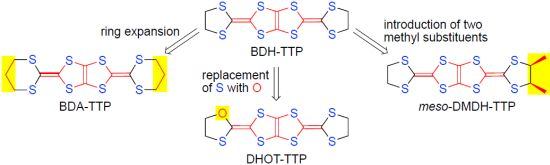
Chemical Modifications of BDH-TTP [2,5-Bis(1,3-dithiolan-2-ylidene)-1,3,4,6-tetrathiapentalene]: Control of Electron Correlation
Abstract
:1. Introduction
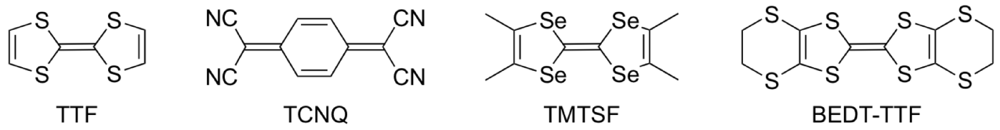
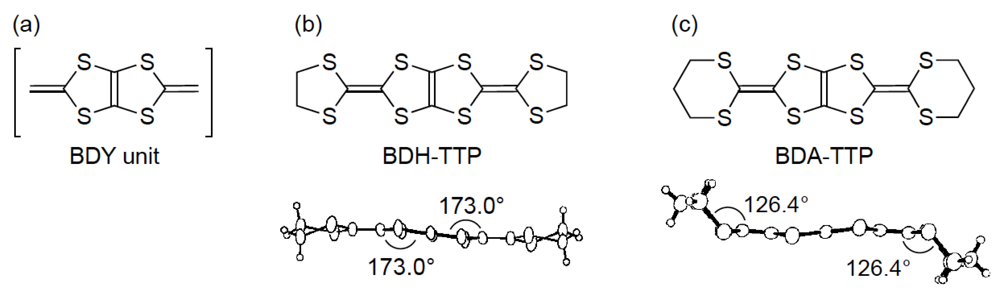
2. Superconductivity in β-(BDA-TTP)2I3
2.1. Effect of Hydrostatic Pressure
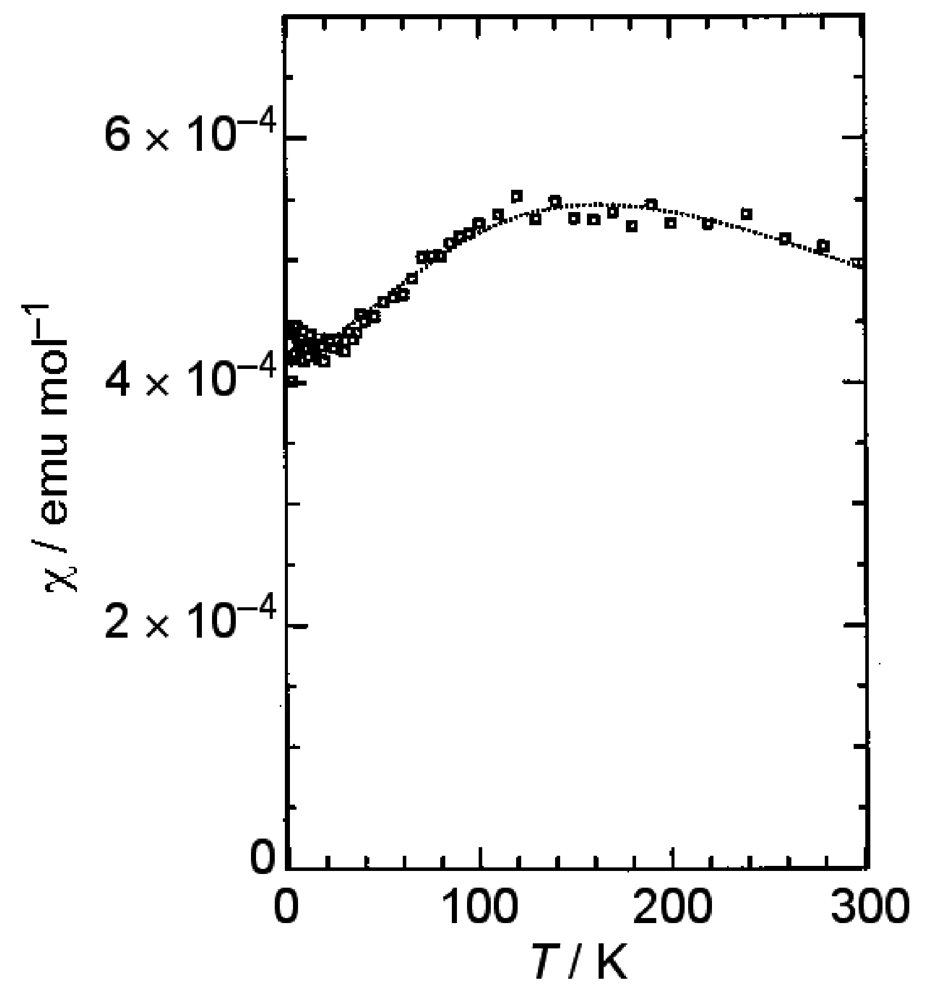
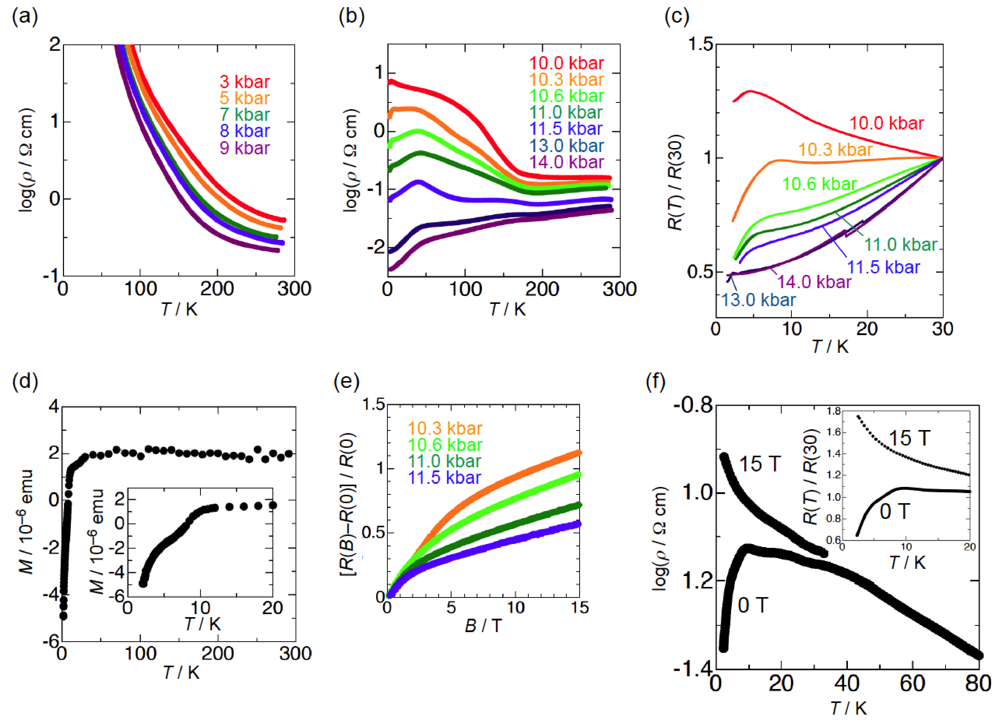
2.2. Effect of Uniaxial Strain

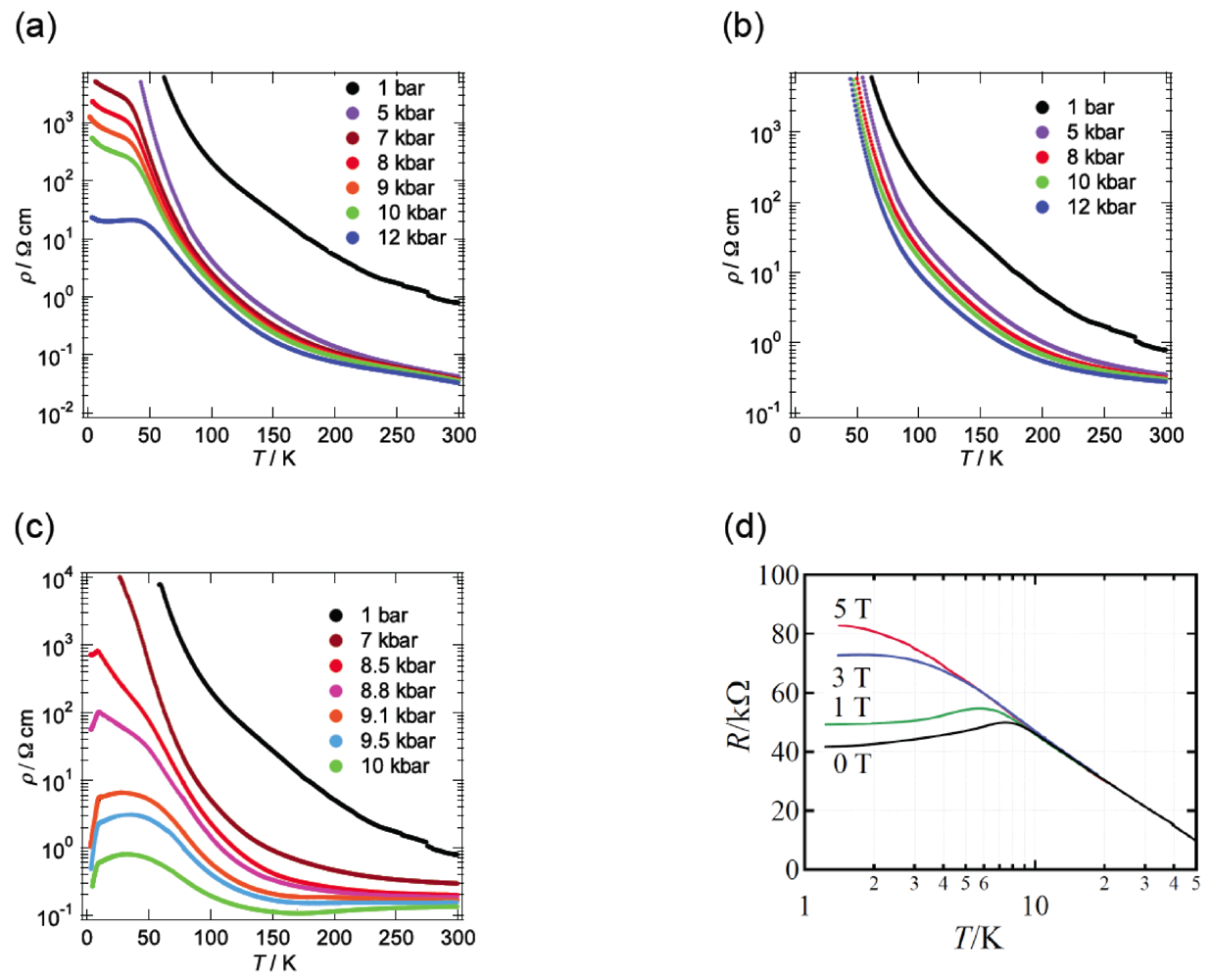
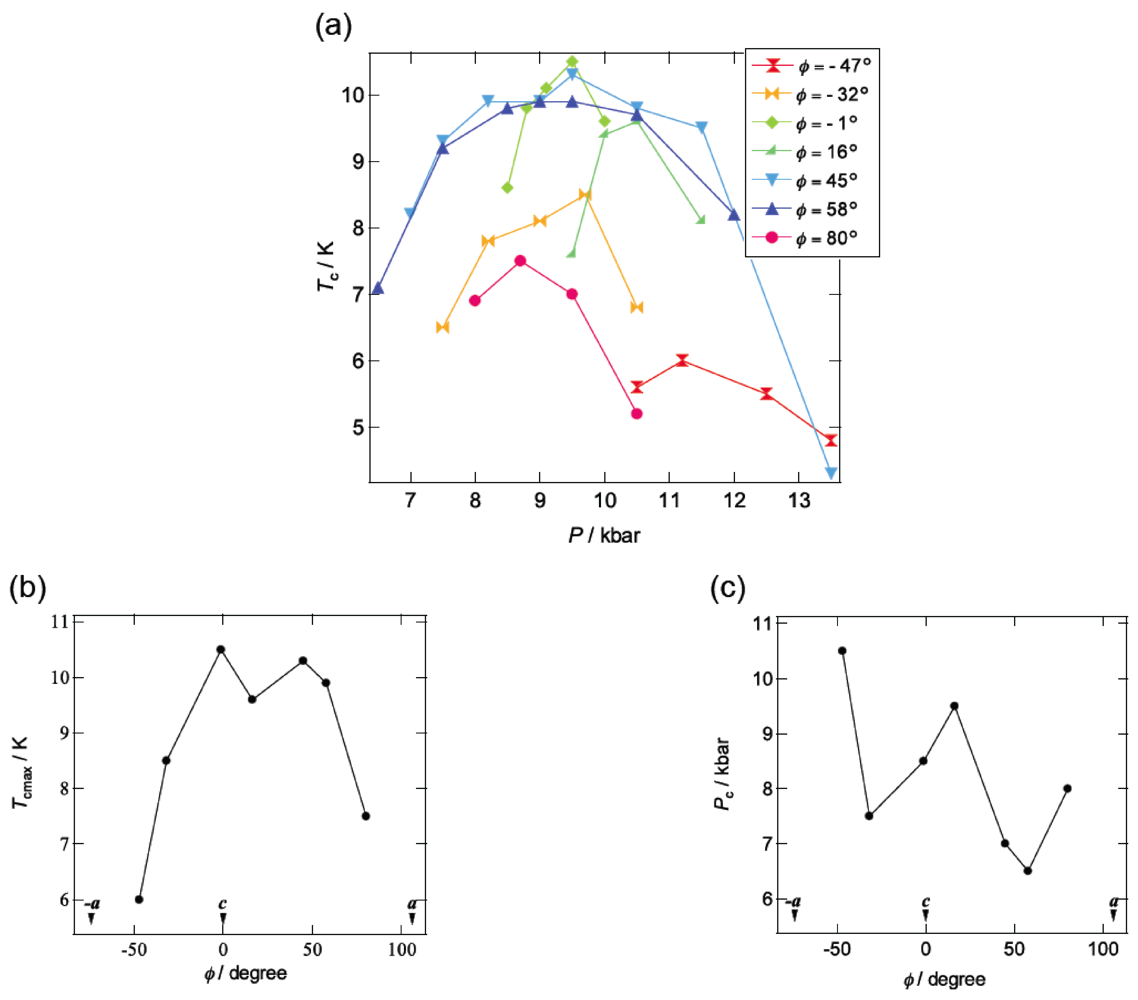
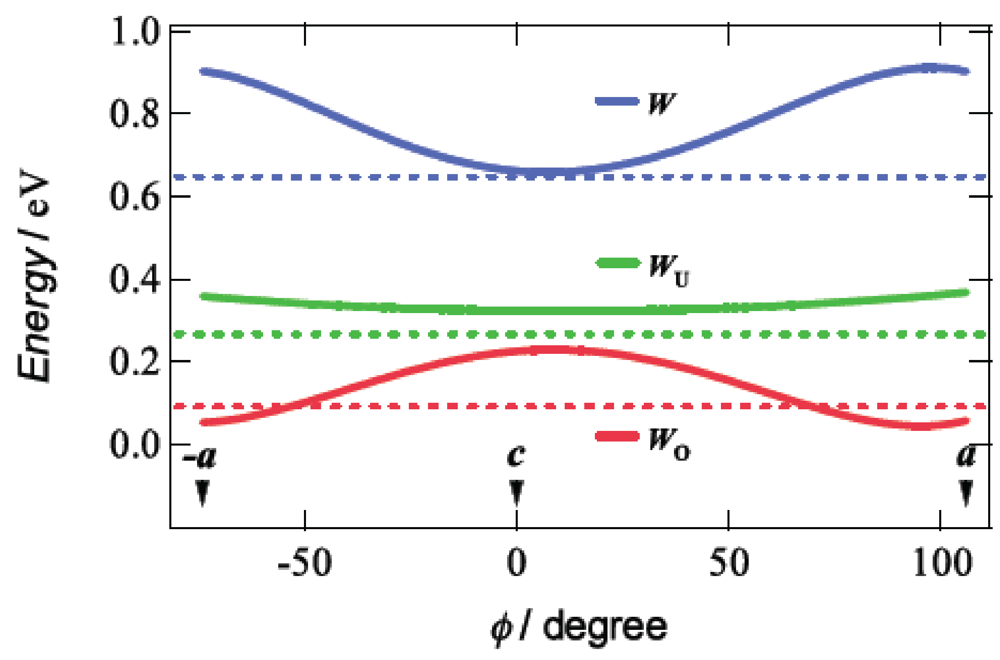
3. DHOT-TTP Salts

3.1. Synthesis, Molecular Structure and Electrochemical Properties of DHDO-TTP
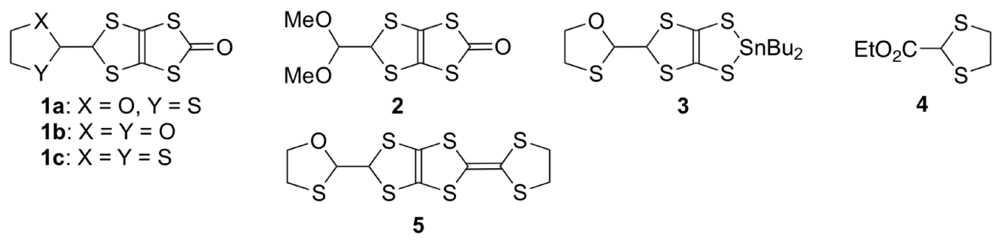
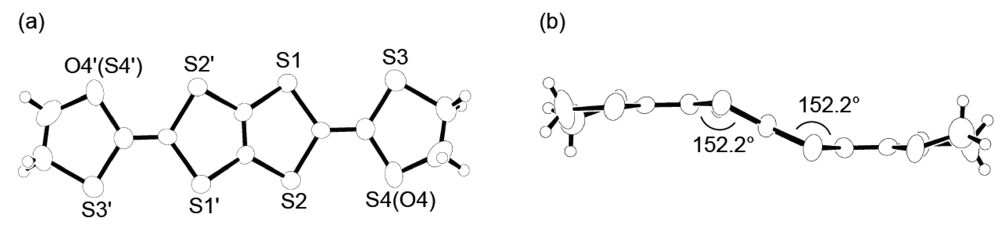
3.2. Physical Properties of DHDO-TTP Salts
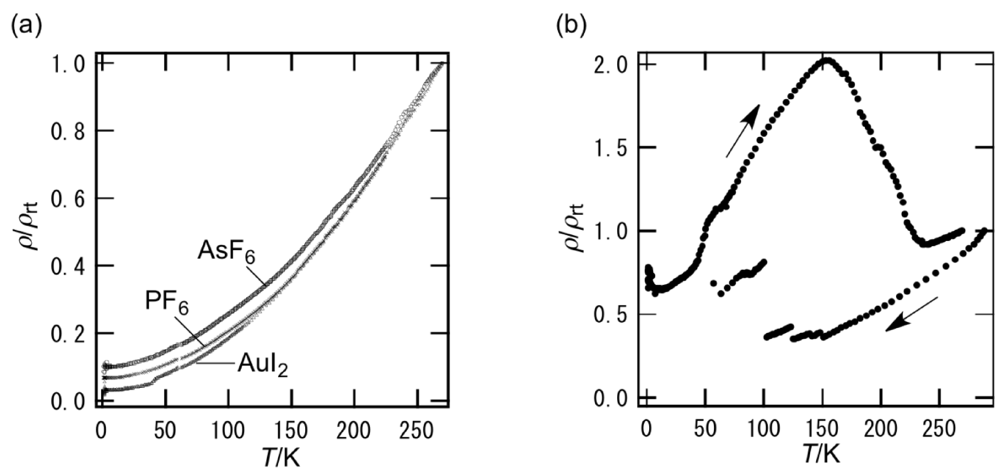
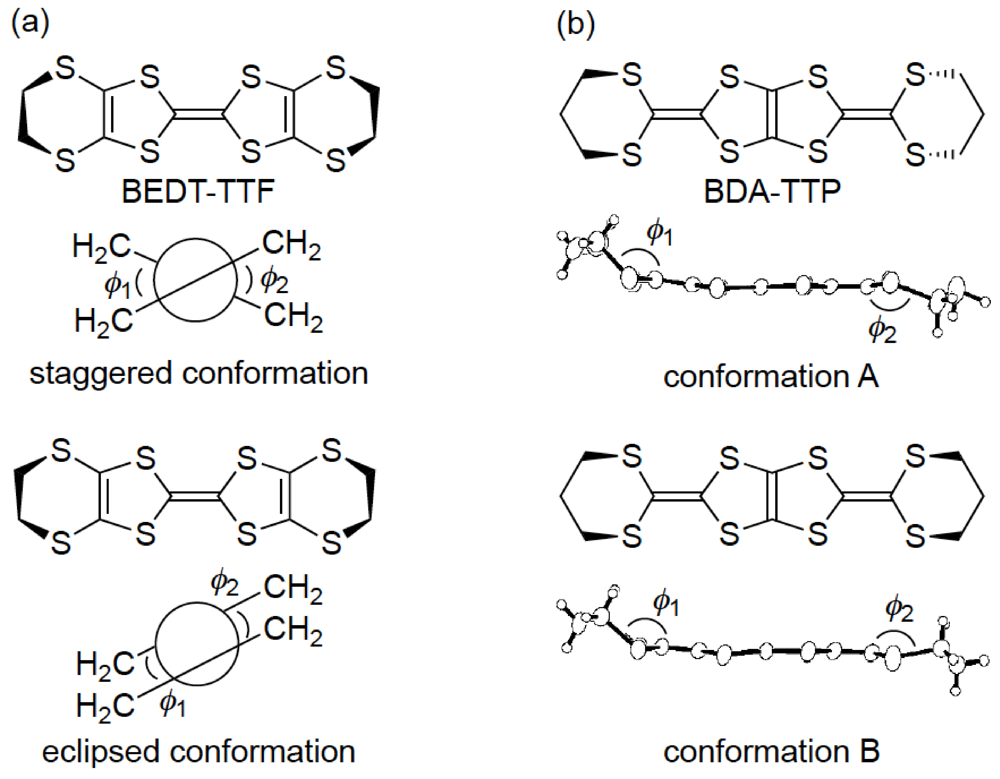
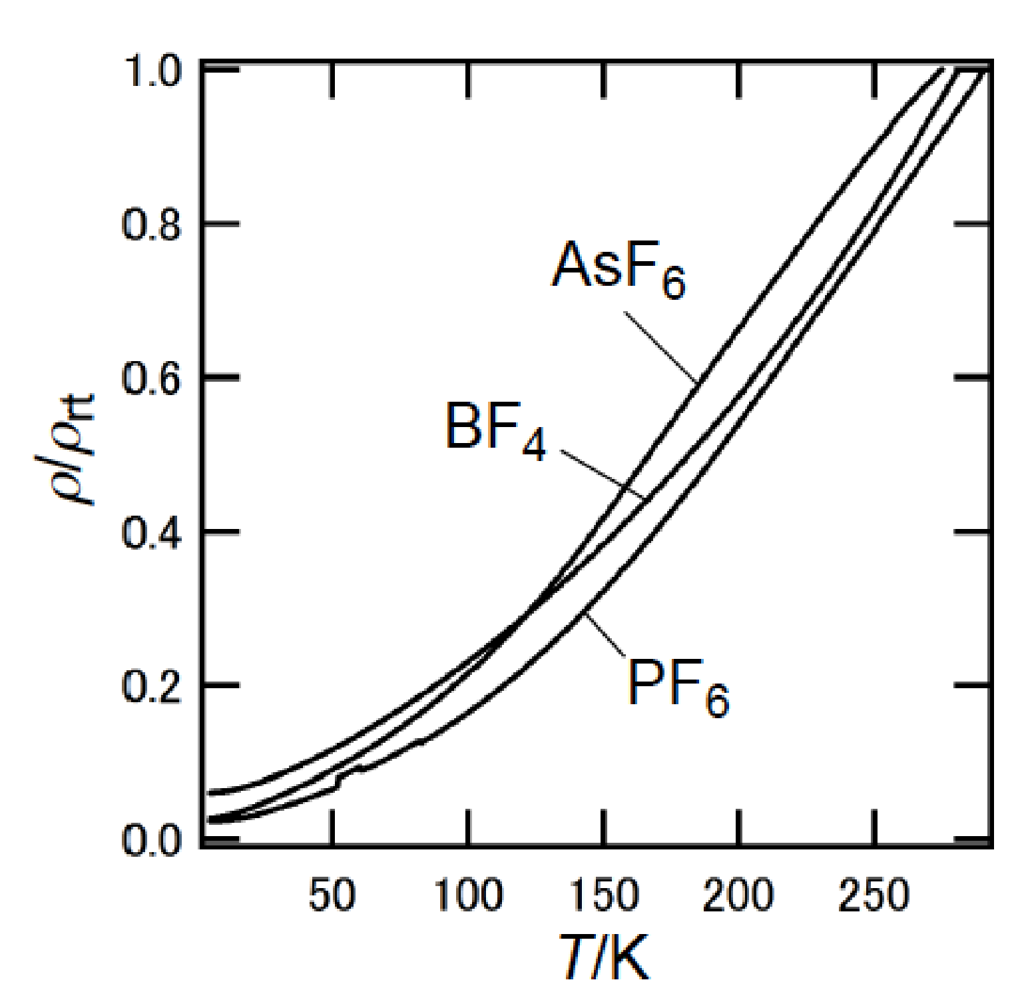
3.3. Structures of DHOT-TTP and BDH-TTP Salts with Linear Anions
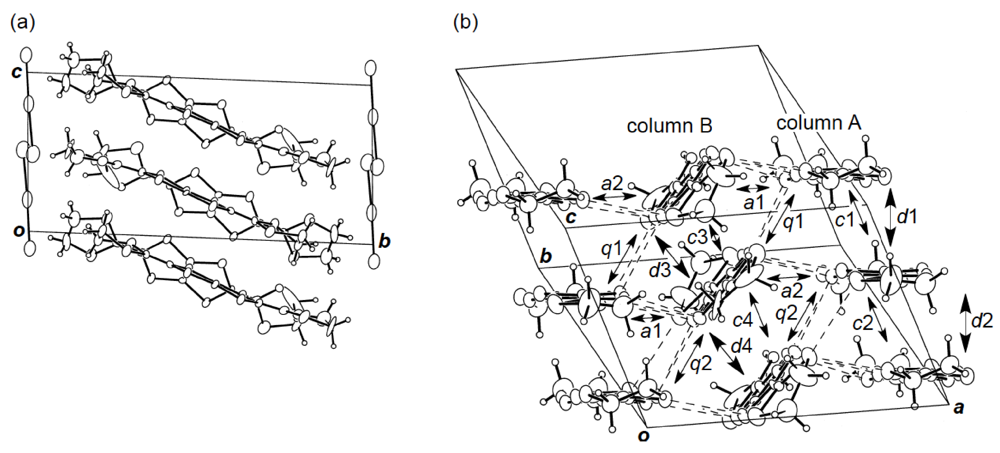
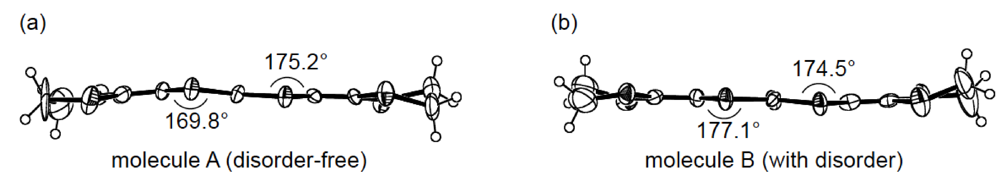
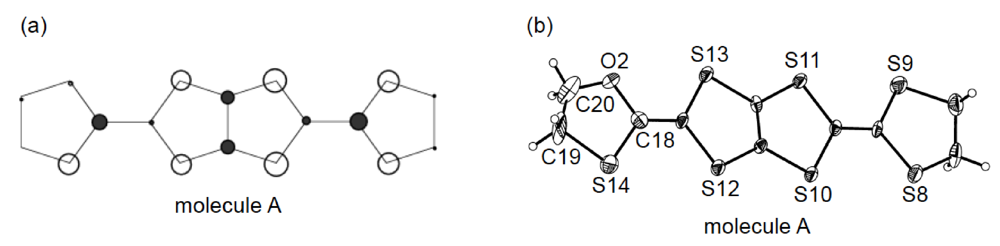
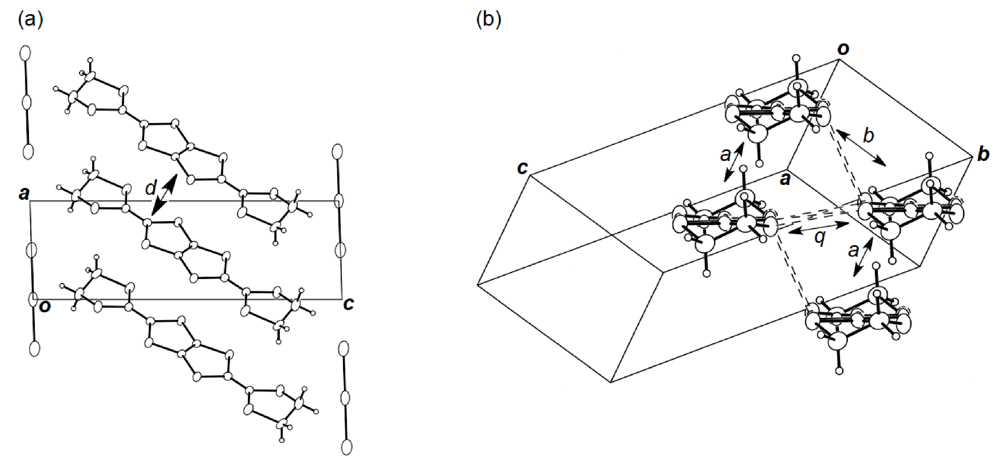


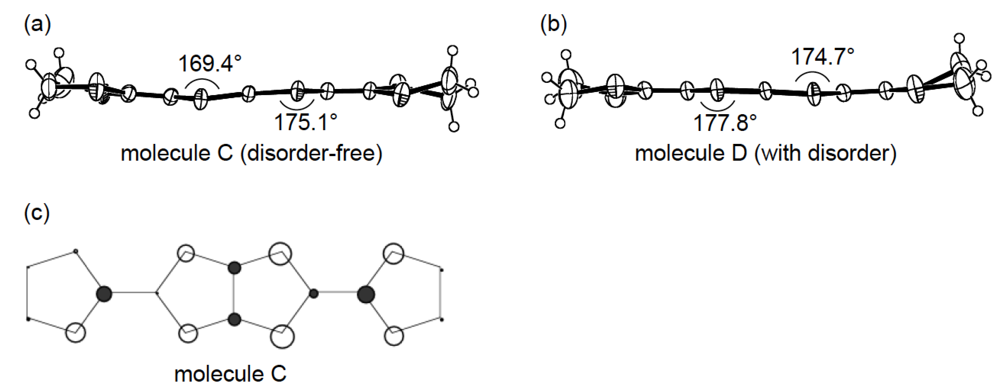

3.4. Structures of κ-Type DHOT-TTP Salts
| V (Å3) | |
|---|---|
| α-(DHOT-TTP)2Au(CN)2 | 1542.1(9) |
| α-(BDH-TTP)2Au(CN)2 | 1591(1) |
| κ-(DHOT-TTP)2PF6 | 3052(5) |
| κ-(DHOT-TTP)2AsF6 | 3070(2) |
| κ-(BDH-TTP)2PF6 | 3125.5(17) |
| κ-(DHOT-TTP)2FeCl4 | 3316(2) |
| κ-(BDH-TTP)2FeCl4 | 3379.1(8) |
4. Meso-DMDH-TTP Salts
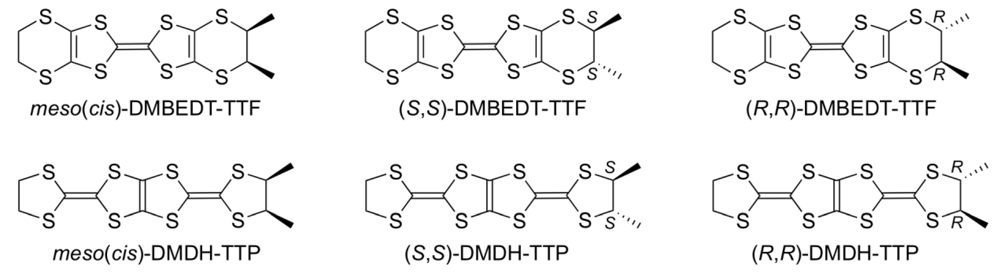
4.1. Synthesis and Electrochemical Properties of DMDH-TTP
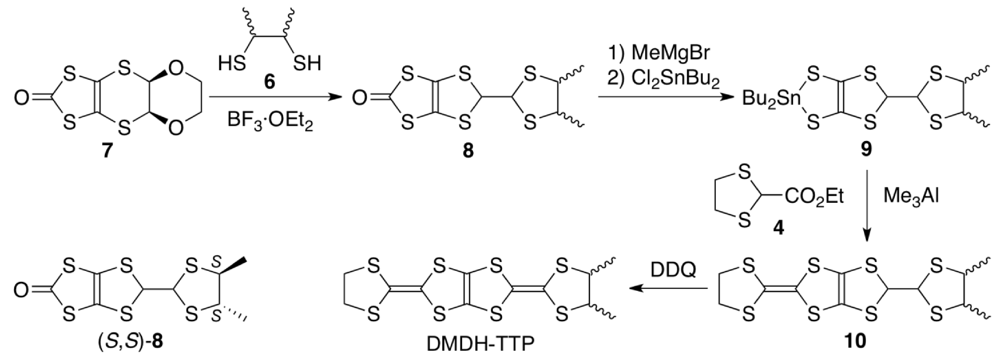
4.2. Preparation, Conductivity and Crystal and Electronic Structures of Meso-DMDH-TTP Salts
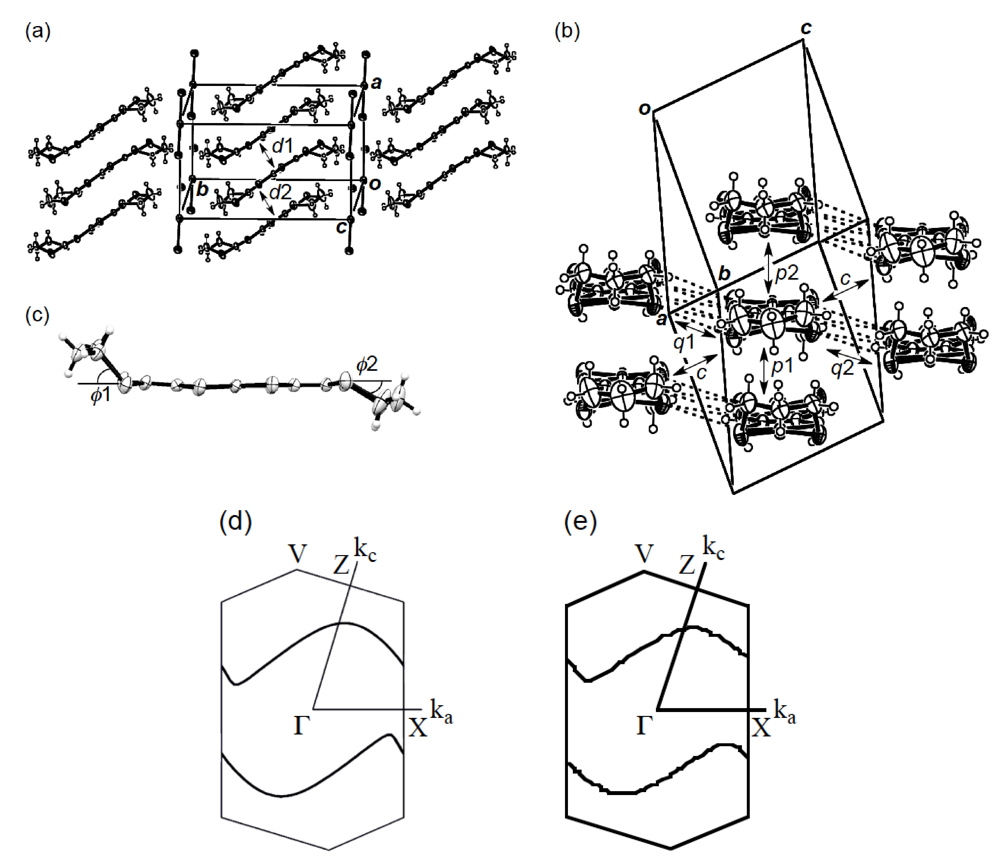
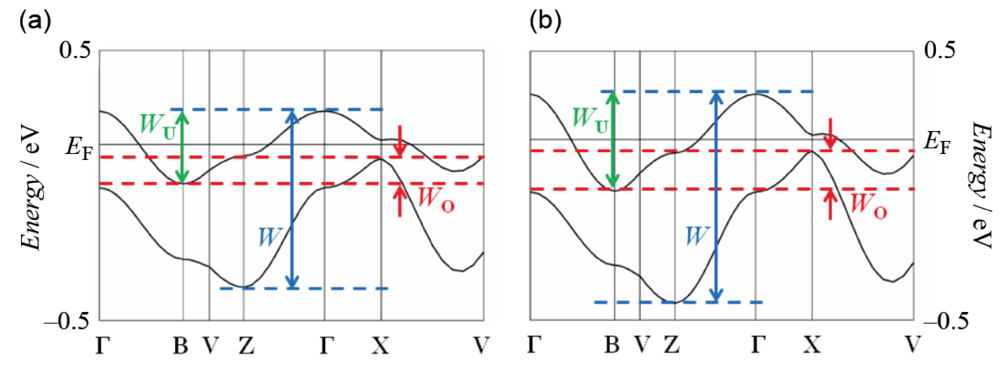
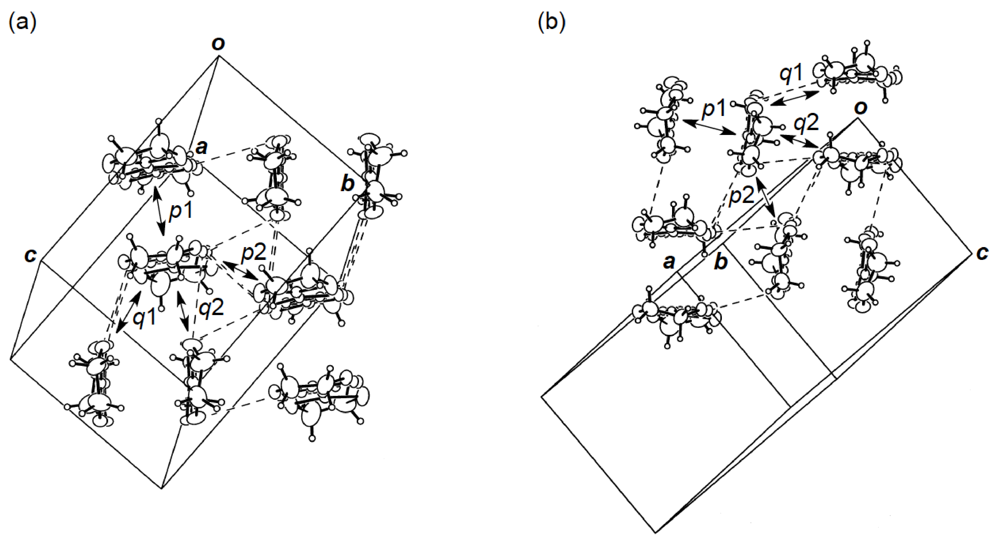
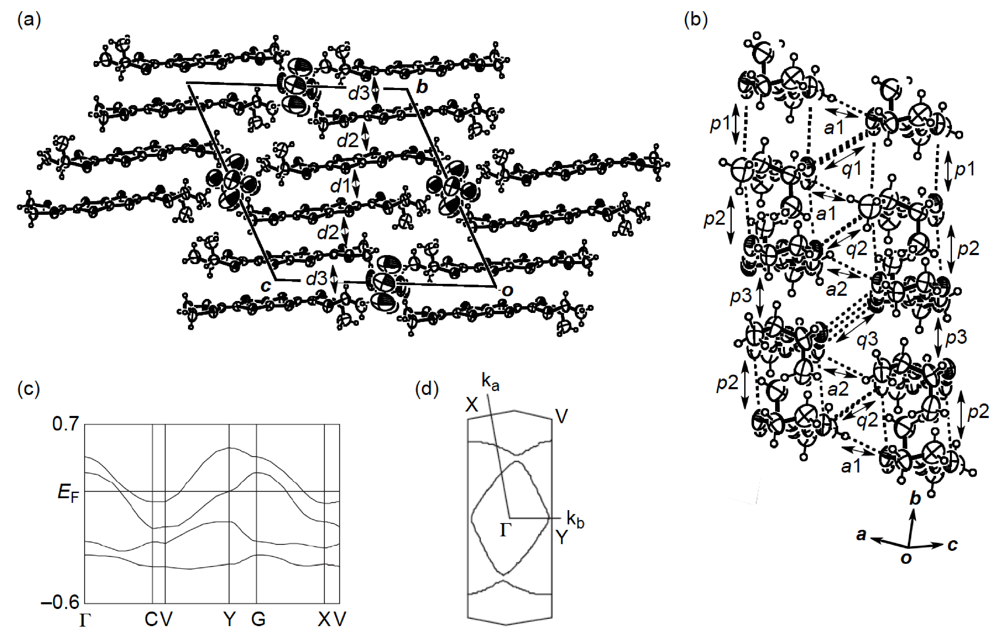
 ] direction in a head-to-tail manner with a four-folded period being 3.87, 3.75 and 3.61 Å apart. Intermolecular S···S contacts shorter than the van der Waals distance (3.70 Å) are observed among three consecutive donormolecules in the four-fold period as well as between donor stacks (Figure 28b). Compared to the absolute values of the interstack overlap integrals a1, a2, q1, q2 and q3, larger absolute values are estimated for all the intrastack overlap integrals p1, p2 and p3. The tight-binding band calculation led to the energy dispersion curve and the Fermi surface shown in Figure 28c,d, respectively. Two highest bands are partially filled, and the Fermi surface associated with these bands contains a pair of wave-like lines and a closed rhombus-like loop centered at Γ. Thus, this salt has both 1D and 2D Fermi surfaces, and the 2D Fermi surface would cause the metallic conductivity down to 4.2 K.
] direction in a head-to-tail manner with a four-folded period being 3.87, 3.75 and 3.61 Å apart. Intermolecular S···S contacts shorter than the van der Waals distance (3.70 Å) are observed among three consecutive donormolecules in the four-fold period as well as between donor stacks (Figure 28b). Compared to the absolute values of the interstack overlap integrals a1, a2, q1, q2 and q3, larger absolute values are estimated for all the intrastack overlap integrals p1, p2 and p3. The tight-binding band calculation led to the energy dispersion curve and the Fermi surface shown in Figure 28c,d, respectively. Two highest bands are partially filled, and the Fermi surface associated with these bands contains a pair of wave-like lines and a closed rhombus-like loop centered at Γ. Thus, this salt has both 1D and 2D Fermi surfaces, and the 2D Fermi surface would cause the metallic conductivity down to 4.2 K.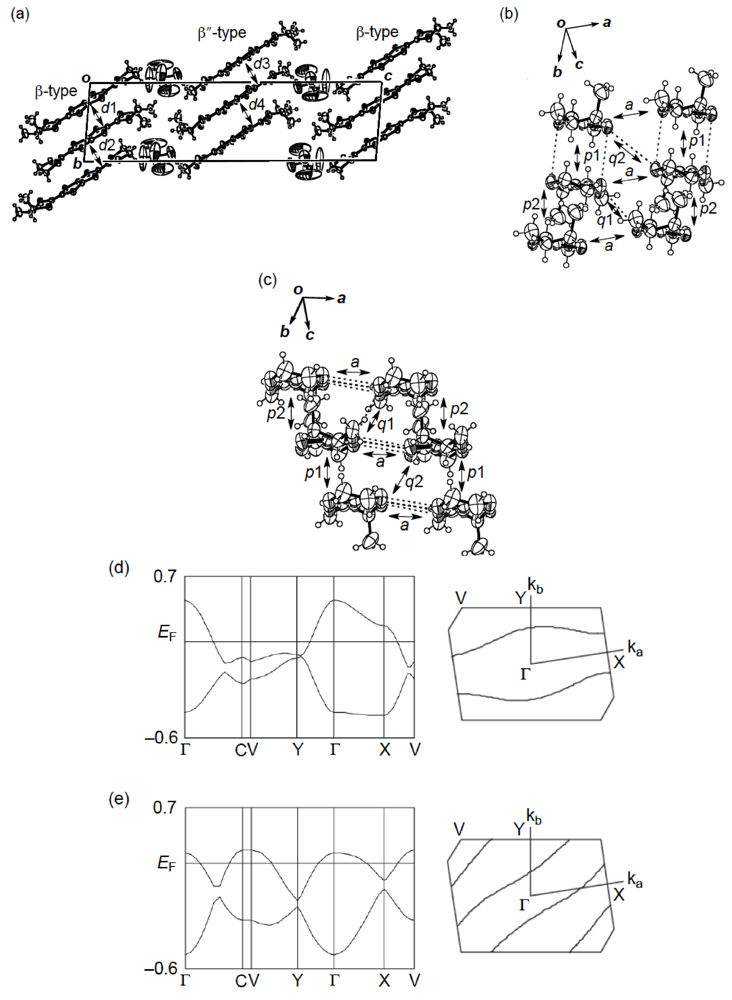
5. Conclusions
Acknowledgments
References and Notes
- Ferraris, J.; Cowan, D.O.; Walatka, V., Jr.; Perlstein, J.H. Electron transfer in a new highly conducting donor-acceptor complex. J. Am. Chem. Soc. 1973, 95, 948–949. [Google Scholar]
- Jérome, D.; Mazaud, A.; Ribault, M. Superconductivity in a synthetic organic conductor (TMTSF)2PF6. J. Phys. 1980, 41, L-95–L-98. [Google Scholar]
- TTF Chemistry–Fundamentals and Applications of Tetrathiafulvalene; Yamada, J.; Sugimoto, T. (Eds.) Kodansha & Springer: Tokyo, Japan, 2004.
- Molecular Conductors. In Chemical Review; Batail, P. (Ed.) American Chemical Society: Washington, DC, USA, 2004.
- Organic Conductors. In Journal of the Physical Society of Japan; Kagoshima, S.; Kanoda, K.; Mori, T. (Eds.) The Physical Society of Japan (JPS): Tokyo, Japan, 2006.
- Focus issue on organic conductors. In Science and Technology of Advanced Materials; Uji, S.; Mori, T.; Takahashi, T. (Eds.) Elsevier: Amsterdam, The Netherlands, 2009.
- Tajima, N.; Kajita, K. Experimental study of organic zero-gap conductor α-(BEDT-TTF)2I3. Sci. Technol. Adv. Mater. 2009, 10, 024308:1–024308:7. [Google Scholar]
- Chollet, M.; Guerin, L.; Uchida, N.; Fukaya, S.; Shimoda, H.; Ishikawa, T.; Matsuda, K.; Hasegawa, T.; Ota, A.; Yamochi, H.; et al. Gigantic photoresponse in 1/4-filled-based organic salt (EDO-TTF)2PF6. Science 2005, 307, 86–89. [Google Scholar]
- Sawano, F.; Terasaki, I.; Mori, H.; Mori, T.; Watanabe, M.; Ikeda, N.; Nogami, Y.; Noda, Y. An organic thyristor. Nature 2005, 437, 522–524. [Google Scholar]
- Endo, H.; Kawamoto, T.; Mori, T.; Terasaki, I.; Kakiuchi, T.; Sawa, H.; Kodani, M.; Takimiya, K.; Otsubo, T. Current-induced metallic state in an organic (EDT-TSF)2GaCl4 conductor. J. Am. Chem. Soc. 2006, 128, 9006–9007. [Google Scholar]
- Kanoda, K. Recent progress in NMR studies on organic conductors. Hyperfine Interactions 1997, 104, 235–249. [Google Scholar] [CrossRef]
- Yamada, J.; Nishikawa, H.; Kikuchi, K. Dihydro-TTFs and Bis-fused 1,3-Dithiol-2-ylidene Donors. In TTF Chemistry–Fundamentals and Applications of Tetrathiafulvalene; Yamada, J., Sugimoto, T., Eds.; Kodansha & Springer: Tokyo, Japan, 2004; pp. 261–286, Chapter 10. [Google Scholar]
- Yamada, J. New approach to the achievement of organic superconductivity. J. Mater. Chem. 2004, 14, 2951–2953. [Google Scholar] [CrossRef]
- Yamada, J.; Akutsu, H.; Nishikawa, H.; Kikuchi, K. New trends in the synthesis of π-electron donors for molecular conductors and superconductors. Chem. Rev. 2004, 104, 5057–5083. [Google Scholar] [CrossRef]
- Yamada, J.; Watanabe, M.; Anzai, H.; Nishikawa, H.; Ikemoto, I.; Kikuchi, K. BDH-TTP as a structural isomer of BEDT-TTF, and its two-dimensional hexafluorophosphate salt. Angew. Chem. Int. Ed. 1999, 38, 810–813. [Google Scholar] [CrossRef]
- Yamada, J.; Watanabe, M.; Akutsu, H.; Nakatsuji, S.; Nishikawa, H.; Ikemoto, I.; Kikuchi, K. New organic superconductors β-(BDA-TTP)2X [BDA-TTP = 2,5-bis(1,3-dithian-2-ylidene)-1,3,4,6-tetrathiapentalene; X− = SbF6−, AsF6−, and PF6−]. J. Am. Chem. Soc. 2001, 123, 4174–4180. [Google Scholar]
- Yamada, J.; Toita, T.; Akutsu, H.; Nakatsuji, S.; Nishikawa, H.; Ikemoto, I.; Kikuchi, K.; Choi, E.S.; Graf, D.; Brooks, J.S. A new organic superconductor, β-(BDA-TTP)2GaCl4 [BDA-TTP = 2,5-bis(1,3-dithian-2-ylidene)-1,3,4,6-tetrathiapentalene]. Chem. Commun. 2003, 2230–2231. [Google Scholar]
- Choi, E.S.; Graf, D.; Brooks, J.S.; Yamada, J.; Akutsu, H.; Kikuchi, K.; Tokumoto, M. Pressure-dependent ground states and fermiology in β-(BDA-TTP)2MCl4 (M = Fe, Ga). Phys. Rev. B 2004, 70, 024517:1–024517:8. [Google Scholar]
- Williams, J.M.; Ferraro, J.R.; Thorn, R.J.; Carlson, D.; Geiser, U.; Wang, H.H.; Kini, A.M.; Whangbo, M.-H. Organic Superconductors (Including Fullerenes): Synthesis, Structure, Properties, and Theory; Prentice Hall: Englewood Cliffs, NJ, USA, 1992. [Google Scholar]
- Yamada, J.; Fujimoto, K.; Akutsu, H.; Nakatsuji, S.; Miyazaki, A.; Aimatsu, M.; Kudo, S.; Enoki, T.; Kikuchi, K. Pressure effect on the electrical conductivity and superconductivity of β-(BDA-TTP)2I3. Chem. Commun. 2006, 1331–1333. [Google Scholar]
- Kikuchi, K.; Isono, T.; Kojima, M.; Yoshino, H.; Kodama, T.; Fujita, W.; Yokogawa, K.; Yoshino, H.; Murata, K.; Kaihatsu, T.; Akutsu, H.; Yamada, J. Uniaxial strain orientation dependence of superconducting transition temperature (Tc) and critical superconducting pressure (Pc) in β-(BDA-TTP)2I3. J. Am. Chem. Soc. 2011, 133, 19590–19593. [Google Scholar]
- Mori, T.; Kobayashi, A.; Sasaki, Y.; Kobayashi, H.; Saito, G.; Inokuchi, H. The intermolecular interaction of tetrathiafulvalene and bis(ethylenedithio)tetrathiafulvalene in organic metals. Calculation of orbital overlaps and models of energy-band structures. Bull. Chem. Soc. Jpn. 1984, 57, 627–633. [Google Scholar] [CrossRef]
- Seo, H.; Hotta, C.; Fukuyama, H. Toward systematic understanding of diversity of electronic properties in low-dimensional molecular solids. Chem. Rev. 2004, 104, 5005–5036. [Google Scholar] [CrossRef]
- Miyagawa, K.; Kanoda, K.; Kawamoto, A. NMR studies on two-dimensional molecular conductors and superconductors: Mott transition in κ-(BEDT-TTF)2X. Chem. Rev. 2004, 104, 5635–5653. [Google Scholar] [CrossRef]
- Mori, H.; Tanaka, S.; Mori, T. Systematic study of the electronic state in θ-type BEDT-TTF organic conductors by changing the electronic correlation. Phys. Rev. B 1998, 57, 12023–12029. [Google Scholar]
- Nishikawa, H.; Sato, Y.; Kikuchi, K.; Kodama, T.; Ikemoto, I.; Yamada, J.; Oshio, H.; Kondo, R.; Kagoshima, S. Charge ordering and pressure-induced superconductivity in β″-(DODHT)2PF6. Phys. Rev. B 2005, 72, 052510:1–052510:4. [Google Scholar]
- Kagoshima, S.; Kondo, R. Control of electronic properties of molecular conductors by uniaxial strain. Chem. Rev. 2004, 104, 5593–5608. [Google Scholar]
- Murata, K.; Kagoshima, S.; Yasuzuka, S.; Yoshino, H.; Kondo, R. High-pressure research in organic conductors. J. Phys. Soc. Jpn. 2006, 75, 051015:1–051015:15. [Google Scholar]
- Uruichi, M.; Nakano, C.; Tanaka, M.; Yakushi, K.; Kaihatsu, T.; Yamada, J. Infrared and Raman spectroscopic study of BDA-TTP [2,5-bis(1,3-dithian-2-ylidene)-1,3,4,6-tetrathiapentalene] and its charge-transfer salts. Solid State Commun. 2008, 147, 484–489. [Google Scholar] [CrossRef]
- Kanoda, K. Metal-insulator transition in κ-(ET)2X and (DCNQI)2M: Two contrasting manifestation of electron correlation. J. Phys. Soc. Jpn. 2006, 75, 051007:1–051007:16. [Google Scholar]
- Kikuchi, K.; Nishikawa, H.; Ikemoto, I.; Toita, T.; Akutsu, H.; Nakatsuji, S.; Yamada, J. Tetrachloroferrare (III) salts of BDH-TTP [2,5-bis(1,3-dithiolan-2-ylidene)-1,3,4,6-tetrathiapentalene] and BDA-TTP [2,5-bis(1,3-dithian-2-ylidene)-1,3,4,6-tetrathiapentalene]: Crystal structures and physical properties. J. Solid State Chem. 2002, 168, 503–508. [Google Scholar] [CrossRef]
- Yamada, J. A new approach in the design of organic superconductors. J. Phys. IV Fr. 2004, 114, 439–443. [Google Scholar] [CrossRef]
- Shevyakova, I.; Buravov, L.; Tkacheva, V.; Zorina., L.; Khasanov, S.; Simonov, S.; Yamada, J.; Canadel, E.; Shibaeva, R.; Yagubskii, E. New organic metals based on BDH-TTP radical cation salts with the photochromic nitroprusside anion [FeNO(CN)5]2−. Adv. Funct. Mater. 2004, 14, 660–668. [Google Scholar] [CrossRef]
- Kushch, N.D.; Kazakova, A.V.; Buravov, L.I.; Yagubskii, E.B.; Simonov, S.V.; Zorina, L.V.; Khasanov, S.S.; Shibaeva, R.P.; Canadell, E.; Son, H.; Yamada, J. The first BDH-TTP radical cation salts with mercuric counterions, κ-(BDH-TTP)4[Hg(SCN)4]·C6H5NO2 and α′-(BDH-TTP)6[Hg(SCN)3][Hg(SCN)4]. Synth. Met. 2005, 155, 588–594. [Google Scholar] [CrossRef]
- Zhilyaeva, E.I.; Flakina, A.M.; Lyubovskaya, R.N.; Fedyanin, I.V.; Lyssenko, K.A.; Antipin, M.Y.; Lyubovskii, R.B.; Yudanova, E.I.; Yamada, J. Synthesis, crystal structures and properties of new radical cation salts based on some tetrathiapentalene derivatives with halogenomercurate anions. Synth. Met. 2006, 156, 991–998. [Google Scholar] [CrossRef]
- Kushch, N.D.; Kazakova, A.V.; Buravov, L.I.; Yagubskii, E.B.; Simonov, S.V.; Zorina, L.V.; Khasanov, S.S.; Shibaeva, R.P.; Yamada, J.; Umemiya, M. New molecular magnetic metals: κ-(BDH-TTP)4CuCl4·(H2O)n and κ-(BDH-TTP)2[CuCl4]0.67·(H2O)0.33 (BDH-TTP is 2,5-bis(1,3-dithiolan-2-ylidene)-1,3,4,6-tetrathiapentalene). Russ. Chem. Bull. Int. Ed. 2010, 59, 1–6. [Google Scholar]
- Misaki, Y. Tetrathiapentalene-based organic conductors. Sci. Technol. Adv. Mater. 2009, 10, 024301:1–02430122:7. [Google Scholar]
- Moge, M.; Hellberg, J.; Törnroos, K.W.; von Schütz, J.-U. Synthesis and crystal structure of a new unsymmetrical oxygen containing TTF. Adv. Mater. 1996, 8, 807–808. [Google Scholar] [CrossRef]
- Yamada, J.; Kunigita, K.; Akutsu, H.; Nakatsuji, S.; Kikuchi, K. Monooxygen-containing analogue of BDH-TTP, DHOT-TTP [2-(1,3-dithiolan-2-ylidene)-5-(1,3-oxathiolan-2-ylidene)-1,3,4,6-tetrathiapentalene], and its metallic AuI2 salt. Chem. Lett. 2005, 34, 32–33. [Google Scholar] [CrossRef]
- Yamada, J.; Kunigita, K.; Akutsu, H.; Nakatsuji, S.; Kikuchi, K. Competitive effect in metallic κ-type DHOT-TTP [2-(1,3-dithiolan-2-ylidene)-5-(1,3-oxathiolan-2-ylidene)-1,3,4,6-tetrathiapentalene] salts. Chem. Lett. 2005, 34, 1126–1127. [Google Scholar] [CrossRef]
- Yamada, J.; Oka, R.; Mangetsu, T.; Akutsu, H.; Nakatsuji, S.; Nishikawa, H.; Ikemoto, I.; Kikuchi, K. Tetrathiafulvalene derivatives linking a dichalcogenolane ring through the σ-bond: New donor molecules for organic metals. Chem. Mater. 2001, 13, 1770–1777. [Google Scholar]
- Kini, A.M.; Mori, T.; Geiser, U.; Budz, S.M.; Williams, J.M. 4,5-Ethylenedioxy-4,5'-ethylenedithiotetrathiafulvalene (EOET): A new unsymmetrical electron donor. J. Chem. Soc. Chem. Commun. 1990, 647–648. [Google Scholar]
- Yamada, J.; Kunigita, K.; Akutsu, H.; Nakatsuji, S.; Kikuchi, K. Unpublished work. 2012; Department of Material Science, Graduate School of Material Science, University of Hyogo, 3-2-1 Kouto, Kamigori-cho, Ako-gun, Hyogo 678-1297, Japan. [Google Scholar]
- Crystal data for (BDH-TTP)2AuI2: C10H8Au0.50IS8, M = 610.04, triclinic, space group P
, a = 4.9044(18), b = 5.581(3), c = 15.543(6) Å, α = 99.157(9)°, β = 91.500(10)° γ = 91.779(11)°, V = 419.6(3) Å3, Z = 1, T = 294 K, Dcalcd = 2.414 g cm–3, R = 0.0697 and Rw = 0.2406 for 1329 observed reflections with I > 2.0σ(I) from 1840 unique reflections. The crystal structure has been deposited at the Cambridge Crystallographic Data Centre (CCDC 885998).
- Crystal data for α-(DHOT-TTP)2Au(CN)2: C22H16AuN2O2S14, M = 986.19, triclinic, space group P
, a = 11.295(4), b = 17.069(5), c = 8.579(4) Å, α = 90.92(3)°, β = 110.04(3)° γ = 96.21(3)°, V = 1542.1(10) Å3, Z = 2, T = 297 K, Dcalcd = 2.124 g cm−3, R = 0.0571 and Rw = 0.1773 for 5419 observed reflections with I > 2.0σ(I) from 7083 unique reflections. The crystal structure has been deposited at the Cambridge Crystallographic Data Centre (CCDC 885999).
- Crystal data for α-(BDH-TTP)2Au(CN)2: C22H16AuN2S16, M = 1018.31, triclinic, space group P
, a = 11.601(5), b = 17.141(9), c = 8.570(3) Å, α = 92.21(4)°, β = 110.01(3)°, γ = 95.04(4)°, V = 1591(1) Å3, Z = 2, T = 299.2 K, Dcalcd = 2.126 g cm−3, R = 0.0672 and Rw = 0.0668 for 5216 observed reflections with I > 3.00σ(I) from 7330 unique reflections. The crystal structure has been deposited at the Cambridge Crystallographic Data Centre (CCDC 885997).
- Yamada, J.; Hiratani, N.; Kunigita, K.; Akutsu, H.; Nakatsuji, S.; Kikuchi, K. Monooxygen-containing Analogues of DHDA-TTP, DHOTA-TTP and OTDA-TTP, and Their Charge-transfer Salts. In Multifunctional Conducting Molecular Materials; Saito, G., Wudl, F., Haddon, R.C., Tanigaki, K., Enoki, T., Katz, H.E., Maesato, M., Eds.; RSC Publishing: Cambridge, UK, 2007; pp. 67–70. [Google Scholar]
- Matsumiya, S.; Izuoka, A.; Sugawara, T.; Taruishi, T.; Kawada, Y. Effect of methyl substitution on conformation and molecular arrangement of BEDT-TTF derivatives in the crystalline environment. Bull. Chem. Soc. Jpn. 1993, 66, 513–522. [Google Scholar]
- Kimura, S.; Maejima, T.; Suzuki, H.; Chiba, R.; Mori, H.; Kawamoto, T.; Mori, T.; Moriyama, H.; Nishio, Y.; Kajita, K. A new organic superconductor β-(meso-DMBEDT-TTF)2PF6. Chem. Commun. 2004, 2454–2455. [Google Scholar]
- Zambounis, J.S.; Mayer, C.W.; Hauenstein, K.; Hilti, B.; Hofherr, W.; Pfeiffer, J. Crystal structure and electrical properties of κ-((S,S)-DMBEDT-TTF)2ClO4. Adv. Mater. 1992, 4, 33–35. [Google Scholar] [CrossRef]
- Yamada, J.; Song, H.; Akutsu, H.; Nakatsuji, S.; Kikuchi, K. Synthesis of dimethyl-substituted BDH-TTP derivative DMDH-TTP as a diastereomeric mixture, and the formation of metallic salts involving only meso-DMDH-TTP. Chem. Lett. 2005, 34, 1404–1405. [Google Scholar] [CrossRef]
- Corey, E.J.; Mitra, R.B. L(+)-2,3-butanedithiol: Synthesis and application to the resolution of racemic carbonyl compounds. J. Am. Chem. Soc. 1962, 84, 2938–2941. [Google Scholar] [CrossRef]
- Anzai, H.; Delrieu, J.M.; Takasaki, S.; Nakatsuji, S.; Yamada, J. Crystal growth of organic charge-transfer complexes by electrocrystallization with controlled applied current. J. Cryst. Growth 1995, 154, 145–150. [Google Scholar] [CrossRef]
- Nishikawa, H.; Sato, T.; Kodama, T.; Ikemoto, I.; Kikuchi, K.; Anzai, H.; Yamada, J. Electrical properties and crystal structures of metallic TMVT salts. J. Mater. Chem. 1999, 9, 693–696. [Google Scholar] [CrossRef]
- Yamada, J.; Song, H.; Akutsu, H.; Nakatsuji, S.; Kikuchi, K. Dimethyl-substituted Analogue of BDH-TTP, DMDH-TTP, and Its Metallic Salts. In Multifunctional Conducting Molecular Materials; Saito, G., Wudl, F., Haddon, R.C., Tanigaki, K., Enoki, T., Katz, H.E., Maesato, M., Eds.; RSC Publishing: Cambridge, UK, 2007; pp. 63–66. [Google Scholar]
- Kimura, S.; Hanazato, S.; Kurai, H.; Mori, T.; Misaki, Y.; Tanaka, K. Stereochemically ordered donor columns in an organic conductor, (Et2BEDT-TTP)2HgI3. Tetrahedron Lett. 2001, 42, 5729–5732. [Google Scholar]
- Schlueter, J.A.; Wiehl, L.; Park, H.; de Souza, M.; Lang, M.; Koo, H.-J.; Whangbo, M.-H. Enhanced critical temperature in a dual-layered molecular superconductor. J. Am. Chem. Soc. 2010, 132, 16308–16310, and references therein.. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamada, J.-i.; Akutsu, H. Chemical Modifications of BDH-TTP [2,5-Bis(1,3-dithiolan-2-ylidene)-1,3,4,6-tetrathiapentalene]: Control of Electron Correlation. Crystals 2012, 2, 812-844. https://doi.org/10.3390/cryst2030812
Yamada J-i, Akutsu H. Chemical Modifications of BDH-TTP [2,5-Bis(1,3-dithiolan-2-ylidene)-1,3,4,6-tetrathiapentalene]: Control of Electron Correlation. Crystals. 2012; 2(3):812-844. https://doi.org/10.3390/cryst2030812
Chicago/Turabian StyleYamada, Jun-ichi, and Hiroki Akutsu. 2012. "Chemical Modifications of BDH-TTP [2,5-Bis(1,3-dithiolan-2-ylidene)-1,3,4,6-tetrathiapentalene]: Control of Electron Correlation" Crystals 2, no. 3: 812-844. https://doi.org/10.3390/cryst2030812