Thermally Activated Paramagnets from Diamagnetic Polymers of Biphenyl-3,5-diyl Bis(tert-butyl Nitroxides) Carrying Methyl and Fluoro Groups at the 2’- and 5’-Positions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization
2.2. Crystal Structure Analysis
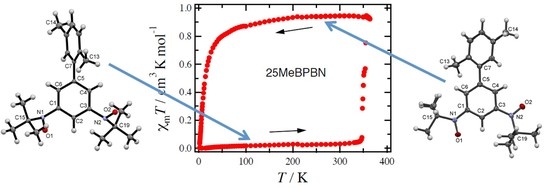
2.3. Magnetic Properties
3. Experimental Section
3.1. Preparation of BPBN Derivatives
3.2. Crystallographic Analysis
3.3. Physical Property Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iwamura, H. What role has organic chemistry played in the development of molecule-based magnets? Polyhedron 2013, 66, 3–14. [Google Scholar] [CrossRef]
- Gallagher, N.M.; Olankitwanit, A.; Rajca, A. High-spin organic molecules. J. Org. Chem. 2015, 80, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Blundell, S.J.; Pratt, F.L. Organic and molecular magnets. J. Phys. Condens. Matter 2004, 16, R771–R828. [Google Scholar] [CrossRef]
- Mukai, K.; Nagai, H.; Ishizu, K. The proof of a triplet ground state in the N,N’-di-t-butyl-m-phenylenebisnitroxide biradical. Bull. Chem. Soc. Jpn. 1975, 48, 2381–2382. [Google Scholar] [CrossRef]
- Ishida, T.; Iwamura, H. Bis[3-tert-butyl-5-(N-oxy-tert-butylamino)phenyl] nitroxide in a quartet grand state: A prototype for persistent high-spin poly[(oxyimino)-1,3-phenylenes]. J. Am. Chem. Soc. 1991, 13, 4238–4241. [Google Scholar] [CrossRef]
- Nishimaki, H.; Mashiyama, S.; Yasui, M.T.; Nogami, T.; Ishida, T. Bistable polymorphs showing diamagnetic and paramagnetic states of an organic crystalline biradical biphenyl-3,5-diyl bis(tert-butylnitroxide). Chem. Mater. 2006, 18, 3602–3604. [Google Scholar] [CrossRef]
- Nishimaki, H.; Ishida, T. Organic two-step spin-transition-like behavior in a linear S = 1 array: 3’-methylbiphenyl-3,5-diyl bis(tert-butylnitroxide) and related compounds. J. Am. Chem. Soc. 2010, 132, 9598–9599. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Kudo, H.; Ishida, T. Intermediate-paramagnetic phases with a half and a quarter spin entities in fluorinated biphenyl-3,5-diyl bis(tert-butyl nitroxides). J. Mater. Chem. C 2015, 3, 7813–7818. [Google Scholar] [CrossRef]
- Kawakami, H.; Tonegawa, A.; Ishida, T. A designed room-temperature triplet ligand from pyridine-2,6-diyl bis(tert-butyl nitroxide). Dalton Trans. 2015, 45, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Tonegawa, A.; Ishida, T. Pyridine-2,6-diyl dinitroxides as room-temperature triplet ligands. AIP Conf. Proc. 2016, 1709, 020017. [Google Scholar]
- Yoshitake, T.; Ishida, T. Ferromagnetic chains of ground triplet 5-methoxy-1,3-phenylene bis(tert-butyl nitroxide). Chem. Lett. 2016. [Google Scholar] [CrossRef]
- Gütlich, P.; Goodwin, H.A. (Eds.) Spin Crossover in Transition Metal Compounds I, II, and III; Springer: Berlin, Germany, 2004.
- Kurokawa, G.; Ishida, T.; Nogami, T. Remarkably strong intermolecular antiferromagnetic couplings in the crystal of biphenyl-3,5-diyl bis(tert-butyl nitroxide). Chem. Phys. Lett. 2004, 392, 74–79. [Google Scholar] [CrossRef]
- Marsh, D. Reaction fields and solvent dependence of the EPR parameters of nitroxides: The microenvironment of spin labels. J. Mag. Res. 2008, 190, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, T.; Shiomi, D.; Sato, K.; Takui, T. Biradical paradox revisited quantitatively: A theoretical model for self-associated biradical molecules as antiferromagnetically exchange coupled spin chains in solution. J. Phys. Chem. B 2012, 116, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Higashiyama, H.; Akutsu, H.; Nakatsuji, S. A functional nitroxide radical displaying unique thermochromism and magnetic phase transition. Angew. Chem. Int. Ed. 2011, 50, 10879–10883. [Google Scholar] [CrossRef] [PubMed]
- Capiomont, A.; Chion, B.; Lajzerowicz, J. Affinement de la structure du radical nitroxyde dimerisé: bicycle [3,3,1] nonanone-3 aza-9 oxyle-9. Acta Cryst. B 1971, 27, 322–326. [Google Scholar] [CrossRef]
- Ishida, T.; Ooishi, M.; Ishii, N.; Mori, H.; Nogami, T. Mono- and dinitroxide radicals from 9, 9’(10H, 10’ H)-spirobiacridine: An approach to a D2d triplet biradical. Polyhedron 2007, 26, 1793–1799. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-coupling reactions of organoboranes: An easy way to construct C-C bonds (Nobel lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef] [PubMed]
- Kanno, F.; Inoue, K.; Koga, N.; Iwamura, H. Persistent 1,3,5-benzenetriyl tris(N-tert-butyl nitroxide) and its analogs with quartet ground states. Intramolecular triangular exchange coupling among three nitroxide radical centers. J. Phys. Chem. 1993, 97, 13267–13272. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Koide, K.; Ishida, T. Biradical chelating host 2,2’-bipyridine-6,6’-diyl bis(tert-butyl nitroxide) showing tunable exchange magnetic coupling. Inorg. Chem. Commun. 2011, 14, 194–196. [Google Scholar] [CrossRef]
- Osada, S.; Hirosawa, N.; Ishida, T. Polyether-bridged bis(tert-butyl nitroxide) paramagnetic hosts showing receptor ability to calcium(II) and barium(II) ions. Tetrahedron 2012, 68, 6193–6197. [Google Scholar] [CrossRef]
- Konno, T.; Koide, K.; Ishida, T. A supramolecular switch between ground high- and low-spin states using 2,2’:6’,2”-terpyridine-6,6”-diyl bis(tert-butyl nitroxide). Chem. Commun. 2013, 49, 5156–5158. [Google Scholar] [CrossRef] [PubMed]
- Okazawa, A.; Nagaichi, Y.; Nogami, T.; Ishida, T. Magneto-structure relationship in copper(II) and nickel(II) complexes chelated with stable tert-butyl 5-phenyl-2-pyridyl nitroxide and related radicals. Inorg. Chem. 2008, 47, 8859–8868. [Google Scholar] [CrossRef] [PubMed]
- Calder, A.; Forrester, A.R.; James, P.G.; Luckhurst, G.R. Nitroxide radicals. V. N,N’-Di-t-butyl-m-phenylenebinitroxide, a stable triplet. J. Am. Chem. Soc. 1969, 91, 3724–3727. [Google Scholar]
- Forrester, A.R.; Thompson, R.H. Stable nitroxide radicals. Nature 1964, 203, 74–75. [Google Scholar] [CrossRef]
- CRYSTALSTRUCTURE, version 4.2; Rigaku/MSC: The Woodlands, TX, USA, 2015.
- Kahn, O. Molecular Magnetism; VCH Publishers, Inc.: Weinheim, Germany, 1993; pp. 257–261. [Google Scholar]










| R in RBPBN | 3Me | α-25Me | β-25Me | 25F | 2F5Me | 5F2Me |
|---|---|---|---|---|---|---|
| Formula | C21H28N2O2 | C22H30N2O2 | C22H30N2O2 | C20H24F2N2O2 | C21H27FN2O2 | C21H27FN2O2 |
| T/K | 116 | 90 | 100 | 296 | 100 | 110 |
| Crystal system | triclinic | triclinic | monoclinic | triclinic | triclinic | triclinic |
| Space group | C2/c | |||||
| a/Å | 9.2411(5) | 9.184(9) | 33.255(8) | 9.549(4) | 8.888(3) | 9.129(3) |
| b/Å | 10.3875(2) | 9.5946(1) | 5.9206(11) | 10.244(5) | 9.232(3) | 9.246(3) |
| c/Å | 11.7540(5) | 12.825(18) | 22.178(5) | 12.036(5) | 12.937(4) | 13.074(4) |
| α/° | 110.621(4) | 112.47(9) | 90 | 111.13(4) | 112.68(2) | 114.16(2) |
| β/° | 96.472(3) | 91.92(15) | 112.325(11) | 93.65(3) | 92.13(2) | 93.73(2) |
| γ/° | 112.361(4) | 106.95(13) | 90 | 116.77(3) | 102.80(2) | 106.31(2) |
| V/Å3 | 936.09(9) | 985(2) | 4039.3(16) | 943.7(8) | 946.2(6) | 945.8(6) |
| Z | 2 | 2 | 8 | 2 | 2 | 2 |
| Dcalc/g cm−3 | 1.208 | 1.195 | 1.166 | 1.275 | 1.258 | 1.259 |
| μ(MoK α)/mm−1 | 0.078 | 0.076 | 0.074 | 0.096 | 0.087 | 0.087 |
| R(F)(I > 2σ (I)) a | 0.0595 | 0.0413 | 0.0621 | 0.0689 | 0.0778 | 0.1187 |
| Rw(F2)(all data) b | 0.1195 | 0.0398 | 0.0906 | 0.0822 | 0.1225 | 0.1626 |
| Goodness-of-fit | 1.102 | 1.003 | 1.002 | 0.997 | 1.023 | 1.222 |
| No. of reflect. | 4175 | 4280 | 4606 | 4292 | 3831 | 4297 |
| Reference | Reference [7] | this work | this work | Reference [8] | this work | this work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshitake, T.; Kudo, H.; Ishida, T. Thermally Activated Paramagnets from Diamagnetic Polymers of Biphenyl-3,5-diyl Bis(tert-butyl Nitroxides) Carrying Methyl and Fluoro Groups at the 2’- and 5’-Positions. Crystals 2016, 6, 30. https://doi.org/10.3390/cryst6030030
Yoshitake T, Kudo H, Ishida T. Thermally Activated Paramagnets from Diamagnetic Polymers of Biphenyl-3,5-diyl Bis(tert-butyl Nitroxides) Carrying Methyl and Fluoro Groups at the 2’- and 5’-Positions. Crystals. 2016; 6(3):30. https://doi.org/10.3390/cryst6030030
Chicago/Turabian StyleYoshitake, Toru, Hiroki Kudo, and Takayuki Ishida. 2016. "Thermally Activated Paramagnets from Diamagnetic Polymers of Biphenyl-3,5-diyl Bis(tert-butyl Nitroxides) Carrying Methyl and Fluoro Groups at the 2’- and 5’-Positions" Crystals 6, no. 3: 30. https://doi.org/10.3390/cryst6030030