Synthesis, Crystal Structure of a Novel Mn Complex with Nicotinoyl-Glycine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Elemental Analysis
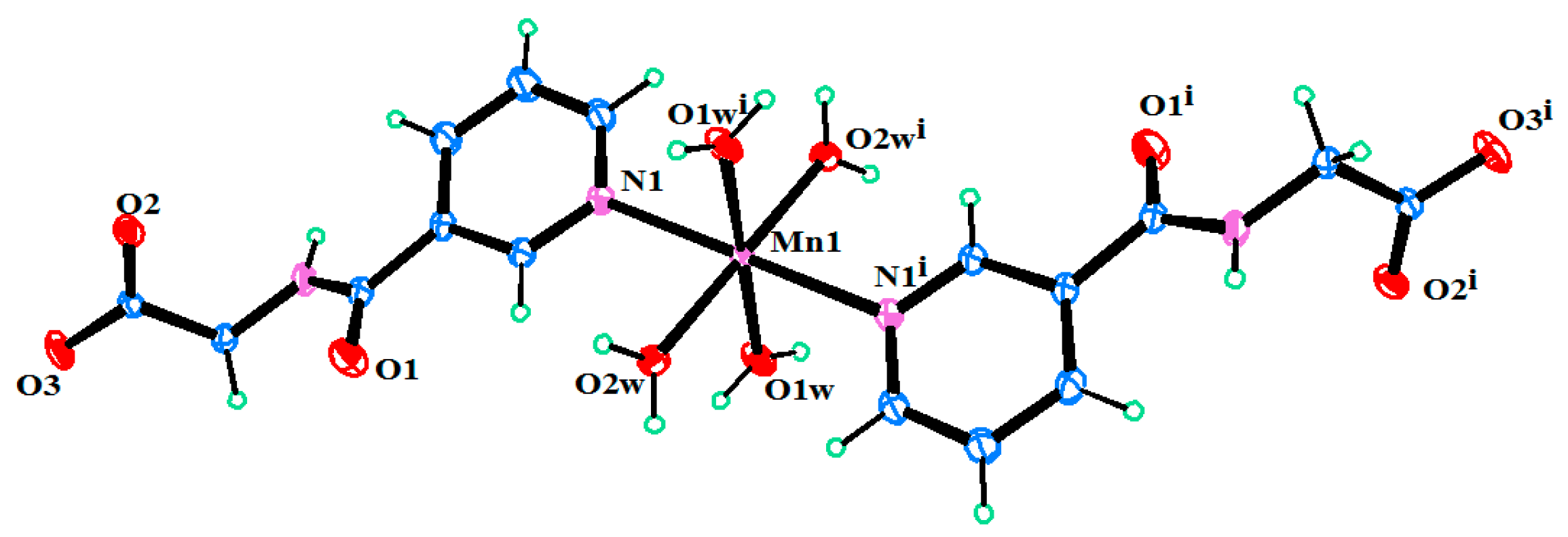
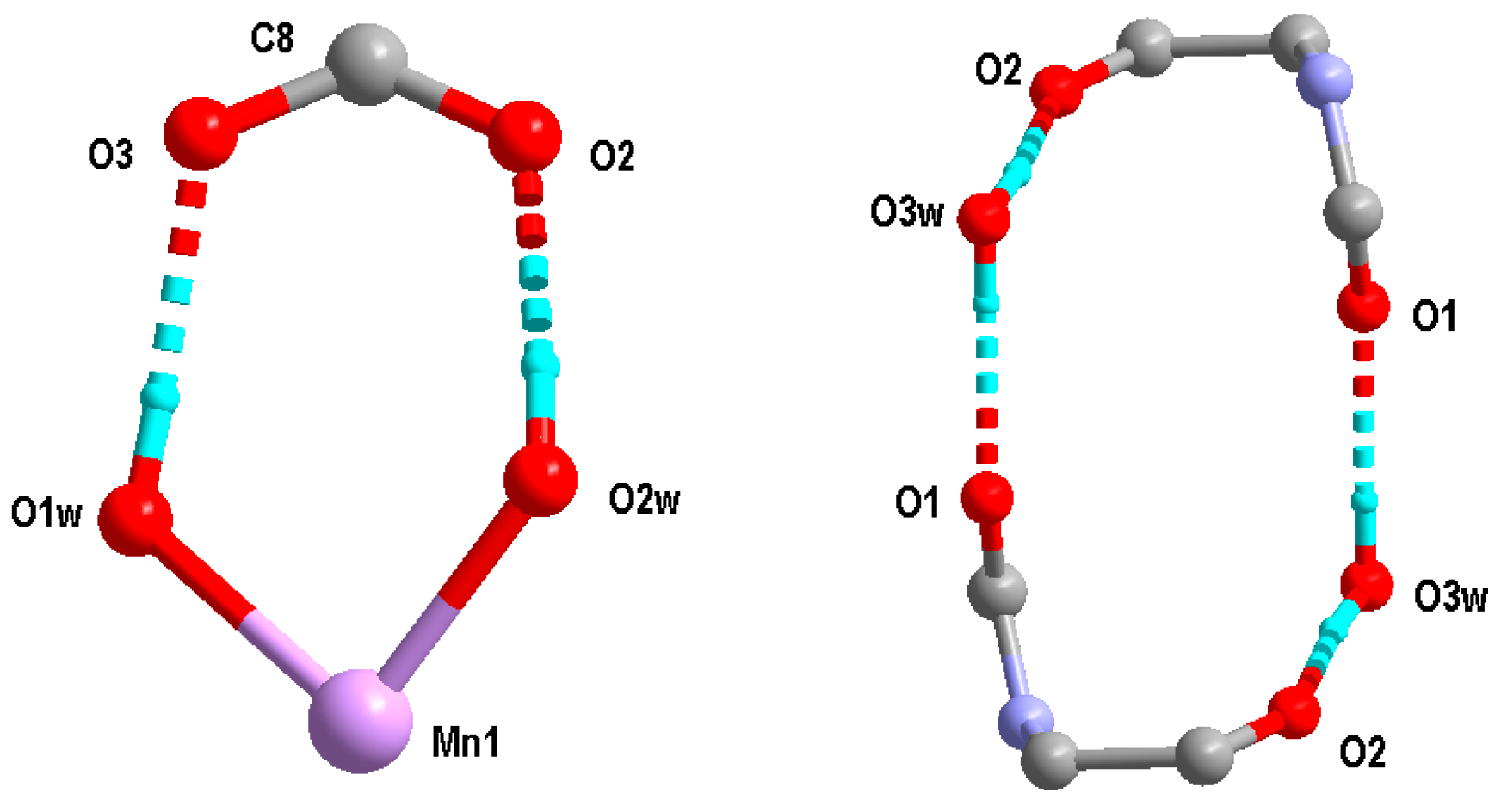
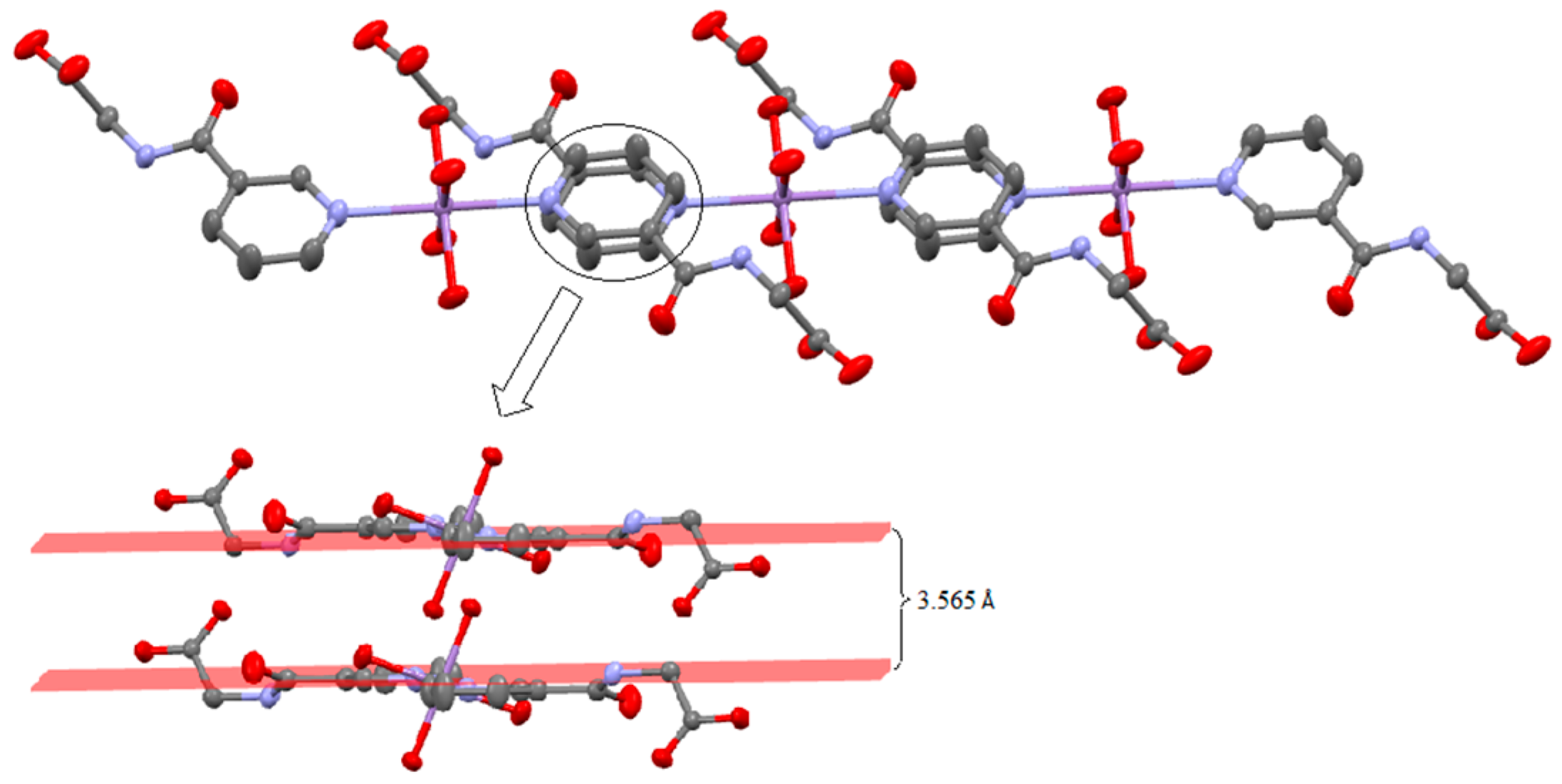
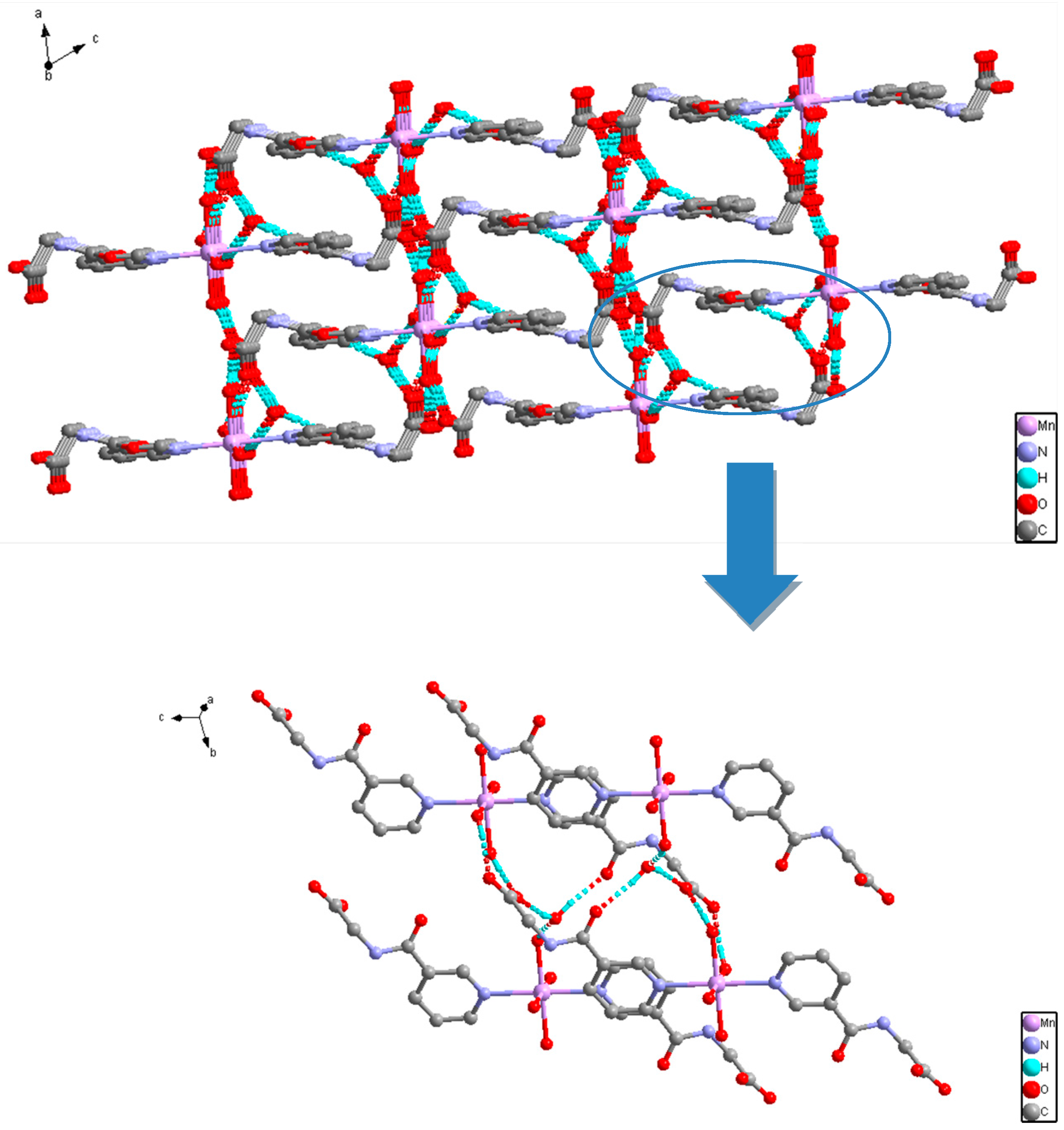
2.2. Structural Description of C16H26MnN4O12 (1)
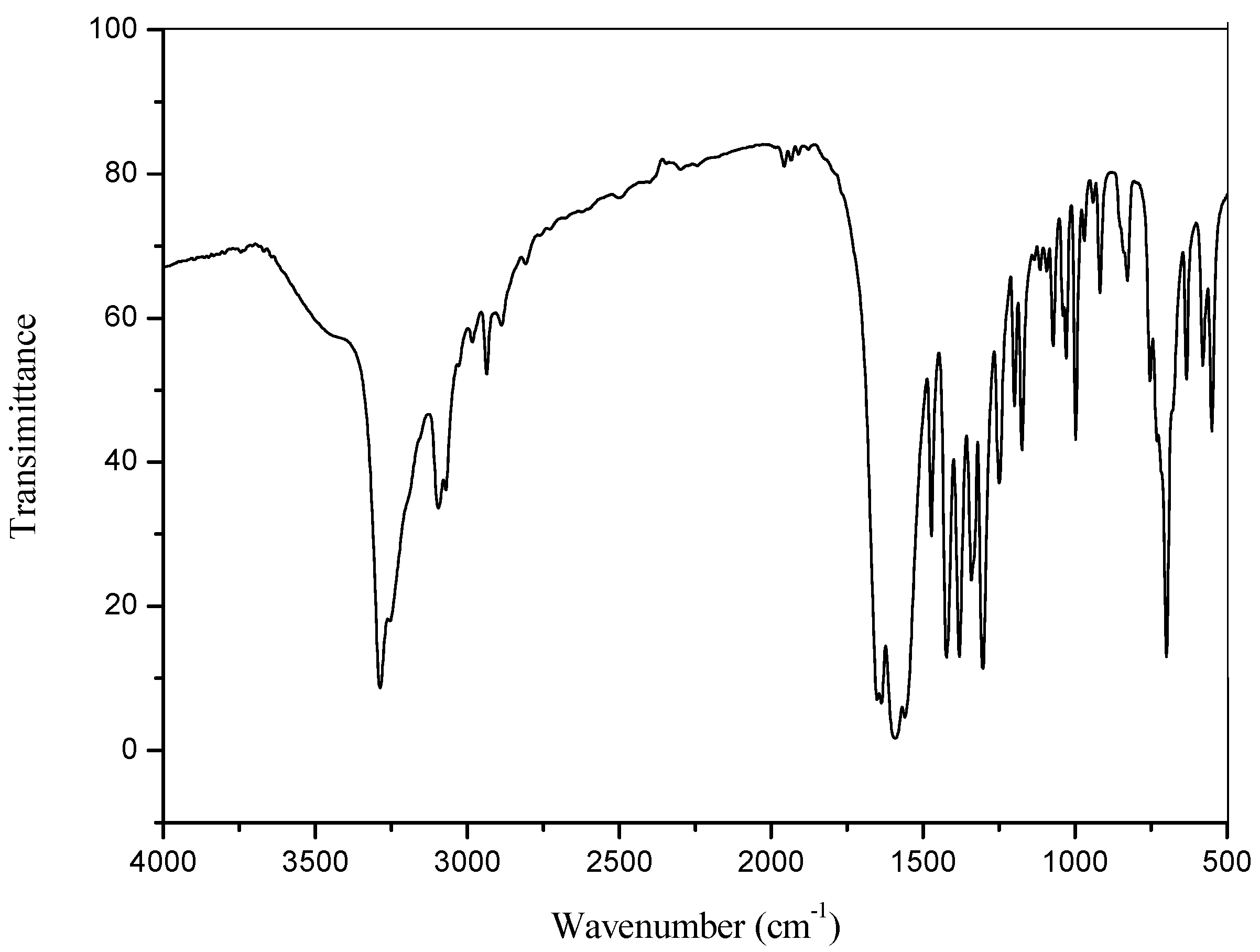
2.3. IR Spectrum
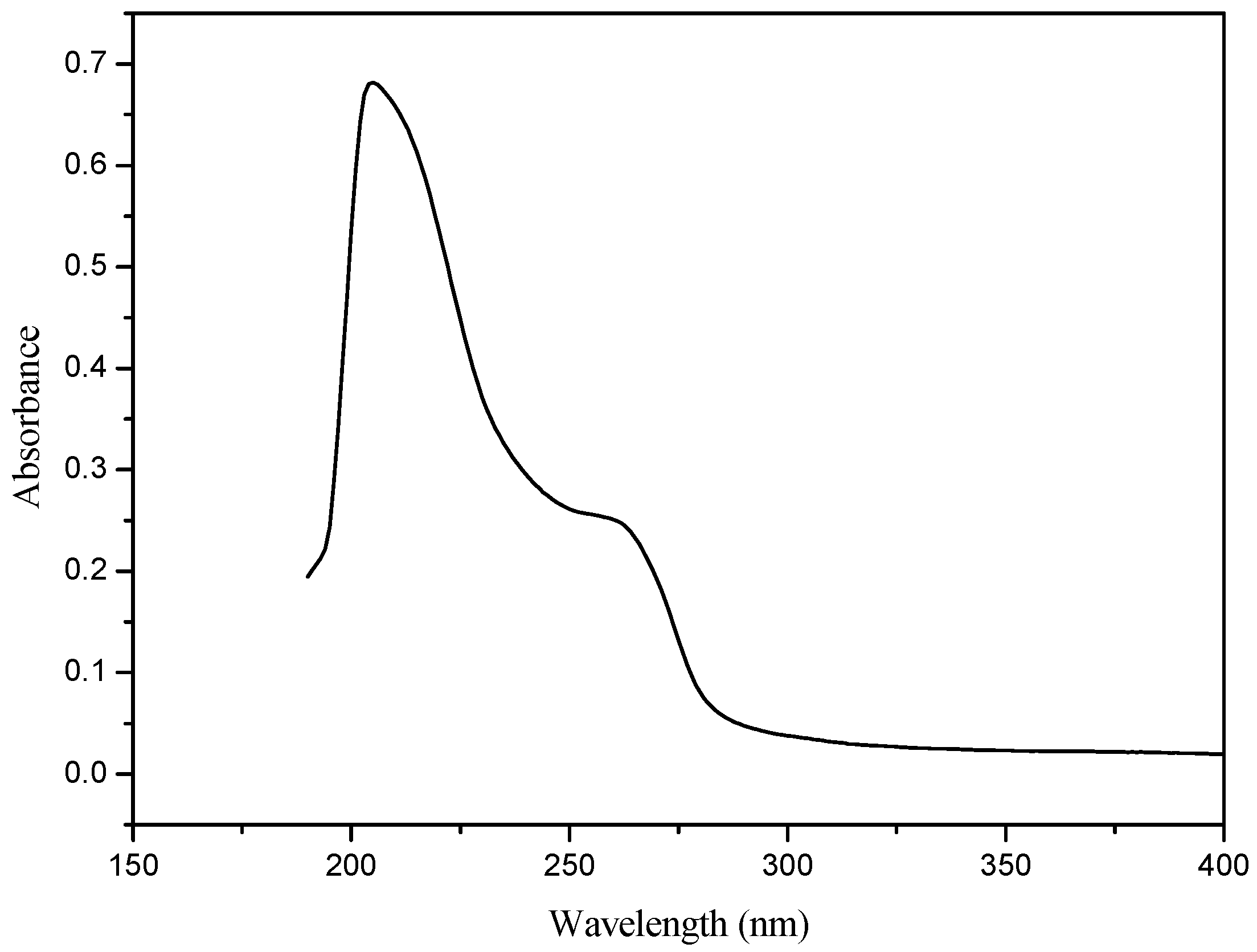
2.4. UV-Vis Spectrum
3. Experimental Section
3.1. Materials and Instrumentation
3.2. Synthesis of C16H26MnN4O12 (1)
3.3. Data Collection, Structural Determination, and Refinement
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hao, J.M.; Zhao, Y.N.; Li, H.H.; Ming, C.L.; Cui, G.H. Synthesis, structures, and characterization of two d 10 metal coordination polymers with a flexible bis(triazole) ligand. Synth. React. Inorg. Met. Org. Nanomet. Chem. 2015, 45, 947–951. [Google Scholar] [CrossRef]
- Ye, W.P.; Chen, M.; Yang, Y.; Zha, L.Q.; Ma, Y.S.; Yuan, R.X. Synthesis, structures and Magnetic Properties of Three Copper Phosphonates Bearing 4,4′-Bipyridine Bridge. Chin. J. Inorg. Chem. 2015, 25, 559–568. [Google Scholar]
- Zhao, G.L.; Zhang, K.T.; Yu, Y.Y. Synthesis and crystal structures of cerium and samarium complexex with 4-methyl-1,2,3-thiadiazol-5-carboxylic acid. J. Zhejiang Normal Univ. (Nat. Sci.) 2013, 23, 1266–1273. [Google Scholar]
- Li, W.; Li, C.H.; Li, Y.L.; Zhang, C.H. Synthesis, Crystal Structure and Spectrum of Trinuclear Manganese Coordination Compound Mn3(2,2′-bipy)2(3,5-DMAB)6. Chin. J. Struct. Chem. 2013, 23, 771–778. [Google Scholar]
- Lee, J.Y.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Mental-organic framework meterials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Tai, X.S.; Zhang, Y.P.; Zhao, W.H. Sythesis, crystal structure and antitumor activity of a dinuclear calcium complex based on 1,5-naphthalenedisulfonate and 2,2′-bipyridine ligands. Res. Chem. Intermed. 2015, 41, 4339–4347. [Google Scholar] [CrossRef]
- Tai, X.S.; Zhao, W.H. Synthesis, structural characterization, and antitumor activity of a Ca(II) coordination polymer based on 1,6-naphthalenedisulfonate and 4,4′-bipyridyl. Materials 2013, 6, 3547–3555. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Song, D.A. Luminescent Metal-Organic Framework as a Turn-On Sensor for DMF Vapor. Angew. Chem. Int. Ed. 2013, 52, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Che, G.B.; Liu, C.B.; Liu, B.; Wang, Q.W.; Xu, Z.L. Syntheses, structures and photoluminescence of a series of metal-organic complexes with 1,3,5-benzenetricarboxylate and pyrazino [2,3-f][1,10]-phenanthroline ligands. CrystEngComm 2008, 10, 184–191. [Google Scholar] [CrossRef]
- Liu, C.B.; Zhao, H.; Wang, S.S.; Cha, X.L.; Li, X.Y.; Che, G.B. Syntheses and Crystal Structures of Co(II) and Mn(II) Complexes with 2,4-Biphenyldicarboxylic Acid and Imidazo [4,5-f][1,10]phenanthroline Ligands. Chin. J. Inorg. Chem. 2013, 29, 1533–1538. [Google Scholar]
- Peddy, V.; Ashwini, N.; Vincent, M.L. Molecular Complexes of Homologous Alkanedicarboxylic Acids with Isonicotinamide: X-ray Crystal Structures, Hydrogen Bond Synthons, and Melting Point Alterna. Cryst. Growth Des. 2003, 3, 783–790. [Google Scholar]
- Rahman, B.; Hassan, H.-M.; Maria, K.; Marta, S.K.; Tadeusz, L. Synthesis and Magnetic Properties of a 1D coordination polymer of Cu(II) containing phenoxido and dicyanamido bridging groups. Polyhedron 2014, 81, 282–289. [Google Scholar]
- Rahman, B.; Ramin, K.; Milosz, S.; Serhiy, D.; Hassan, H.-M.; Tadeusz, L. Magnetic and spectroscopic properties of a 2D Mn(II) coordination polymer with carbohydrazone ligand. Inorg. Chem. Commun. 2016, 70, 219–222. [Google Scholar]
- Nader, N.; Azam, H.; Fakhri, H.; Rahman, B.; Tadeusz, L. Chiral lactic hydrazone derivatives as potential bioactive antibacterial agents: Synthesis, spectroscopic, structural and molecular docking studies. J. Mol. Struct. 2017, 1128, 391–399. [Google Scholar]
- Antony, A.; Fasna, F.; Ajil, P.A.; Varkey, J.T. Amino Acid based Schiff Bases and its Zn(II) Complexes. Res. Rev. J. Chem. 2016, 5, 37–44. [Google Scholar]
- Anam, F.; Abbas, A.; Lo, K.M.; Hameed, S.; Ramasami, P.; Umar, Y.; Ullah, A.; Naseer, M.M. Synthesis, crystal structure, experimental and theoretical investigations of 3-(4-ethoxy-3-methoxyphenyl)-1-phenylprop-2-en-1-one. J. Mol. Struct. 2017, 1127, 742–750. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, C.W.; Ren, N.; Zhang, J.J. Synthesis, crystal structures, thermal decomposition mechanism and thermal properties of mononuclear ternry lanthanide comples with 2,4-dichlorobenzoic acid and 2,2′:6,2″-terpyridine. Chin. Sci. Bull. 2016, 61, 3146–3154. [Google Scholar]
- Shao, D.J.; Li, D.L.; Liu, H.M. New Cd(II) complexes of preparation, physical and chemical properities and separation of cadmium contaminated in soil. Appl. Chem. Ind. 2016, 45, 1998–2001. [Google Scholar]
- Pang, H.X.; Liu, W.; Xiong, H.; Guan, J.F. Synthesis, characterization and properties of a quad-core lead(II) complex. J. Cent. China Normal Univ. (Nat. Sci.) 2016, 50, 726–731. [Google Scholar]
- Xin, Q.P.; Li, S.L.; Liu, X.J. Synthesis and Crystal Structures of Cadmiun(II) Coordination Compounds of Large Ring and Bicyclo Framework with a Bidentate Betaine Derivative. Chin. J. Inorg. Chem. 2012, 28, 815–822. [Google Scholar]
- Cui, F.H.; Ping, R.P.; Xuan, X.P. Density Functional Theory Studies on FT-IR and Raman Spectra of 4-Acetylpyridine. J. Light Scatt. 2014, 26, 282–287. [Google Scholar]
- Rahman, B.; Pavio, A.; Hassan, H.-M.; Joaquin, S.; Ritta, S.; Tadeusz, L. Synthesis, structure, magnetic properties and EPR spectroscopy of a copper(II) coordination polymer with a ditopic hydrazone ligand and acetate bridges. Dalton Trans. 2015, 44, 1782–1789. [Google Scholar]
- Rahman, B.; Hassan, H.-M.; Leslaw, S.; Angel, G. Synthesis, crystal structure, spectroscopic study, and magnetic behavior of the first dinuclear Mn(II) complex of hydrazone-based ligand-containing dicyanamide bridging groups. J. Coord. Chem. 2013, 66, 4023–4031. [Google Scholar]
- Rahman, B.; Hassan, H.-M.; Vera, V.; Joaquin, S.; Javier, A.; Jose, M.B.; Christoph, J. Heteronuclear, mixed-metal Ag(I)–Mn(II) coordination polymers with bridging N-pyridinylisonicotinohydrazide ligands: Synthesis, crystal structures, magnetic and photoluminescence properties. Dalton Trans. 2014, 43, 11925–11935. [Google Scholar]
- Rahman, B.; Hassan, H.-M.; Pavlo, A.; Ritta, S.; Milosz, S.; Tadeusz, L. Single crystal EPR spectroscopy, magnetic studies and catalytic activity of a self-assembled [2 × 2] Cu II 4 cluster obtained from a carbohydrazone based ligand. Polyhedron 2013, 66, 4023–4031. [Google Scholar]
- Tai, X.S.; You, H.Y. A new 1D chained coordination polymer: Synthesis, crystal structure, antitumor activity and luminescent property. Crystals 2015, 5, 608–616. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
| Bond | Distance | Bond | Distance |
|---|---|---|---|
| Mn1–O1w | 2.1309 (13) | O2–C8 | 1.241 (2) |
| Mn1–O1wi | 2.1309 (13) | O3–C8 | 1.253 (2) |
| Mn1–O2w | 2.1444 (13) | O3w–H3wB | 0.851 |
| Mn1–O2wi | 2.1444 (13) | O3w–H3wA | 0.8491 |
| Mn1–N1 | 2.3489 (16) | N2–H2B | 0.86 |
| Mn1–N1i | 2.3489 (16) | O1W–H1wB | 0.8515 |
| N1–C5 | 1.339 (2) | O1W–H1wA | 0.8449 |
| N1–C1 | 1.340 (3) | O1–C6 | 1.222 (2) |
| N2–C6 | 1.334 (2) | O2w–H2wA | 0.8518 |
| N2–C7 | 1.445 (2) | O2w–H2wB | 0.8592 |
| Angle | Angle | ||
| O1wi–Mn1–O2wi | 90.01 (6) | O2w–Mn1–O2wi | 180 |
| O1w–Mn1–N1 | 90.58 (6) | O1wi–Mn1–N1 | 89.42 (6) |
| O2w–Mn1–N1 | 89.20 (6) | O2wi–Mn1–N1 | 90.80 (6) |
| O1w–Mn1–N1i | 89.42 (6) | O1w–Mn1–N1i | 90.58 (6) |
| O2w–Mn1–N1i | 90.80 (6) | O2wi–Mn1–N1i | 89.20 (6) |
| N1–Mn–N1i | 180.00 (7) | O1–C6–N2 | 121.78 (16) |
| C5–N1–Mn1 | 121.76 (12) | O1–C6–C4 | 121.07 (16) |
| C1–N1–Mn1 | 121.05 (12) | N2–C6–C4 | 117.11 (15) |
| C6–N2–C7 | 121.44 (15) | N2–C7–C8 | 114.59 (14) |
| Mn1–O1w–H1wB | 121.2 | Mn1–O1w–H1wA | 129.9 |
| Donor–H…Acceptor | D–H | H…A | D…A | ∠D–H…A | |
|---|---|---|---|---|---|
| 1 | O1w–H1wB…O3 | 0.85 | 1.82 | 2.6711 (5) | 176 |
| 2 | O1w–H1wA…O3 | 0.84 | 1.87 | 2.6882 (6) | 163 |
| 3 | O2w–H2wA…O3w | 0.85 | 1.83 | 2.6718 (5) | 169 |
| 4 | O2w–H2wB…O2 | 0.86 | 1.81 | 2.6671 (6) | 175 |
| 5 | O3w–H3wB…O2 | 0.85 | 1.92 | 2.7589 (6) | 167 |
| 6 | O3w–H3wA…O1 | 0.85 | 1.89 | 2.7345 (6) | 171 |
| 7 | Intra C5–H5A…O1 | 0.93 | 2.50 | 2.8144 (6) | 100 |
| Empirical Formula | C16H26MnN4O12 |
| Temperature/K | 293 (2) |
| Crystal system | Triclinic |
| Space group | P1 |
| a/Å | 7.8192 (16) |
| b/Å | 8.8800 (18) |
| c/Å | 9.0142 (18) |
| α/° | 83.14 (3) |
| β/° | 65.27 (3) |
| γ/° | 81.67 (3) |
| Volume/Å3 | 561.3 (2) |
| Z | 1 |
| Dx (mg/m−3) | 1.542 |
| μ/mm−1 | 0.66 |
| S | 1.06 |
| F(000) | 271 |
| Index ranges | −10 ≤ h ≤ 10 |
| −11 ≤ k ≤ 11 | |
| −11 ≤ l ≤ 11 | |
| Reflections collected | 5481 |
| Reflections with I > 2σ(I) | 2467 |
| Independent reflections | 2559 [R(int) = 0.027] |
| Data/restraints/parameters | 2559/6/151 |
| Goodness-of-fit on F2 | 1.061 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0381, wR2 = 0.0964 |
| Final R indexes [all data] | R1 = 0.0388, wR2 = 0.0969 |
| Largest diff. peak/hole/e Å−3 | 0.41/−0.38 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, B.; He, M. Synthesis, Crystal Structure of a Novel Mn Complex with Nicotinoyl-Glycine. Crystals 2017, 7, 3. https://doi.org/10.3390/cryst7010003
Wang X, Chen B, He M. Synthesis, Crystal Structure of a Novel Mn Complex with Nicotinoyl-Glycine. Crystals. 2017; 7(1):3. https://doi.org/10.3390/cryst7010003
Chicago/Turabian StyleWang, Xin, Biqing Chen, and Min He. 2017. "Synthesis, Crystal Structure of a Novel Mn Complex with Nicotinoyl-Glycine" Crystals 7, no. 1: 3. https://doi.org/10.3390/cryst7010003






