process Development Strategy
- The relevant batch process conditions can be suitably mapped in the continuous process;
- The continuous and robust operation can be ensured;
- The batch and continuous process produces the (interim) product with similar properties.
2.2. Optimization Framework for Continuous Operation
- Volumetric flow rate of the reactant ;
- Volumetric flow rate of water ;
- Reactant concentration (concentrations from the batch protocol are referred to as );
- Temperature in the micromixer via ;
- Temperature in the reaction vessel ;
- Ripening time after initial dosing in the reaction vessel;
- Design of the micromixer
- XRD patterns;
- Average crystal size D according to Section 2.4;
- General operability.
2.3. Process and Equipment Design
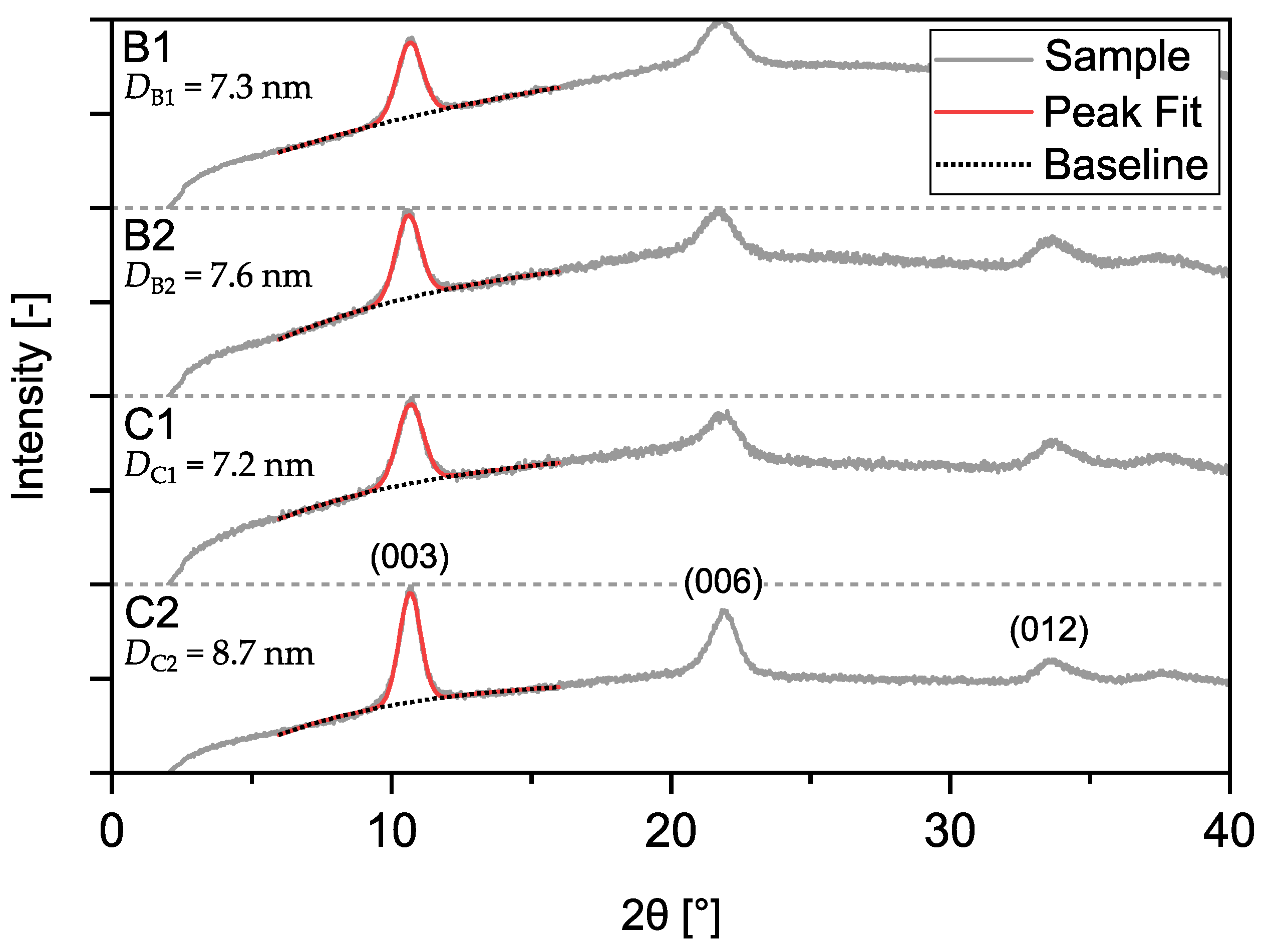
2.4. Measurement of Critical Product and Process Attributes
3. Discussion of the Equipment Design Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FEP | Fluorinated Ethylene Propylene |
| FWHM | Full Width at Half Maximum |
| HT | Hydrothermal |
| LDH | Layered Double Hydroxides |
| NP | Nano Particles |
| PDMS | Polydimethylsiloxan |
Appendix A
Appendix A.1. Hardware Used
| Substance | Supplier | Purity | Lot |
|---|---|---|---|
| NaOH | Sigma Aldrich®, MO, USA | ≥97% | MB2029962 201 |
| FeCl3 | Sigma Aldrich®, MO, USA | ≥98% | S8262845 225 |
| MgCl2 · 6H2O | VWR Chemicals, PA, USA | ≥98% | 22A054107 |
| Equipment | Supplier | Name | Label |
| Pressure sensor | AFRISO-EURO-INDEX GmbH | DMU 01 ST − 1/+3 bar | |
| Lab Automation | HiTec Zang GmbH | LabManager® | - |
| pH-electrode | WTW | SenTix® 940 | - |
| Stirrer Motor | Nanotec Electronic GmbH & Co. KG | Nema 17 | |
| Motor driver | Pololu, Las Vegas, USA | Tic T825 | - |
| Syringe | Henke-Sass, Wolf GmbH | HENKE-JECT® Luer Lock 50 mL | - |
| Syringe pump | LAMBDA Instruments GmbH | Lambda Vit-Fit | , |
| Temperature sensor | Rössel-Messtechnik GmbH | Pt-B-100-2 | , |
| Thermostat | Huber Kältemaschinenbau AG | Ministat 125 | - |
| Vacuum oven | Memmert GmbH & Co. KG | Memmert VO400 | - |
| Vacuum pump | VACUUBRAND GmbH & Co. KG | PC3001 VARIOpro | - |
| Centrifuge | Witeg Labortechnik GmbH | WiseSpin CF-10 | - |
| 3D printer | Formlabs | Form3+ | - |
Appendix A.2. Modification to Syringe Pumps
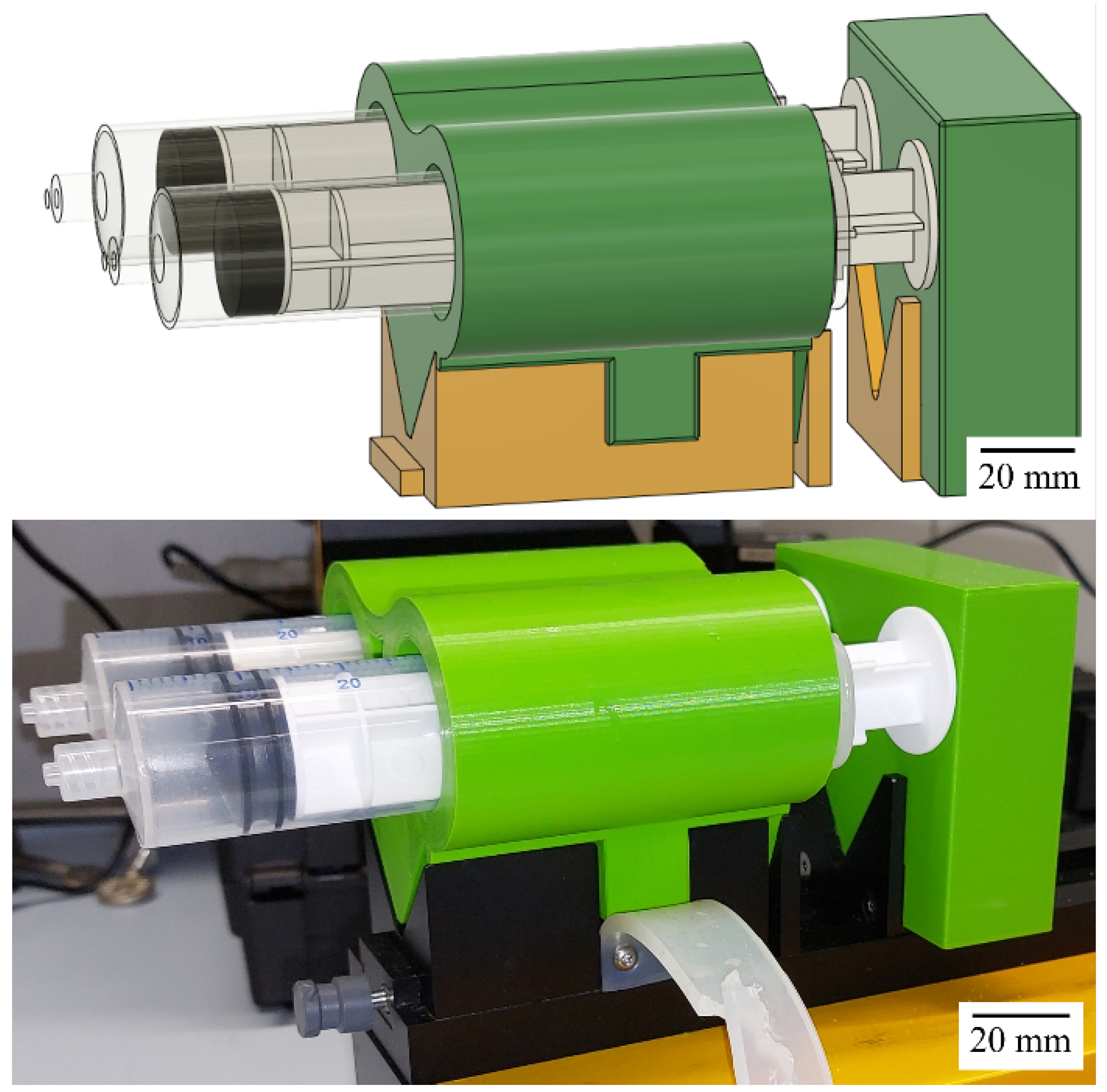
Appendix A.3. PXRD Measurements and Scherrer Equation
- 1.
- Centrifuging of samples (12,000 rpm, 3 min) and discarding the liquid;
- 2.
- Washing (resuspending) solids with water with subsequent centrifugation (12,000 rpm, 3 min) and discarding of the liquid;
- 3.
- Repetition of washing until a total of three washing steps was reached;
- 4.
- Drying of solids in a vacuum oven (at least 20 h, 200 mbar, 30 °C).
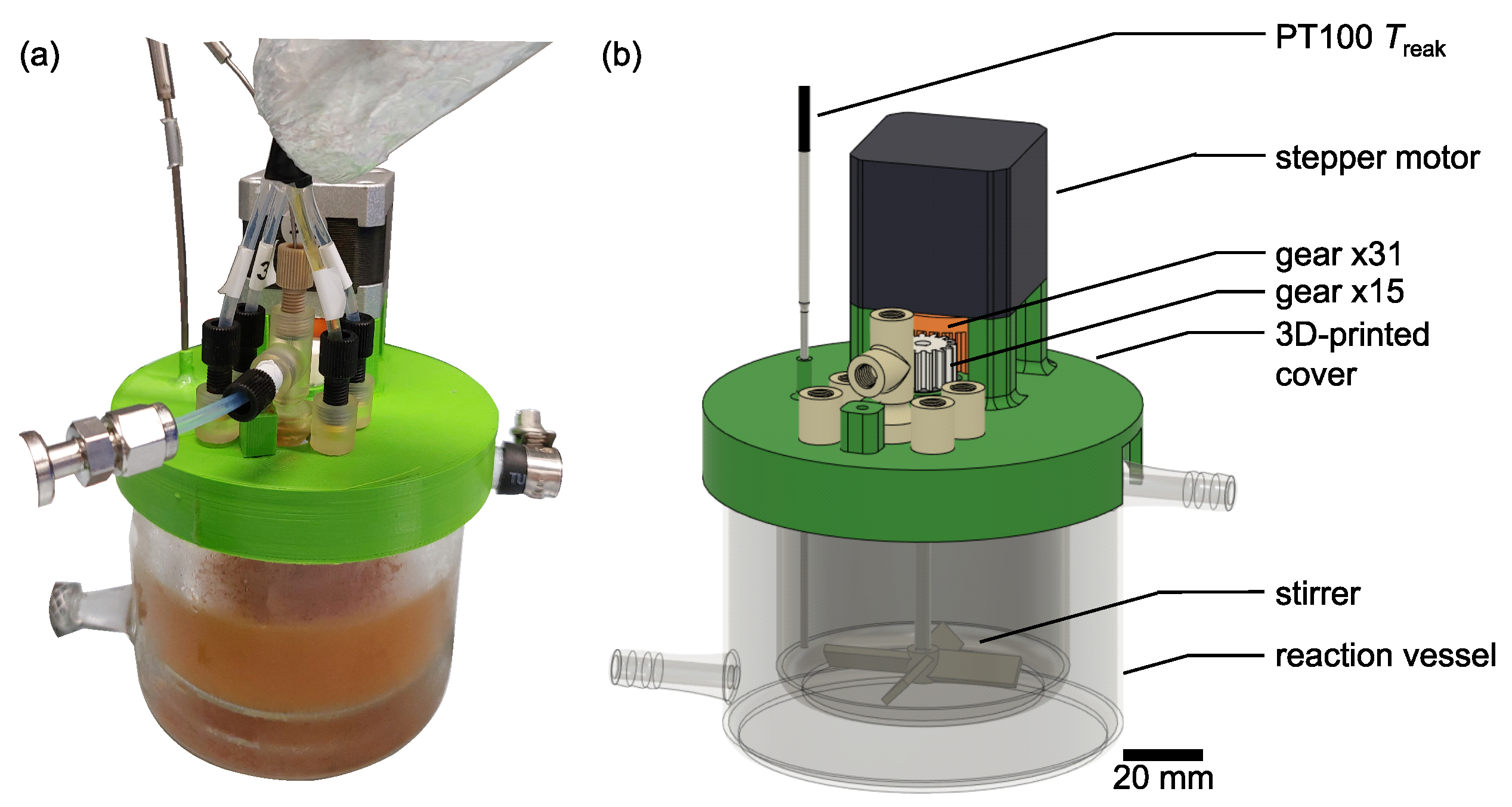
Appendix A.4. Reactor Setup

References
- Suryawanshi, P.L.; Gumfekar, S.P.; Bhanvase, B.A.; Sonawane, S.H.; Pimplapure, M.S. A review on microreactors: Reactor fabrication, design, and cutting-edge applications. Chem. Eng. Sci. 2018, 189, 431–448. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Wang, B.; Cai, Y.; Song, Q. An overview on state-of-art of micromixer designs, characteristics and applications. Anal. Chim. Acta 2023, 1279, 341685. [Google Scholar] [CrossRef] [PubMed]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.L. Managing Solids in Microreactors for the Upstream Continuous Processing of Fine Chemicals. Org. Process Res. Dev. 2012, 16, 870–887. [Google Scholar] [CrossRef]
- Zong, J.; Yue, J. Continuous Solid Particle Flow in Microreactors for Efficient Chemical Conversion. Ind. Eng. Chem. Res. 2022, 61, 6269–6291. [Google Scholar] [CrossRef]
- Abiev, R.; Almjasheva, O.; Popkov, V.; Proskurina, O. Microreactor synthesis of nanosized particles: The role of micromixing, aggregation, and separation processes in heterogeneous nucleation. Chem. Eng. Res. Des. 2022, 178, 73–94. [Google Scholar] [CrossRef]
- Abiev, R.S.; Kudryashova, Y.S.; Zdravkov, A.V.; Fedorenko, N.Y. Micromixing and Co-Precipitation in Continuous Microreactors with Swirled Flows and Microreactors with Impinging Swirled Flows. Inorganics 2023, 11, 49. [Google Scholar] [CrossRef]
- Arrabito, G.; Bonasera, A.; Prestopino, G.; Orsini, A.; Mattoccia, A.; Martinelli, E.; Pignataro, B.; Medaglia, P. Layered Double Hydroxides: A Toolbox for Chemistry and Biology. Crystals 2019, 9, 361. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhao, L.; Zhang, S.; Zhang, F.; Dong, M.; Xu, S. Morphologies, Preparations and Applications of Layered Double Hydroxide Micro-/Nanostructures. Materials 2010, 3, 5220–5235. [Google Scholar] [CrossRef]
- Sun, X.; Neuperger, E.; Dey, S.K. Insights into the synthesis of layered double hydroxide (LDH) nanoparticles: Part 1. Optimization and controlled synthesis of chloride-intercalated LDH. J. Colloid Interface Sci. 2015, 459, 264–272. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Grzybek, T.; Galvez, M.; Da Costa, P. A Short Review on the Catalytic Activity of Hydrotalcite-Derived Materials for Dry Reforming of Methane. Catalysts 2017, 7, 32. [Google Scholar] [CrossRef]
- Xiong, P.; Ji, X.; Zhao, X.; Lv, W.; Liu, T.; Lu, W. Materials design and control synthesis of the layered double hydroxide with the desired basal spacing. Chemometr. Intell. Lab. 2015, 144, 11–16. [Google Scholar] [CrossRef]
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Layered Materials; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Vucelic, M. Cation Ordering in Synthetic Layered Double Hydroxides. Clay Clay Miner. 1997, 45, 803–813. [Google Scholar] [CrossRef]
- Wijitwongwan, R.; Intasa-ard, S.; Ogawa, M. Preparation of Layered Double Hydroxides toward Precisely Designed Hierarchical Organization. Chem. Eng. 2019, 3, 68. [Google Scholar] [CrossRef]
- Ladewig, K.; Niebert, M.; Xu, Z.P.; Gray, P.P.; Lu, G.Q.M. Efficient siRNA delivery to mammalian cells using layered double hydroxide nanoparticles. Biomaterials 2010, 31, 1821–1829. [Google Scholar] [CrossRef]
- Balcomb, B.; Singh, M.; Singh, S. Synthesis and characterization of layered double hydroxides and their potential as nonviral gene delivery vehicles. ChemistryOpen 2015, 4, 137–145. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Ellison, C.J. Layered Double Hydroxides. In Polymer Green Flame Retardants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 675–707. [Google Scholar] [CrossRef]
- Andrea, K.A.; Wang, L.; Carrier, A.J.; Campbell, M.; Buhariwalla, M.; Mutch, M.; MacQuarrie, S.L.; Bennett, C.; Mkandawire, M.; Oakes, K.; et al. Adsorption of Oligo-DNA on Magnesium Aluminum-Layered Double-Hydroxide Nanoparticle Surfaces: Mechanistic Implication in Gene Delivery. Langmuir 2017, 33, 3926–3933. [Google Scholar] [CrossRef]
- Jain, R.G.; Fletcher, S.J.; Manzie, N.; Robinson, K.E.; Li, P.; Lu, E.; Brosnan, C.A.; Xu, Z.P.; Mitter, N. Foliar application of clay-delivered RNA interference for whitefly control. Nat. Plants 2022, 8, 535–548. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Tathod, A.P.; Gazit, O.M. Fundamental Insights into the Nucleation and Growth of Mg–Al Layered Double Hydroxides Nanoparticles at Low Temperature. Cryst. Growth Des. 2016, 16, 6709–6713. [Google Scholar] [CrossRef]
- Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring Hybrid Layered Double Hydroxides for the Development of Innovative Applications. Adv. Funct. Mater. 2018, 28, 1703868. [Google Scholar] [CrossRef]
- Ameena Shirin, V.K.; Sankar, R.; Johnson, A.P.; Gangadharappa, H.V.; Pramod, K. Advanced drug delivery applications of layered double hydroxide. J. Control. Release 2021, 330, 398–426. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys (Revised Reprint); CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Zhang, R.; Evans, D.G.; Duan, X. Preparation of Layered Double-Hydroxide Nanomaterials with a Uniform Crystallite Size Using a New Method Involving Separate Nucleation and Aging Steps. Chem. Mater. 2002, 14, 4286–4291. [Google Scholar] [CrossRef]
- Yun, S.K.; Pinnavaia, T.J. Water Content and Particle Texture of Synthetic Hydrotalcite-like Layered Double Hydroxides. Chem. Mater. 1995, 7, 348–354. [Google Scholar] [CrossRef]
- Sun, X.; Dey, S.K. Insights into the synthesis of layered double hydroxide (LDH) nanoparticles: Part 2. Formation mechanisms of LDH. J. Colloid Interface Sci. 2015, 458, 160–168. [Google Scholar] [CrossRef]
- Oh, J. The effect of synthetic conditions on tailoring the size of hydrotalcite particles. Solid State Ion. 2002, 151, 285–291. [Google Scholar] [CrossRef]
- Van der Pol, A.; Mojet, B.L.; van de Ven, E.; de Boer, E. Ordering of Intercalated Water and Carbonate Anions in Hydrotalcite. An NMR Study. J. Phys. Chem. 1994, 98, 4050–4054. [Google Scholar] [CrossRef]
- Miyata, S. Physico-Chemical Properties of Synthetic Hydrotalcites in Relation to Composition. Clay Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Grégoire, B.; Ruby, C.; Carteret, C. Hydrolysis of mixed Ni2+-Fe3+ and Mg2+-Fe3+ solutions and mechanism of formation of layered double hydroxides. Dalton Trans. 2013, 42, 15687–15698. [Google Scholar] [CrossRef]
- Radha, A.V.; Kamath, P.V.; Shivakumara, C. Order and disorder among the layered double hydroxides: Combined Rietveld and DIFFaX approach. Acta Crystallogr. B 2007, 63, 243–250. [Google Scholar] [CrossRef]
- Hessel, V.; Löwe, H.; Schönfeld, F. Micromixers—A review on passive and active mixing principles. Chem. Eng. Sci. 2005, 60, 2479–2501. [Google Scholar] [CrossRef]
- Tonomura, O.; Nishida, A.; Wang, L.; Hasebe, S. Optimal channel design and sensor placement in flow distributors for detecting blockage of parallelized microreactors. In 11th International Symposium on Process Systems Engineering; Elsevier: Amsterdam, The Netherlands, 2012; Volume 31, pp. 1281–1285. [Google Scholar] [CrossRef]
- Kockmann, N. Transport Processes and Exchange Equipment. In Micro Process Engineering; Kockmann, N., Ed.; Advanced Micro and Nanosystems, Wiley: Hoboken, NJ, USA, 2006; pp. 71–113. [Google Scholar] [CrossRef]
- Veser, G.; Friedrich, G.; Freygang, M.; Zengerle, R. A Simple and Flexible Micro Reactor for Investigations on Heterogeneous Catalytic Gas Phase Reactions. In Proceedings of the Micro-Electro-Mechanical Systems (MEMS), Anaheim, CA, USA, 15–20 November 1998; pp. 199–206. [Google Scholar] [CrossRef]
- VDI (Ed.) VDI-Wärmeatlas; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Aralekallu, S.; Boddula, R.; Singh, V. Development of glass-based microfluidic devices: A review on its fabrication and biologic applications. Mater. Des. 2023, 225, 111517. [Google Scholar] [CrossRef]
- Jähnisch, K.; Hessel, V.; Löwe, H.; Baerns, M. Chemistry in Microstructured Reactors. Angew. Chem. Int. Ed. 2004, 43, 406–446. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Du, L.; Liu, J.; Yao, J. Review of the applications of microreactors. Renew. Sustain. Energy Rev. 2015, 47, 519–539. [Google Scholar] [CrossRef]
- Kockmann, N.; Roberge, D.M. Scale-up concept for modular microstructured reactors based on mixing, heat transfer, and reactor safety. Chem. Eng. Process. Process Intensif. 2011, 50, 1017–1026. [Google Scholar] [CrossRef]
- Bojang, A.A.; Wu, H.S. Design, Fundamental Principles of Fabrication and Applications of Microreactors. Processes 2020, 8, 891. [Google Scholar] [CrossRef]
- Amores, I.D.; González-Gutiérrez, J.; García, I.M.; Franco, J.M.; Gallegos, C. 3D printing–Present and future–A Chemical Engineering perspective. Chem. Eng. Res. Des. 2022, 187, 598–610. [Google Scholar] [CrossRef]
- Sun, H.; Jia, Y.; Dong, H.; Dong, D.; Zheng, J. Combining additive manufacturing with microfluidics: An emerging method for developing novel organs-on-chips. Curr. Opin. Chem. Eng. 2020, 28, 1–9. [Google Scholar] [CrossRef]
- Parra-Cabrera, C.; Achille, C.; Kuhn, S.; Ameloot, R. 3D printing in chemical engineering and catalytic technology: Structured catalysts, mixers and reactors. Chem. Soc. Rev. 2018, 47, 209–230. [Google Scholar] [CrossRef]
- Wang, H.; Mustaffar, A.; Phan, A.N.; Zivkovic, V.; Reay, D.; Law, R.; Boodhoo, K. A review of process intensification applied to solids handling. Chem. Eng. Process. Process Intensif. 2017, 118, 78–107. [Google Scholar] [CrossRef]
- Poe, S.L.; Cummings, M.A.; Haaf, M.P.; McQuade, D.T. Solving the Clogging Problem: Precipitate-Forming Reactions in Flow. Angew.-Chem.-Int. Ed. 2006, 118, 1574–1578. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, X.; Ling, C.; Chen, S.; Wu, Z. High-performance Cu/ZnO catalysts prepared using a three-channel microreactor. Appl. Catal. A 2019, 570, 192–199. [Google Scholar] [CrossRef]
- Kockmann, N.; Kastener, J.; Woias, P. Reactive particle precipitation in liquid microchannel flow. Chem. Eng. J. 2008, 135, S110–S116. [Google Scholar] [CrossRef]
- Castro, F.; Kuhn, S.; Jensen, K.; Ferreira, A.; Rocha, F.; Vicente, A.; Teixeira, J.A. Continuous-flow precipitation of hydroxyapatite in ultrasonic microsystems. Chem. Eng. J. 2013, 215–216, 979–987. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, M.; Zhao, Q.; Yao, C.; Chen, G. Scale-up of antisolvent precipitation process with ultrasonic microreactors: Cavitation patterns, mixing characteristics and application in nanoparticle manufacturing. Chem. Eng. J. 2023, 475, 146040. [Google Scholar] [CrossRef]
- Giri, G.; Yang, L.; Mo, Y.; Jensen, K.F. Adding Crystals To Minimize Clogging in Continuous Flow Synthesis. Cryst. Growth Des. 2018, 19, 98–105. [Google Scholar] [CrossRef]
- Hohmann, L.; Greinert, T.; Mierka, O.; Turek, S.; Schembecker, G.; Bayraktar, E.; Wohlgemuth, K.; Kockmann, N. Analysis of Crystal Size Dispersion Effects in a Continuous Coiled Tubular Crystallizer: Experiments and Modeling. Cryst. Growth Des. 2018, 18, 1459–1473. [Google Scholar] [CrossRef]
- Schmalenberg, M.; Kreis, S.; Weick, L.K.; Haas, C.; Sallamon, F.; Kockmann, N. Continuous Cooling Crystallization in a Coiled Flow Inverter Crystallizer Technology—Design, Characterization, and Hurdles. Processes 2021, 9, 1537. [Google Scholar] [CrossRef]
- Coliaie, P.; Kelkar, M.S.; Korde, A.; Langston, M.; Liu, C.; Nazemifard, N.; Patience, D.; Skliar, D.; Nere, N.K.; Singh, M.R. On-the-spot quenching for effective implementation of cooling crystallization in a continuous-flow microfluidic device. React. Chem. Eng. 2022, 7, 1179–1190. [Google Scholar] [CrossRef]
- Kramer, A.L.M.S.H.; van Rosmalen, G. Precipitation and anti-solvent crystallization. In Industrial Crystallization: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2015; Chapter 11; pp. 234–260. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Kuhn, S.; Hartman, R.L.; Sultana, M.; Nagy, K.D.; Marre, S.; Jensen, K.F. Teflon-Coated Silicon Microreactors: Impact on Segmented Liquid-Liquid Multiphase Flows. Langmuir 2011, 27, 6519–6527. [Google Scholar] [CrossRef]
- Kuhn, S.; Noël, T.; Gu, L.; Heider, P.L.; Jensen, K.F. A Teflon microreactor with integrated piezoelectric actuator to handle solid forming reactions. Lab Chip 2011, 11, 2488. [Google Scholar] [CrossRef]
- Flowers, B.S.; Hartman, R.L. Particle Handling Techniques in Microchemical Processes. Challenges 2012, 3, 194–211. [Google Scholar] [CrossRef]
- Delacour, C.; Lutz, C.; Kuhn, S. Pulsed ultrasound for temperature control and clogging prevention in micro-reactors. Ultrason. Sonochem. 2019, 55, 67–74. [Google Scholar] [CrossRef]
- Banakar, V.; Sabnis, S.; Gogate, P.; Raha, A.; Saurabh. Ultrasound assisted continuous processing in microreactors with focus on crystallization and chemical synthesis: A critical review. Chem. Eng. Res. Des. 2022, 182, 273–289. [Google Scholar] [CrossRef]
- Wu, K.; Kuhn, S. Strategies for solids handling in microreactors. Chim. Oggi. 2014, 32, 62. [Google Scholar]
- Kurt, S.K.; Akhtar, M.; Nigam, K.D.P.; Kockmann, N. Continuous Reactive Precipitation in a Coiled Flow Inverter: Inert Particle Tracking, Modular Design, and Production of Uniform CaCO3 Particles. Ind. Eng. Chem. Res. 2017, 56, 11320–11335. [Google Scholar] [CrossRef]
- Kumar, V.; Mridha, M.; Gupta, A.; Nigam, K. Coiled flow inverter as a heat exchanger. Chem. Eng. Sci. 2007, 62, 2386–2396. [Google Scholar] [CrossRef]
- Mridha, M.; Nigam, K. Coiled flow inverter as an inline mixer. Chem. Eng. Sci. 2008, 63, 1724–1732. [Google Scholar] [CrossRef]
- Johnson, B.K.; Prud’homme, R.K. Chemical processing and micromixing in confined impinging jets. AlChE J. 2003, 49, 2264–2282. [Google Scholar] [CrossRef]
- Mahajan, A.J.; Kirwan, D.J. Micromixing effects in a two-impinging-jets precipitator. AlChE J. 1996, 42, 1801–1814. [Google Scholar] [CrossRef]
- Han, J.; Zhu, Z.; Qian, H.; Wohl, A.R.; Beaman, C.J.; Hoye, T.R.; Macosko, C.W. A simple confined impingement jets mixer for flash nanoprecipitation. J. Pharm. Sci. 2012, 101, 4018–4023. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Madane, K.; Kulkarni, A.A. Antisolvent based precipitation: Batch, capillary flow reactor and impinging jet reactor. Chem. Eng. J. 2019, 369, 1161–1171. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, C.; Liu, Y.; Prud’homme, R.K.; Fox, R.O. Mixing in a multi-inlet vortex mixer (MIVM) for flash nano-precipitation. Chem. Eng. Sci. 2008, 63, 2829–2842. [Google Scholar] [CrossRef]
- Markwalter, C.E.; Prud’homme, R.K. Design of a Small-Scale Multi-Inlet Vortex Mixer for Scalable Nanoparticle Production and Application to the Encapsulation of Biologics by Inverse Flash NanoPrecipitation. J. Pharm. Sci. 2018, 107, 2465–2471. [Google Scholar] [CrossRef]
- Fernandez Rivas, D.; Kuhn, S. Synergy of Microfluidics and Ultrasound: Process Intensification Challenges and Opportunities. Top. Curr. Chem. 2016, 374, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, Q.; Yao, C.; Chen, G. Investigation of anti-clogging mechanism of ultrasound-driven oscillating slugs/bubbles and its application on continuous crystallization process. Chem. Eng. Sci. 2024, 290, 119898. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley Series in Metallurgy and Materials; Addison-Wesley: Boston, MA, USA, 1978. [Google Scholar]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd ed.; John Wiley: New York, NY, USA, 1974. [Google Scholar]
- Krischner, H. Einführung in die Röntgenfeinstrukturanalyse; Vieweg+Teubner Verlag: Wiesbaden, Germany, 1990. [Google Scholar] [CrossRef]
- Spieß, L.; Teichert, G.; Schwarzer, R.; Behnken, H.; Genzel, C. Moderne Röntgenbeugung; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2019. [Google Scholar] [CrossRef]
- Lu, M.; Shan, Z.; Andrea, K.; MacDonald, B.; Beale, S.; Curry, D.E.; Wang, L.; Wang, S.; Oakes, K.D.; Bennett, C.; et al. Chemisorption Mechanism of DNA on Mg/Fe Layered Double Hydroxide Nanoparticles: Insights into Engineering Effective SiRNA Delivery Systems. Langmuir 2016, 32, 2659–2667. [Google Scholar] [CrossRef]
- Hargreaves, J.S.J. Some considerations related to the use of the Scherrer equation in powder X-ray diffraction as applied to heterogeneous catalysts. Catal. Struct. React. 2016, 2, 33–37. [Google Scholar] [CrossRef]



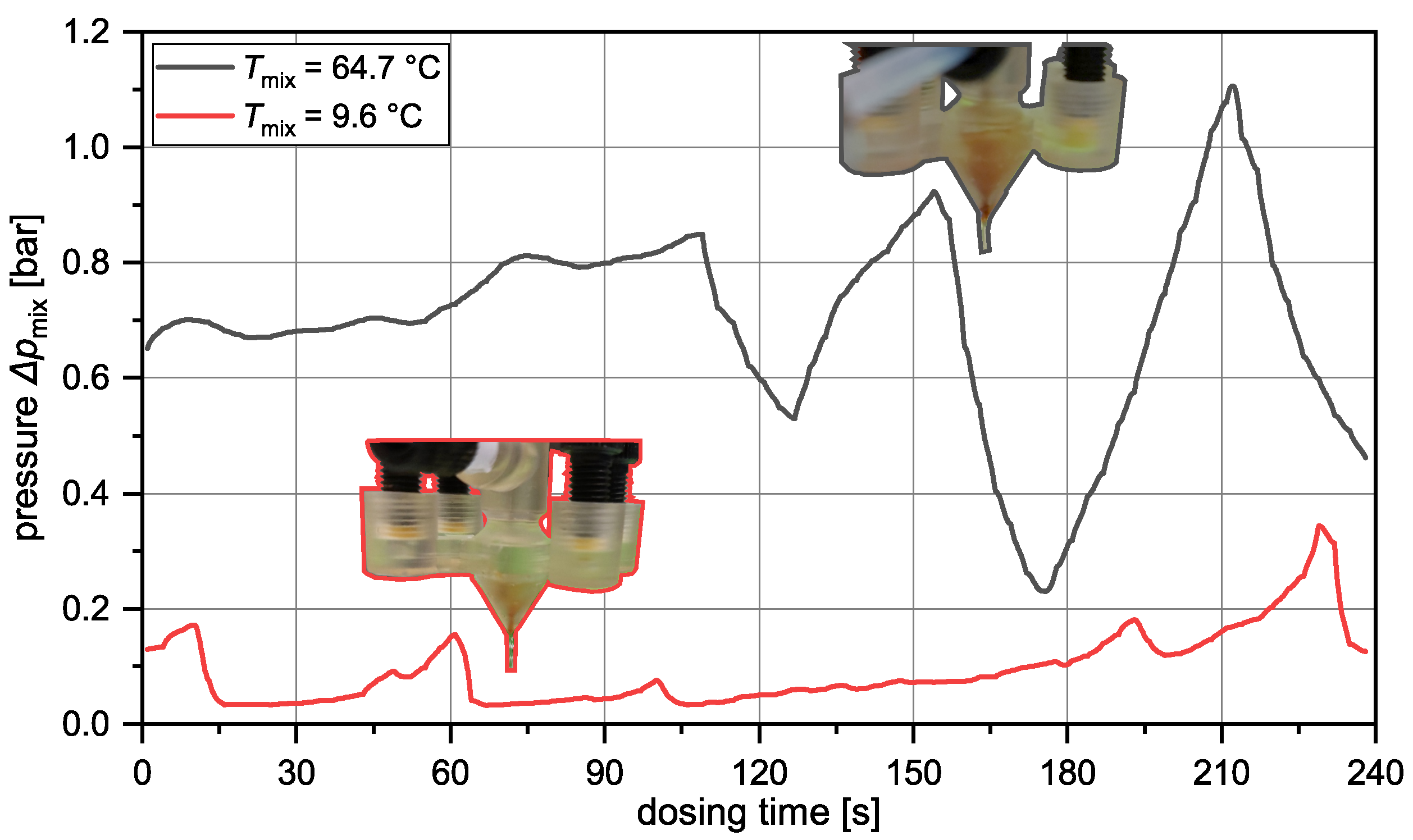

| Prototype | CAD Sketch | Channel Geometry | Changelog * | Remarks |
|---|---|---|---|---|
| P1.0 |  |  | – | Temperature sensor from above |
| P1.1 |  |  | Pressure sensor connector added | Pressure sensor from the side |
| P1.2 |  |  | Pointed outlet, sharp outlet rim to avoid solid adhesion | – |
| P2.0 |  |  | Tangential merging of fluid streams to maintain flow profile | Most successful in operation |
| P2.1 |  |  | Larger mixing chamber ( = 10 mm to = 14 mm) | Mixing chamber not filling up |
| Conditions | ||||||
|---|---|---|---|---|---|---|
| Batch Reference | 0.8 mol·L−1 | 0.3 mol·L−1 | 0.1 mol·L−1 | 50 mL | – | 100 mL |
| Continuous Set A (5 min) | 1.6 mol·L−1 | 0.6 mol·L−1 | 0.2 mol·L−1 | 5 mL·min−1 | 5 mL·min−1 | 100 mL |
| Continuous Set B (4 min) | 2 mol·L−1 | 0.75 mol·L−1 | 0.25 mol·L−1 | 5 mL·min−1 | 7.5 mL·min−1 | 100 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höving, S.; Ronnewinkel, P.; Kockmann, N. From Batch to Continuous Small-Scale Production of Particles: Mixer Design Methodology for Robust Operation. Crystals 2024, 14, 398. https://doi.org/10.3390/cryst14050398
Höving S, Ronnewinkel P, Kockmann N. From Batch to Continuous Small-Scale Production of Particles: Mixer Design Methodology for Robust Operation. Crystals. 2024; 14(5):398. https://doi.org/10.3390/cryst14050398
Chicago/Turabian StyleHöving, Stefan, Philipp Ronnewinkel, and Norbert Kockmann. 2024. "From Batch to Continuous Small-Scale Production of Particles: Mixer Design Methodology for Robust Operation" Crystals 14, no. 5: 398. https://doi.org/10.3390/cryst14050398





