A Review on the Properties of Iron Aluminide Intermetallics
Abstract
:1. Introduction


2. Characteristics of Fe-Al Intermetallics
2.1. Phase Diagram
| Phase | Label in Figure 4 | Pearson Symbol | Space Group | Prototype | Lattice Parameters (nm) | Elastic Constants (eV/Å3) | ||
|---|---|---|---|---|---|---|---|---|
| Liquid | L | |||||||
| a0 = b0 = c0 = 0.40496 [43] | C11 = 0.6492, C12 = 0.4619 C44 = 0.2684 [44] | |||||||
| a0 = b0 = c0 = 0.36599 [40] | -- | |||||||
| a0 = b0 = c0 = 0.28665 [43] | C11 = 1.4357, C12 = 0.8426 C44 = 0.73 [44] | |||||||
| a0 = b0 = c0 = 0.2895 [40], 0.5904/2 [44], 0.5792/2 [43] | [44] | [45] | ||||||
| C11 = 0.945, C12 = 0.892, C44 = 0.788 | 1.067 0.822 0.815 | |||||||
| a0 = b0 = c0 = 0.291 [41,43,46], 0.283 [9], 0.3031 [44] | [46] | [9] | [44] | |||||
| C11 = 1.2 C12 = 0.75 C44 = 0.73 | 1.8 0.8 1.029 | 0.883 0.846 0.691 | ||||||
| n.a. [42] | -- | |||||||
| a0 = 0.4872, b0 = 0.6459, c0 = 0.8794, α = 91.76 β = 73.35, γ = 96.89 [41] | -- | |||||||
| -- | a0 = 0.7652, b0 = 0.6463, c0 = 0.4229 [41] | -- | ||||||
| -- | n.a. [41] | -- | ||||||

2.2. Point Defects in the Super Cells

| Defects | D03 | B2 |
|---|---|---|
| 1.25 [63], 1.583 [44] | 0.97 [57], 1.06, [64], 0.80 [65], 0.653 [66] | |
| 2.27 [63], 1.388 [44] | ||
| 1.4 [63], 2.221 [44] | 4.00 [57], 3.46, [64] 2.80 [65], 1.493 [66] | |
| 0.430 [44] | 1.03 [57], 0.78[65], 0.95 [66] | |
| 0.047 [44] | 0.95 [57], 0.76 [65], 1.03 [66] | |
| 0.218 [44] |

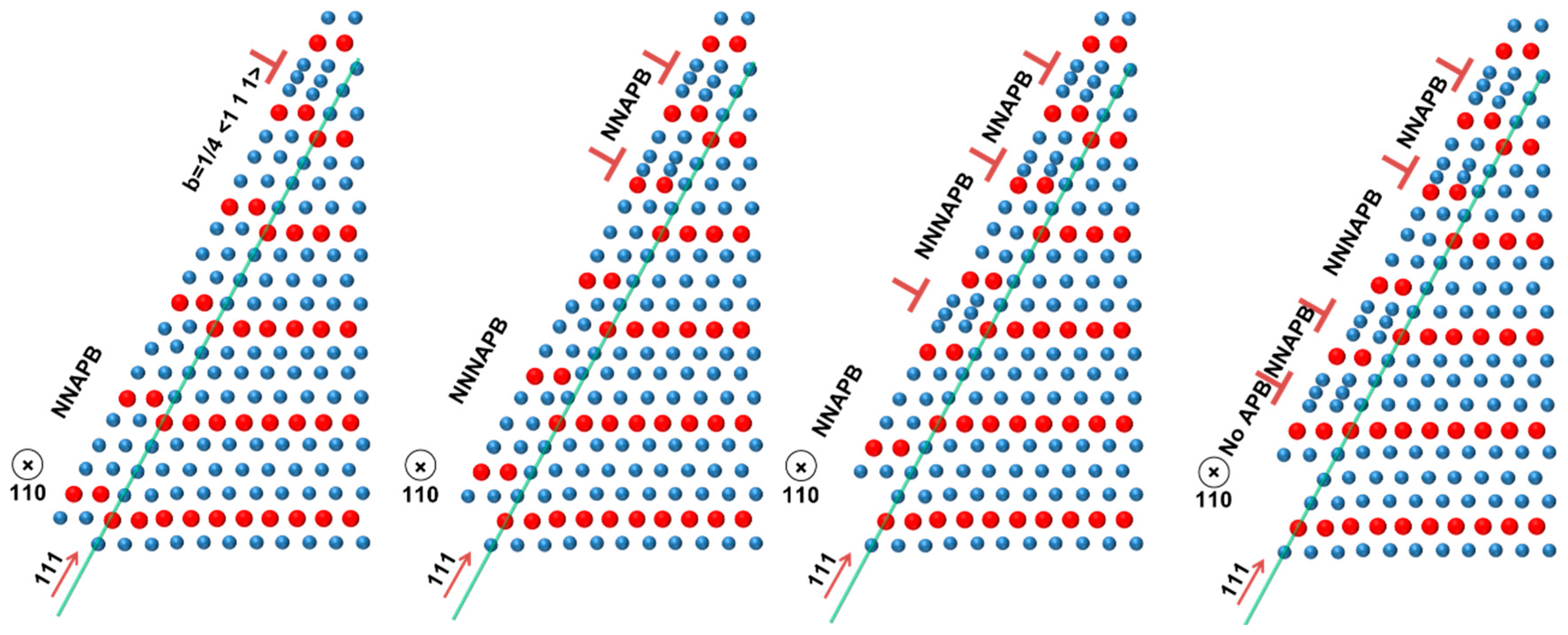
2.3. Dislocations in Fe-Al
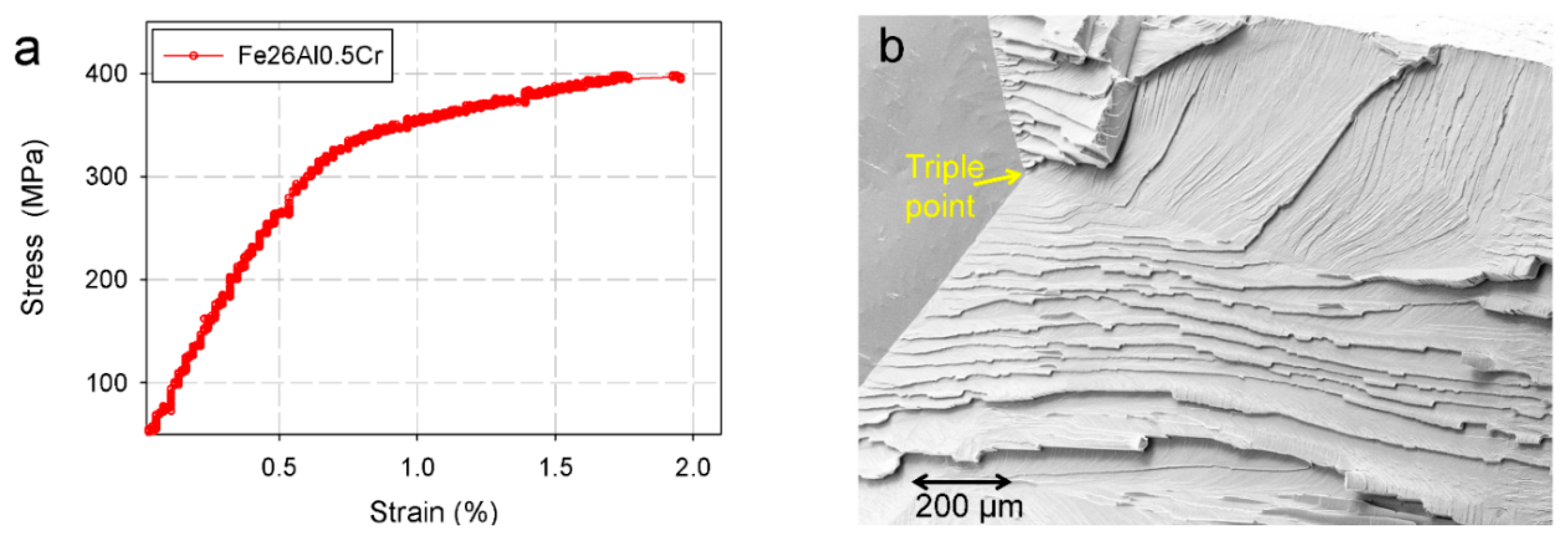
2.4. Mechanical Properties at High Temperatures
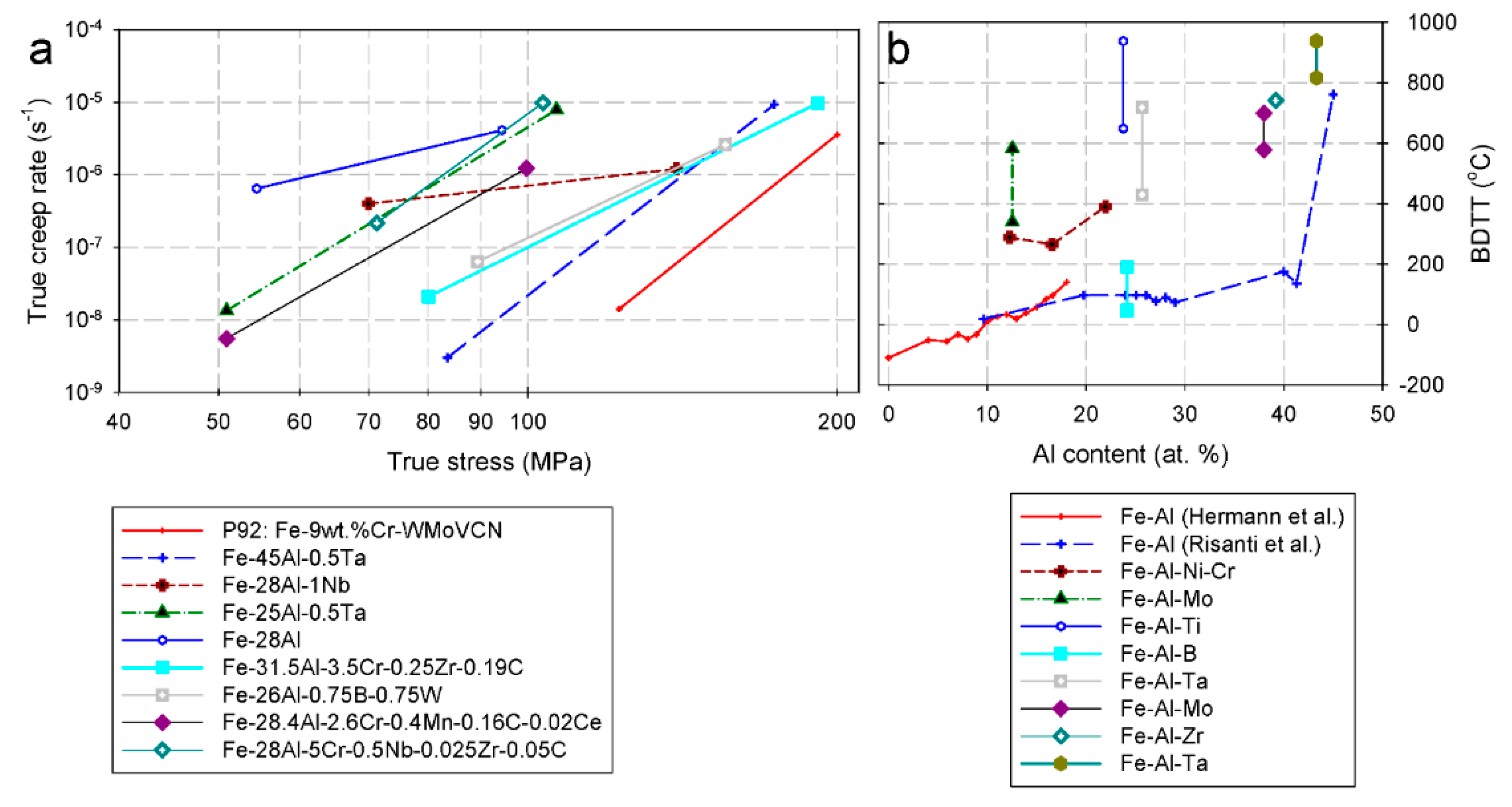
2.5. Alloying Elements
| -- | Formation Energy at Different Sublattices (meV/atom) [40] | Relative Changes of the Lattice Parameter with Respect to the Solute Content | Relative Changes of the Young’s Modulus [40] | ||||
|---|---|---|---|---|---|---|---|
| β | α | γ | Theory | Exp. T = 77 K | Exp. T = 300 K | ||
| Ti | −242 | −191 | −194 | 0.05 [40], 0.03 [88], 0.05 [86] | 0.02 | 0.16 | 0.17 |
| W | −198 | −124 | −147 | 0.03 [40] | 0.05 | 0.13 | 0.15 |
| V | −229 | −182 | −188 | −0.03 [40], −0.02 [88] | 0.04 | 0.07 | 0.08 |
| Cr | −185 | −175 | −156 | −0.05 [40], −0.02 [88], 0.01 [86] | 0.02 | 0.02 | 0.02 |
| Si | −227 | −194 | −231 | -- | 0.03 | 0.09 | 0.08 |

2.5.1. Influence of Solute Atoms on Mechanical Properties
2.5.2. Effect of Cr on the Mechanical Properties of Fe-Al Intermetallics

3. Environmental Degradation
3.1. Oxidation and Corrosion of Iron-Aluminides
3.1.1. Oxidation Resistance

3.1.2. Electrochemical Properties of Fe-Al Intermetallics


3.2. Hydrogen Embrittlement (HE)
3.2.1. Mechanism of Hydrogen Ingress
| Material | Activation Barrier (eV) | Diffusion Coefficient at Room Temperature (m2/s) |
|---|---|---|
| Fe | -- | 10−8 |
| Fe-18Al | -- | 10−11 [146,148] |
| Fe-25Al | 0.42 [144] | 1.45 × 10−13 [146] |
| Fe-37Al | -- | 5.57 × 10−10 [149] |
| Fe-40Al | 0.22 [145] | 4.4 × 10−13 [147], 5.07 × 10−10 [149] |
| Fe-43Al | -- | 4.46 × 10−10 [149] |
| Fe-46Al | -- | 3.62 × 10−10 [149] |
| Fe-50Al | 0.26 [142] | 2.257 × 10−10 [149] |
3.2.2. Hydrogen Interaction with Defects
| Host Material | Type of Trap | Binding Energy (eV) | Reference (s) |
|---|---|---|---|
| α-Fe | Vacancy | 0.63, 0.48 | [160,161] |
| substitutional (Ti) | 0.19 | [160] | |
| interstitional (C) | 0.03 | [160] | |
| interstitional (N) | >0.13 | [160] | |
| Grain boundary | 0.10 | [161] | |
| Dislocation elastic stress field | 0.21 | [161] | |
| Dislocation core | 0.61 | [161] | |
| Free surface | 0.73 | [161] | |
| bcc carbon steel | Fe3C phase interface | 0.11 | [160] |
| Al | Vacancy | 0.52, 0.53 | [160,161,162,163,164] |
| Grain boundary | 0.15 | [160] | |
| Al2O3-Al phase interface | 0.7, 1.0–1.4 | [160] |
3.2.3. Hydrogen Embrittlement of Fe-Al Intermetallics


| Sample Orientation | Elongation (%) | Fracture Strength (MPa) | ||
|---|---|---|---|---|
| Air | Vacuum | Air | Vacuum | |
| Edge | 4.0 | 9.4 | 336 | 460 |
| 3.3 | 5.7 | 335 | 425 | |
| 5.9 | 12.0 | 320 | 422 | |
| Screw | 1.1 | 3.0 | 235 | 435 |
| 2.6 | 10.8 | 335 | 495 | |
| 5.7 | 10.1 | 345 | 470 | |
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ziegler, N. Resistance of iron-aluminum alloys to oxidation at high temperatures. Trans. Am. Inst. Min. Met. Eng. 1932, 100, 267–271. [Google Scholar]
- Blau, P.J.; Meyer, H.M. Characteristics of wear particles produced during friction tests of conventional and unconventional disc brake materials. Wear 2003, 255, 1261–1269. [Google Scholar] [CrossRef]
- Judkins, R.R.; Rao, U.S. Fossil energy applications of intermetallic alloys. Intermetallics 2000, 8, 1347–1354. [Google Scholar] [CrossRef]
- Stoloff, N.; Liu, C.; Deevi, S. Emerging applications of intermetallics. Intermetallics 2000, 8, 1313–1320. [Google Scholar] [CrossRef]
- Baker, I.; Munroe, P.R. Mechanical properties of FeAl. Int. Mater. Rev. 1997, 42, 181–205. [Google Scholar] [CrossRef]
- Noebe, R.D.; Bowman, R.R.; Nathal, M.V. Physical and mechanical-properties of the B2 compound nial. Int. Mater. Rev. 1993, 38, 193–232. [Google Scholar] [CrossRef]
- Sundararajan, V.; Sahu, B.R.; Kanhere, D.G.; Panat, P.V.; Das, G.P. Cohesive, electronic and magnetic-properties of the transition-metal aluminides FeAl, CoAl and NiAl. J. Phys.Condens. Matter 1995, 7, 6019–6034. [Google Scholar] [CrossRef]
- Fu, C.L.; Wang, X.D. The effect of electronic structure on the defect properties of FeAl. Mat. Sci. Eng. A-Struct. 1997, 240, 761–768. [Google Scholar] [CrossRef]
- Fu, C.L.; Yoo, M.H. Electronic structure and mechanical behavior of transition-metal aluminides: A first-principles total-energy investigation. Mater. Chem. Phys. 1992, 32, 25–36. [Google Scholar] [CrossRef]
- Miracle, D.; Darolia, R. NiAl and Its Alloys, Intermetallic Compounds, Vol. 3: Structural Applications of Intermetallic Compounds; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Huang, S.C.; Chesnutt, C.J. Gamma TiAl and Its Alloys, Intermetallic Compounds, Vol. 3: Structural Applications of Intermetallic Compounds; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Briant, C.L. Intergranular and Cleavage Fracture, Intermetallic Compounds, Vol. 2: Basic Mechanical Properties and Lattice Defects of Intermetallic Compounds; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Vedula, K. FeAl and Fe3Al, Intermetallic Compounds, Vol. 3: Structural Applications of Intermetallic Compounds; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Mckamey, C.G.; Devan, J.H.; Tortorelli, P.F.; Sikka, V.K. A review of recent developments in Fe3Al-based alloys. J. Mater. Res. 1991, 6, 1779–1805. [Google Scholar] [CrossRef]
- Liu, C.T. Recent advances in ordered intermetallics. Mater. Chem. Phys. 1995, 42, 77–86. [Google Scholar] [CrossRef]
- Liu, C.; Lee, E.; McKamey, C. An environmental effect as the major cause for room-temperature embrittlement in FeAl. Scr. Metall. Mater. 1989, 23, 875–880. [Google Scholar] [CrossRef]
- Prescott, R.; Graham, M. The oxidation of iron-aluminum alloys. Oxid. Metals 1992, 38, 73–87. [Google Scholar] [CrossRef]
- Tortorelli, P.; Natesan, K. Critical factors affecting the high-temperature corrosion performance of iron aluminides. Mater. Sci. Eng.: A 1998, 258, 115–125. [Google Scholar] [CrossRef]
- Alven, D.; Stoloff, N. The influence of composition on the environmental embrittlement of Fe3Al alloys. Mater. Sci. Eng.: A 1997, 239, 362–368. [Google Scholar] [CrossRef]
- Castagna, A.; Stoloff, N. Hydrogen embrittlement of Fe3Al alloys. Mater. Sci. Eng. A 1995, 192, 399–406. [Google Scholar] [CrossRef]
- Balasubramaniam, R. Hydrogen in iron aluminides. J. Alloys Compd. 2002, 330, 506–510. [Google Scholar] [CrossRef]
- Barnoush, A.; Dake, J.; Kheradmand, N.; Vehoff, H. Examination of hydrogen embrittlement in FeAl by means of in situ electrochemical micropillar compression and nanoindentation techniques. Intermetallics 2010, 18, 1385–1389. [Google Scholar] [CrossRef]
- Zamanzade, M.; Barnoush, A. An overview of the hydrogen embrittlement of iron aluminides. Procedia Mater. Sci. 2014, 3, 2016–2023. [Google Scholar] [CrossRef]
- Zamanzade, M.; Vehoff, H.; Barnoush, A. Cr effect on hydrogen embrittlement of Fe3Al-based iron aluminide intermetallics: Surface or bulk effect. Acta Mater. 2014, 69, 210–223. [Google Scholar] [CrossRef]
- McKamey, C.; Horton, J.; Liu, C. Effect of chromium on properties of Fe3Al. J. Mater. Res. 1989, 4, 1156–1163. [Google Scholar] [CrossRef]
- Keddam, M.; Mattos, O.; Takenouti, H. Mechanism of anodic dissolution of iron-chromium alloys investigated by electrode impedances—I. Experimental results and reaction model. Electrochim. Acta 1986, 31, 1147–1158. [Google Scholar] [CrossRef]
- Keddam, M.; Mattos, O.; Takenouti, H. Mechanism of anodic dissolution of iron-chromium alloys investigated by electrode impedances—II. Elaboration of the reaction model. Electrochim. Acta 1986, 31, 1159–1165. [Google Scholar] [CrossRef]
- Epelboin, I.; Keddam, M.; Mattos, O.; Takenouti, H. The dissolution and passivation of Fe and Fe-Cr alloys in acidified sulphate medium: Influences of pH and Cr content. Corros. Sci. 1979, 19, 1105–1112. [Google Scholar] [CrossRef]
- Barnoush, A.; Vehoff, H. Recent developments in the study of hydrogen embrittlement: Hydrogen effect on dislocation nucleation. Acta Mater. 2010, 58, 5274–5285. [Google Scholar] [CrossRef]
- McQueen, H.J.; Kuczynski, G.C. Order-disorder transformations in iron-aluminum alloys. Trans. Am. Inst. Min. Metall. Eng. 1959, 215, 619–622. [Google Scholar]
- Eguchi, T.; Matsuda, H.; Oki, K.; Kiyoto, S.-i.; Yasutake, K. Order-disorder transformation in Fe-Al alloys. Trans. Jpn. Inst. Metals 1967, 8, 174–179. [Google Scholar] [CrossRef]
- Koster, W.; Godecke, T. Physical measurements on iron—Aluminum alloys between 10 and 50 at.% Al. I.—Confirmation of and additional conribution to the iron—Aluminum phase diagram. Z. Metallkd. 1980, 71, 765–769. [Google Scholar]
- Taylor, A.; Jones, R. Constitution and magnetic properties of iron-rich iron-aluminum alloys. J. Phys. Chem. Solids 1958, 6, 16–37. [Google Scholar] [CrossRef]
- Davies, R. An X-ray and dilatometric study of order and the “k-state” in iron-aluminum alloys. J. Phys. Chem. Solids 1963, 24, 985–992. [Google Scholar] [CrossRef]
- Stein, F.; Schneider, A.; Frommeyer, G. Flow stress anomaly and order–disorder transitions in Fe3Al-based Fe-Al-Ti-X alloys with X = V, Cr, Nb, or Mo. Intermetallics 2003, 11, 71–82. [Google Scholar] [CrossRef]
- Kubaschewski, O. Iron-Binary Phase Diagrams; Springer: Berlin, Germany, 1982. [Google Scholar]
- Palm, M. Concepts derived from phase diagram studies for the strengthening of Fe-Al-based alloys. Intermetallics 2005, 13, 1286–1295. [Google Scholar] [CrossRef]
- Morris, D.; Liu, C.; George, E. Pinning of dislocations and the origin of the stress anomaly in FeAl alloys. Intermetallics 1999, 7, 1059–1068. [Google Scholar] [CrossRef]
- Morris, D.G.; Munoz-Morris, M. The stress anomaly in FeAl-Fe3Al alloys. Intermetallics 2005, 13, 1269–1274. [Google Scholar] [CrossRef]
- Friák, M.; Deges, J.; Krein, R.; Frommeyer, G.; Neugebauer, J. Combined ab initio and experimental study of structural and elastic properties of Fe3Al-based ternaries. Intermetallics 2010, 18, 1310–1315. [Google Scholar] [CrossRef]
- Palm, M.; Inden, G.; Thomas, N. The Fe-Al-Ti system. J. Phase Equilib. 1995, 16, 209–222. [Google Scholar] [CrossRef]
- Ducher, R.; Stein, F.; Viguier, B.; Palm, M.; Lacaze, J. A re-examination of the liquidus surface of the Al-Fe-Ti system. Z. Metallkd. 2003, 94, 396–410. [Google Scholar] [CrossRef]
- Calvert, L.; Villars, P. Pearson’s Handbook of Crystallographic Data for Intermetallic Phases; ASM International: Materials Park, OH, USA, 1991. [Google Scholar]
- Shu, X.L.; Hu, W.Y.; Xiao, H.N.; Deng, H.Q.; Zhang, B.W. Vacancies and antisites in B2 FeAl and D03 Fe3Al with a modified analytic eam model. J. Mater. Sci. Technol. 2001, 17, 601–604. [Google Scholar]
- Leamy, H.; Gibson, E.; Kayser, F. The elastic stiffness coefficients of iron-aluminum alloys-I experimental results and thermodynamic analysis. Acta Metall. Mater. 1967, 15, 1827–1838. [Google Scholar] [CrossRef]
- Vailhe, C.; Farkas, D. Shear faults and dislocation core structure simulations in B2 FeAl. Acta Mater. 1997, 45, 4463–4473. [Google Scholar] [CrossRef]
- Stein, F. Summer School on Iron Aluminides Part 1: The binary Fe-Al system. In Proceedings of the 5th Discussion Meeting on the Development of Innovative Iron Aluminum Alloys in Prague, Prague, Czech Republic, 21–24 September 2009.
- Bradley, A.; Jay, A. The formation of superlattices in alloys of iron and aluminium. Proc. R. Soc. Lond. Ser. A 1932, 210–232. [Google Scholar] [CrossRef]
- Lawley, A.; Cahn, R. A high temperature X-ray study of ordering in iron-aluminium alloys. J. Phys. Chem. Solids 1961, 20, 204–221. [Google Scholar] [CrossRef]
- Fu, C.L. Origin of ordering in B2-type transition-metal aluminides: Comparative study of the defect properties of PdAl, NiAl, and FeAl. Physical Review B 1995, 52, 3151–3158. [Google Scholar] [CrossRef]
- Herrmann, J.; Inden, G.; Sauthoff, G. Deformation behaviour of iron-rich iron-aluminum alloys at low temperatures. Acta Mater. 2003, 51, 2847–2857. [Google Scholar] [CrossRef]
- Herrmann, J.; Inden, G.; Sauthoff, G. Deformation behaviour of iron-rich iron-aluminium alloys at high temperatures. Acta Mater. 2003, 51, 3233–3242. [Google Scholar] [CrossRef]
- Hasemann, G.; Schneibel, J.H.; George, E.P. Dependence of the yield stress of Fe3Al on heat treatment. Intermetallics 2012, 21, 56–61. [Google Scholar] [CrossRef]
- Hasemann, G.; Schneibel, J.H.; Krüger, M.; George, E.P. Vacancy strengthening in Fe3Al iron aluminides. Intermetallics 2014, 54, 95–103. [Google Scholar] [CrossRef]
- Neumann, J.; Chang, Y.; Lee, C. Thermodynamics of intermetallic phases with the triple-defect B2 structure. Acta Metall. Mater. 1976, 24, 593–604. [Google Scholar] [CrossRef]
- Foiles, S.; Daw, M. Application of the embedded atom method to Ni3Al. J. Mater. Res. 1987, 2, 5–15. [Google Scholar] [CrossRef]
- Fu, C.; Ye, Y.-Y.; Yoo, M.; Ho, K. Equilibrium point defects in intermetallics with the B2 structure: NiAl and FeAl. Phys. Rev. B 1993, 48, 6712. [Google Scholar] [CrossRef]
- Stein, F.; Palm, M. Re-determination of transition temperatures in the Fe-Al system by differential thermal analysis. Int J. Mater. Res. 2007, 98, 580–588. [Google Scholar] [CrossRef]
- Schaefer, H.-E.; Frenner, K.; Würschum, R. High-temperature atomic defect properties and diffusion processes in intermetallic compounds. Intermetallics 1999, 7, 277–287. [Google Scholar] [CrossRef]
- Hehenkamp, T.; Scholz, P.; Köhler, B.; Kerl, R. Vacancy formation and diffusion in FeAl-alloys. Defect Diffus. Forum 2001, 194–199, 389–396. [Google Scholar] [CrossRef]
- Würschum, R.; Grupp, C.; Schaefer, H.-E. Simultaneous study of vacancy formation and migration at high temperatures in B2-type Fe aluminides. Phys. Rev. Lett. 1995, 75, 97. [Google Scholar] [CrossRef] [PubMed]
- Schneibel, J. Strengthening of iron aluminides by vacancies and/or nickel. Mater. Sci. Eng. A 1998, 258, 181–186. [Google Scholar] [CrossRef]
- Mayer, J.; Meyer, B.; Oehrens, J.S.; Bester, G.; Börnsen, N.; Fähnle, M. Effective formation energies of atomic defects in D03 Fe3Al: An ab-initio study. Intermetallics 1997, 5, 597–600. [Google Scholar] [CrossRef]
- Fähnle, M.; Mayer, J.; Meyer, B. Theory of atomic defects and diffusion in ordered compounds, and application to B2-FeAl. Intermetallics 1999, 7, 315–323. [Google Scholar] [CrossRef]
- Besson, R.; Morillo, J. Development of a semiempirical n-body noncentral potential for Fe-Al alloys. Phys. Rev. B 1997, 55, 193–204. [Google Scholar] [CrossRef]
- Bakker, H.; Modder, I.; Kuin, M. Extension of miedema’s semiempirical model to estimates of the formation enthalpies of point defects in intermetallic compounds with the B2 structure. Intermetallics 1997, 5, 535–546. [Google Scholar] [CrossRef]
- Yasuda, H.Y.; Umakoshi, Y. Pseudoelastic behaviour of Fe3Al single crystals with D03 structure. Intermetallics 2010, 18, 1273–1278. [Google Scholar] [CrossRef]
- Yasuda, H.; Nakajima, T.; Nakano, K.; Yamaoka, K.; Ueda, M.; Umakoshi, Y. Effect of Al concentration on pseudoelasticity in Fe3Al single crystals. Acta Mater. 2005, 53, 5343–5351. [Google Scholar] [CrossRef]
- Yasuda, H.Y.; Nakajima, T.; Umakoshi, Y. Temperature dependence of pseudoelasticity in Fe3Al single crystals. Intermetallics 2007, 15, 819–823. [Google Scholar] [CrossRef]
- Wolff, J.; Franz, M.; Hehenkamp, T. Defect analysis with positron annihilation-Applications to Fe aluminides. Microchim. Acta 1997, 125, 263–268. [Google Scholar] [CrossRef]
- Saburi, T.; Yamauchi, I.; Nenno, S. Electron microscope observation of dislocations and antiphase boundaries in iron-aluminum alloys. J. Phys. Soc. Jpn. 1972, 32, 694–701. [Google Scholar] [CrossRef]
- Crawford, R.C.; Ray, I.L.F.; Cockayne, D.J. Weak-beam technique applied to superlattice dislocations in iron-aluminum alloys. 2. Fourfold dissociation in D03-type order. Philos. Mag. 1973, 27, 1–7. [Google Scholar] [CrossRef]
- Crawford, R.C.; Ray, I.L.F. Antiphase boundary energies in iron-aluminum alloys. Philos. Mag. 1977, 35, 549–565. [Google Scholar] [CrossRef]
- Král, F.; Schwander, P.; Kostorz, G. Superdislocations and antiphase boundary energies in deformed Fe3Al single crystals with chromium. Acta Mater. 1997, 45, 675–682. [Google Scholar] [CrossRef]
- Morris, D.; Dadras, M.; Morris, M. The influence of cr addition on the ordered microstructure and deformation and fracture behaviour of a Fe 28% Al intermetallic. Acta Metall. Mater. 1993, 41, 97–111. [Google Scholar] [CrossRef]
- Brinck, A.; Engelke, C.; Neuhäuser, H.; Molénat, G.; Rösner, H.; Langmaack, E.; Nembach, E. Dislocation processes in Fe3Al investigated by transmission electron, scanning force and optical microscopy. Mater. Sci. Eng. A 1998, 258, 32–36. [Google Scholar] [CrossRef]
- Yoshimi, K.; Hanada, S.; Yoo, M. Yielding and plastic flow behavior of B2-type Fe-39.5 mol.% Al single crystals in compression. Acta Metall. Mater. 1995, 43, 4141–4151. [Google Scholar] [CrossRef]
- Schröer, W.; Hartig, C.; Mecking, H. Plasticity of D03-ordered Fe-Al and Fe-Al-Si single-crystals. Z. Metallkd. 1993, 84, 294–300. [Google Scholar]
- Yoo, M.; Koeppe, M.; Hartig, C.; Mecking, H.; Hermann, W.; Sockel, H.-G. Effect of temperature on elastic constants and dislocation properties of Fe 30% Al single crystals. Acta Mater. 1997, 45, 4323–4332. [Google Scholar] [CrossRef]
- Yoo, M.; Horton, J.; Liu, C. Micromechanisms of yield and flow in ordered intermetallic alloys. Acta Metall. Mater. 1988, 36, 2935–2946. [Google Scholar] [CrossRef]
- Koster, W.; Godecke, T. Physical measurements on iron-aluminium alloys between 10 and 50 at.-% Al. Iv.—The modulus of elasticity of the alloys. Z. Metallkd. 1982, 73, 111–114. [Google Scholar]
- Palm, M. The Al-Cr-Fe system–phases and phase equilibria in the Al-rich corner. J. Alloys Compd. 1997, 252, 192–200. [Google Scholar] [CrossRef]
- Alonso, P.R.; Gargano, P.H.; Bozzano, P.B.; Ramírez-Caballero, G.E.; Balbuena, P.B.; Rubiolo, G.H. Combined ab initio and experimental study of A2 + L21 coherent equilibria in the Fe-Al-X (X = Ti, Nb, V) systems. Intermetallics 2011, 19, 1157–1167. [Google Scholar] [CrossRef]
- Stein, F.; Sauthoff, G.; Palm, M. Phases and phase equilibria in the Fe-Al-Zr system: Dedicated to professor dr. Peter neumann on the occasion of his 65th birthday. Z. Metallkd. 2004, 95, 469–485. [Google Scholar] [CrossRef]
- Fu, C.L.; Zou, J. Site preference of ternary alloying additions in FeAl and NiAl by first-principles calculations. Acta. Mater 1996, 44, 1471–1478. [Google Scholar] [CrossRef]
- Zuqing, S.; Wangyue, Y.; Lizhen, S.; Yuanding, H.; Baisheng, Z.; Jilian, Y. Neutron diffraction study on site occupation of substitutional elements at sub lattices in Fe3Al intermetallics. Mater. Sci. Eng.: A 1998, 258, 69–74. [Google Scholar] [CrossRef]
- Nishino, Y.; Asano, S.; Ogawa, T. Phase stability and mechanical properties of Fe3Al with addition of transition elements. Mater. Sci. Eng.: A 1997, 234, 271–274. [Google Scholar] [CrossRef]
- Nishino, Y.; Kumada, C.; Asano, S. Phase stability of Fe3Al with addition of 3d transition elements. Scr. Mater. 1997, 36, 461–466. [Google Scholar] [CrossRef]
- Krein, R.; Schneider, A.; Sauthoff, G.; Frommeyer, G. Microstructure and mechanical properties of Fe3Al-based alloys with strengthening boride precipitates. Intermetallics 2007, 15, 1172–1182. [Google Scholar] [CrossRef]
- Connetable, D.; Lacaze, J.; Maugis, P.; Sundman, B. A calphad assessment of Al-C-Fe system with the κ carbide modelled as an ordered form of the fcc phase. Calphad 2008, 32, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Schneider, A.; Falat, L.; Sauthoff, G.; Frommeyer, G. Constitution and microstructures of Fe-Al-M-C (M = Ti, V, Nb, Ta) alloys with carbides and laves phase. Intermetallics 2003, 11, 443–450. [Google Scholar] [CrossRef]
- Kobayashi, S.; Zaefferer, S.; Schneider, A. Carbide Precipitation in an Fe3Al-Cr-Mo-C Alloy. In Proceedings of an International Conference on Solid-Solid Phase Transformations in Inorganic Materials 2005, Vol. 1: Diffusional transformations; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Doucakis, T.; Kumar, K. Formation and stability of refractory metal diborides in an Fe3Al matrix. Intermetallics 1999, 7, 765–777. [Google Scholar] [CrossRef]
- Morris, M.; Morris, D. Dispersoid additions and their effect on high temperature deformation of FeAl. Acta Metall. Mater. 1990, 38, 551–559. [Google Scholar] [CrossRef]
- Palm, M. Fe-Al materials for structural applications at high temperatures: Current research at mpie. Int. J. Mater. Res. 2009, 100, 277–287. [Google Scholar] [CrossRef]
- Risanti, D.; Deges, J.; Falat, L.; Kobayashi, S.; Konrad, J.; Palm, M.; Pöter, B.; Schneider, A.; Stallybrass, C.; Stein, F. Dependence of the brittle-to-ductile transition temperature (BDTT) on the Al content of Fe-Al alloys. Intermetallics 2005, 13, 1337–1342. [Google Scholar] [CrossRef]
- Anthony, L.; Fultz, B. Effects of early transition metal solutes on the D03-B2 critical temperature of Fe3Al. Acta Metall. Mater. 1995, 43, 3885–3891. [Google Scholar] [CrossRef]
- Fleischer, R.L. Substitutional solutes in AlRu-I. Effects of solute on moduli, lattice parameters and vacancy production. Acta Metall. Mater. 1993, 41, 863–869. [Google Scholar] [CrossRef]
- Fleischer, R.L. Substitutional solutes in AlRu-II. Hardening and correlations with defect structure. Acta Metall. Mater. 1993, 41, 1197–1205. [Google Scholar] [CrossRef]
- Schneibel, J.; Specht, E.; Simpson, W. Solid solution strengthening in ternary B2 iron aluminides containing 3d transition elements. Intermetallics 1996, 4, 581–583. [Google Scholar] [CrossRef]
- Zamanzade, M.; Barnoush, A. Effect of chromium on the electrochemical properties of iron aluminide intermetallics. Corros. Sci. 2014, 78, 223–232. [Google Scholar] [CrossRef]
- Rao, V.S. A review of the electrochemical corrosion behaviour of iron aluminides. Electrochim. Acta 2004, 49, 4533–4542. [Google Scholar] [CrossRef]
- Balasubramaniam, R. On the role of chromium in minimizing room temperature hydrogen embrittlement in iron aluminides. Scr. Mater. 1996, 34, 127–133. [Google Scholar] [CrossRef]
- Hakiki, N. Comparative study of structural and semiconducting properties of passive films and thermally grown oxides on AISI 304 stainless steel. Corros. Sci. 2011, 53, 2688–2699. [Google Scholar] [CrossRef]
- Rose, J.H.; Smith, J.R.; Ferrante, J. Universal features of bonding in metals. Phys. Rev. B 1983, 28, 1835. [Google Scholar] [CrossRef]
- Rose, J.H.; Smith, J.R.; Guinea, F.; Ferrante, J. Universal features of the equation of state of metals. Phys. Rev. B 1984, 29, 2963. [Google Scholar] [CrossRef]
- Morris, D.G.; Dadras, M.; Morris, M. The influence of chromium additions on order and ductility in Fe3Al intermetallic. J. Phys. IV 1993, 3, C7-429–C427-434. [Google Scholar] [CrossRef] [Green Version]
- Zamanzade, M.; Vehoff, H.; Barnoush, A. Effect of chromium on elastic and plastic deformation of Fe3Al intermetallics. Intermetallics 2013, 41, 28–34. [Google Scholar] [CrossRef]
- Zamanzade, M.; Rafael-Velayarce, J.; Torrents-Abad, O.; Motz, C.; Barnoush, A. Mechanical behavior of iron aluminides: A comparison of nanoindentation, compression and bending of micropillars. Mater. Sci. Eng. A 2015. [Google Scholar] [CrossRef]
- Barnoush, A.; Zamanzade, M. Effect of substitutional solid solution on dislocation nucleation in Fe3Al intermetallic alloys. Philos. Mag. 2012, 92, 3257–3268. [Google Scholar] [CrossRef]
- Motz, C.; Schoberl, T.; Pippan, R. Mechanical properties of micro-sized copper bending beams machined by the focused ion beam technique. Acta Mater. 2005, 53, 4269–4279. [Google Scholar] [CrossRef]
- Ellinger, C.S. In situ oxidation study of flat and stepped binary alloy surfaces. Ph.D. Thesis, Institut für Theoretische und Angewandte Physik &Max-Planck-Institut für Intelligente Systeme, Stuttgart, Germany, 2010. [Google Scholar]
- Hammer, L.; Meier, W.; Blum, V.; Heinz, K. Equilibration processes in surfaces of the binary alloy Fe-Al. J. Phys. Condens. Matter 2002, 14. [Google Scholar] [CrossRef]
- Baddorf, A.; Chandavarkar, S. Identification of an incommensurate FeAl2 overlayer on FeAl (110) using X-ray diffraction and reflectivity. Phys. B: Condens. Matter 1996, 221, 141–144. [Google Scholar] [CrossRef]
- Voges, D.; Taglauer, E.; Dosch, H.; Peisl, J. Surface segregation on Fe3Al (110) near the order-disorder transition temperature. Surf. Sci. 1992, 269, 1142–1146. [Google Scholar] [CrossRef]
- Dosch, H.; Mailänder, L.; Johnson, R.; Peisl, J. Critical phenomena at the Fe3Al (110) surface: A glancing angle X-ray scattering study. Surf. Sci. 1992, 279, 367–379. [Google Scholar] [CrossRef]
- Hutchings, R.; Loretto, M. Compositional dependence of oxidation rates of NiAl and CoAl. Metal Sci. 1978, 12, 503–510. [Google Scholar] [CrossRef]
- Pöter, B.; Stein, F.; Wirth, R.; Spiegel, M. Early stages of protective oxide layer growth on binary iron aluminides. Z. Phys. Chem. 2005, 219, 1489–1503. [Google Scholar] [CrossRef]
- Velon, A.; Olefjord, I. Oxidation behavior of Ni3Al and Fe3Al: I. Early stage of oxide growth. Oxid. Metals 2001, 56, 425–452. [Google Scholar] [CrossRef]
- Velon, A.; Yi, D.-Q. Influence of Cr on the oxidation of Fe3Al and Ni3Al at 500 °C. Oxid. Metals 2002, 57, 13–31. [Google Scholar] [CrossRef]
- Lee, W.; Lin, R. Oxidation, sulfidation and hot corrosion of intermetallic compound Fe3Al at 605 °C and 800 °C. Mater. Chem. Phys. 1999, 58, 231–242. [Google Scholar] [CrossRef]
- Vogel, D.; Hotař, A.; Vogel, A.; Palm, M.; Renner, F.U. Corrosion behaviour of Fe-Al(-Ti) alloys in steam. Intermetallics 2010, 18, 1375–1378. [Google Scholar] [CrossRef]
- Pint, B.A.; Wright, I.G. The Oxidation Behavior of Fe-Al Alloys. Mater. Sci. Forum 2004, 461–464, 799–806. [Google Scholar] [CrossRef]
- Janda, D. Mechanical Properties and Oxidation Behavior of Micro-Alloyed Iron Aluminides. Ph.D. Thesis, Karlsruher Institut für Technologie (KIT), Karlsruhe, Germany, 18 February 2015. [Google Scholar]
- Grabke, H. Oxidation of NiAl and FeAl. Intermetallics 1999, 7, 1153–1158. [Google Scholar] [CrossRef]
- Kim, I.; Cho, W.D.; Kim, H.J. High-temperature oxidation of Fe3Al containing yttrium. J. Mater. Sci. 2000, 35, 4695–4703. [Google Scholar] [CrossRef]
- Frangini, S.; Giorgi, R.; Lascovich, J.; Mignone, A. XPS study of passive films formed on an iron-aluminium intermetallic compound in acid solution. Surf. Interface Anal. 1994, 21, 435–441. [Google Scholar] [CrossRef]
- McCafferty, E. Thermodynamics of corrosion: Pourbaix diagrams. In Introduction to Corrosion Science; Springer: Berlin, Germany, 2010; pp. 95–117. [Google Scholar]
- De Cristofaro, N.; Frangini, S.; Mignone, A. Passivity and passivity breakdown on a β-FeAl intermetallic compound in sulphate and chloride containing solutions. Corros. Sci. 1996, 38, 307–315. [Google Scholar] [CrossRef]
- Frangini, S.; De Cristofaro, N.; Lascovich, J.; Mignone, A. On the passivation characteristics of a β-FeAl intermetallic compound in sulphate solutions. Corros. Sci. 1993, 35, 153–159. [Google Scholar] [CrossRef]
- Moshier, W.; Davis, G.; Ahearn, J. The corrosion and passivity of aluminum exposed to dilute sodium sulfate solutions. Corros. Sci. 1987, 27, 785–801. [Google Scholar] [CrossRef]
- George, F.O. Chromium-Free Conversion Coating of Aluminium-Copper Alloys. Ph.D. Thesis, The University of Manchester, Manchester, UK, 4 February 2011. [Google Scholar]
- Vargel, C. Corrosion of Aluminium; Elsevier: Amsterdam, The Nietherlands, 2004. [Google Scholar]
- García-Alonso, M.C.; López, M.F.; Escudero, M.L.; González-Carrasco, J.L.; Morris, D.G. Corrosion behaviour of an Fe3Al-type intermetallic in a chloride containing solution. Intermetallics 1999, 7, 185–191. [Google Scholar] [CrossRef]
- Rosalbino, F.; Carlini, R.; Parodi, R.; Zanicchi, G.; Scavino, G. Investigation of passivity and its breakdown on Fe3Al-Si and Fe3Al-Ge intermetallics in chloride-containing solution. Corros. Sci. 2014, 85, 394–400. [Google Scholar] [CrossRef]
- Johnson, W.H. On some remarkable changes produced in iron and steel by the action of hydrogen and acids. Proc. R. Soc. Lond. 1874, 23, 168–179. [Google Scholar] [CrossRef]
- Lynch, S. Comments on “a unified model of environment-assisted cracking”. Scr. Mater. 2009, 61, 331–334. [Google Scholar] [CrossRef]
- Gangloff, R.P.; Somerday, B.P. Gaseous Hydrogen Embrittlement of Materials in Energy Technologies: Mechanisms, Modelling and Future Developments; Elsevier: Amsterdam, The Nietherlands, 2012; Volume 2. [Google Scholar]
- McKamey, C.; Liu, C. Chromium addition and environmental embrittlement in Fe3Al. Scr. Metall. Mater. 1990, 24, 2119–2122. [Google Scholar] [CrossRef]
- Stroe, M.E. Hydrogen Embrittlement of Ferrous Materials. Ph.D. Thesis, Universite Libre De Bruxelles, Brussel, Belgium, 31 March 2006. [Google Scholar]
- Fu, C.; Painter, G. First principles investigation of hydrogen embrittlement in FeAl. J. Mater. Res. 1991, 6, 719–723. [Google Scholar] [CrossRef]
- Johnson, D.F.; Carter, E.A. First-principles assessment of hydrogen absorption into FeAl and Fe3Si: Towards prevention of steel embrittlement. Acta Mater. 2010, 58, 638–648. [Google Scholar] [CrossRef]
- Hirth, J.P. Effects of hydrogen on the properties of iron and steel. Metall. Trans. A 1980, 11, 861–890. [Google Scholar] [CrossRef]
- Cheng, X.; Wan, X. Hydrogen diffusivity in a Fe3Al-based alloy. Scr. Mater. 1998, 38, 1505–1509. [Google Scholar] [CrossRef]
- Kupka, M.; Stępień, K. Hydrogen permeation in Fe-40at.% Al alloy at different temperatures. Corros. Sci. 2009, 51, 699–702. [Google Scholar] [CrossRef]
- Banerjee, P.; Balasubramaniam, R. Hydrogen diffusivity in iron aluminides determined by subscale microhardness profiling. Scr. Mater. 1998, 39, 1215–1219. [Google Scholar] [CrossRef]
- Yang, Y.; Hanada, S. Absorption and desorption of hydrogen in Fe-40Al intermetallic. Scr. Metall. Mater. 1995, 32, 1719–1724. [Google Scholar] [CrossRef]
- Luu, W.; Wu, J. Hydrogen transport and environmental embrittlement effects in iron aluminides. J. Mater. Sci. 2000, 35, 4121–4127. [Google Scholar] [CrossRef]
- Stępień, K.; Kupka, M. Diffusivity of hydrogen in B2 iron aluminides. Scr. Mater. 2006, 55, 585–588. [Google Scholar] [CrossRef]
- Prakash, U.; Parvathavarthini, N.; Dayal, R. Effect of composition on hydrogen permeation in Fe-Al alloys. Intermetallics 2007, 15, 17–19. [Google Scholar] [CrossRef]
- Kimizuka, H.; Mori, H.; Ogata, S. Effect of temperature on fast hydrogen diffusion in iron: A path-integral quantum dynamics approach. Phys. Rev. B 2011, 83, 094110. [Google Scholar] [CrossRef]
- Bockris, J.M.; Fullenwider, M. The electro-permeation of hydrogen into metals. Electrochim. Acta 1970, 15, 47–60. [Google Scholar] [CrossRef]
- Song, R.H.; Pyun, S.; Oriani, R. Hydrogen permeation through the passivation film on iron by time-lag method. J. Electrochem. Soc. 1990, 137, 1703–1706. [Google Scholar] [CrossRef]
- Ruetschi, P.; Giovanoli, R. Cation vacancies in MnO2 and their influence on electrochemical reactivity. J. Electrochem. Soc. 1988, 135, 2663–2669. [Google Scholar] [CrossRef]
- Song, R.-H.; Pyun, S.-I.; Oriani, R. The hydrogen permeation through passivating film on iron by modulation method. Electrochim. Acta 1991, 36, 825–831. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, X.; Yang, F.; Shi, Y.; Song, J.; Lai, X. Energetics and diffusion of hydrogen in hydrogen permeation barrier of α-Al2O3/ FeAl with two different interfaces. Int. J. Hydrog. Energy 2013, 38, 7550–7560. [Google Scholar] [CrossRef]
- Hollenberg, G.; Simonen, E.; Kalinin, G.; Terlain, A. Tritium/hydrogen barrier development. Fusion Eng. Des. 1995, 28, 190–208. [Google Scholar]
- Barnoush, A.; Vehoff, H. Hydrogen embrittlement of aluminum in aqueous environments examined by in situ electrochemical nanoindentation. Scr. Mater. 2008, 58, 747–750. [Google Scholar] [CrossRef]
- Pundt, A.; Kirchheim, R. Hydrogen in metals: Microstructural aspects. Annu. Rev. Mater. Res. 2006, 36, 555–608. [Google Scholar] [CrossRef]
- Myers, S.M.; Baskes, M.; Birnbaum, H.; Corbett, J.W.; De Leo, G.; Estreicher, S.; Haller, E.E.; Jena, P.; Johnson, N.M.; Kirchheim, R. Hydrogen interactions with defects in crystalline solids. Rev. Mod. Phys. 1992, 64, 559–617. [Google Scholar] [CrossRef]
- Ryu, J.H. Hydrogen Embrittlement in Trip and Twip Steels. Ph.D. Thesis, Pohang University of Science and Technology, Pohang, South Korea, 29 May 2012. [Google Scholar]
- Myers, S.; Besenbacher, F.; No, J. Immobilization mechanisms for ion-implanted deuterium in aluminum. J. Appl. Phys. 1985, 58, 1841–1850. [Google Scholar] [CrossRef]
- Ziegler, R.; Schaefer, H. Vacancies and interstitials in metals and alloys. In Proceedings of the 1987 International Conference on Vacancies and Interstititials in Metals and Alloys, Berlin, Germany, 14–19 September 1987.
- Linderoth, S.; Rajainmäki, H.; Nielsen, B.; Hansen, H.; Nieminen, R.M.; Petersen, K. Hydrogen in Vacancies in Mo and Ni. Mater. Sci. Forum 1987, 15–18, 751–756. [Google Scholar] [CrossRef]
- Wittmann, M.; Wu, D.; Baker, I.; George, E.; Heatherly, L. The role of edge and screw dislocations on hydrogen embrittlement of Fe-40Al. Mater. Sci. Eng.: A 2001, 319, 352–355. [Google Scholar] [CrossRef]
- Tien, J.; Thompson, A.W.; Bernstein, I.; Richards, R.J. Hydrogen transport by dislocations. Metall. Trans. A 1976, 7, 821–829. [Google Scholar] [CrossRef]
- Louthan, M.; Caskey, G.; Donovan, J.; Rawl, D. Hydrogen embrittlement of metals. Mater. Sci. Eng. 1972, 10, 357–368. [Google Scholar] [CrossRef]
- Donovan, J.A. Accelerated evolution of hydrogen from metals during plastic deformation. Metall. Trans. A 1976, 7, 1677–1683. [Google Scholar] [CrossRef]
- Nathal, M.; Liu, C. Intrinsic ductility of FeAl single crystals. Intermetallics 1995, 3, 77–81. [Google Scholar] [CrossRef]
- Lynch, R.; Gee, K.; Heldt, L. Environmental embrittlement of single crystal and thermomechanically processed B2-ordered iron aluminides. Scr. Metall. Mater. 1994, 30, 945–950. [Google Scholar] [CrossRef]
- Saka, H.; Nishizaki, T. Roles of edge and screw dislocations in the environmental embrittlement of an Fe [sbnd] Al intermetallic compound. Philos. Mag. A 1996, 73, 1173–1179. [Google Scholar] [CrossRef]
- Yang, Y.; Baker, I.; George, E. Effect of vacancies on the tensile properties of Fe-40Al single crystals in air and vacuum. Mater. Charact. 1999, 42, 161–167. [Google Scholar] [CrossRef]
- Munroe, P.; Baker, I. Observation of <001> dislocations and a mechanism for transgranular fracture on {001} in FeAl. Acta Metall. Mater. 1991, 39, 1011–1017. [Google Scholar] [CrossRef]
- Li, J.; Liu, C. Crack nucleation in hydrogen embrittlemet. Scr. Metall. Mater. 1992, 27, 1701–1705. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamanzade, M.; Barnoush, A.; Motz, C. A Review on the Properties of Iron Aluminide Intermetallics. Crystals 2016, 6, 10. https://doi.org/10.3390/cryst6010010
Zamanzade M, Barnoush A, Motz C. A Review on the Properties of Iron Aluminide Intermetallics. Crystals. 2016; 6(1):10. https://doi.org/10.3390/cryst6010010
Chicago/Turabian StyleZamanzade, Mohammad, Afrooz Barnoush, and Christian Motz. 2016. "A Review on the Properties of Iron Aluminide Intermetallics" Crystals 6, no. 1: 10. https://doi.org/10.3390/cryst6010010









