High-Pressure Reactivity of Kr and F2—Stabilization of Krypton in the +4 Oxidation State
Abstract
:1. Introduction
2. Results
2.1. Pressures Up to 50 GPa
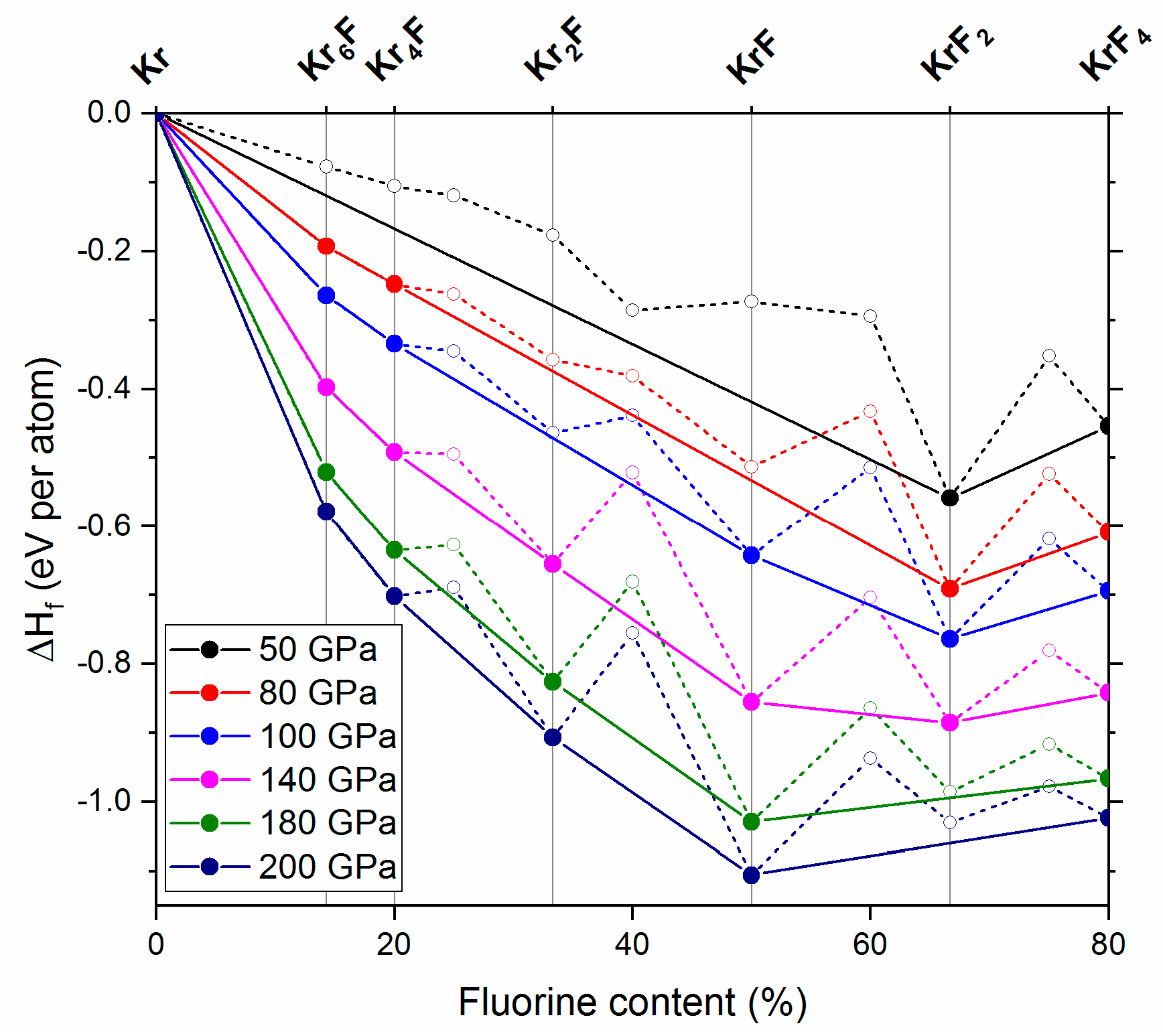
2.2. The 50–200 GPa Pressure Range
3. Discussion
4. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tramšek, M.; Žemva, B. Synthesis, properties and chemistry of xenon(II) fluoride. Acta Chim. Slov. 2006, 105–116. [Google Scholar] [CrossRef]
- Grochala, W. Atypical compounds of gases, which have been called “noble”. Chem. Soc. Rev. 2007, 36, 1632–1655. [Google Scholar] [CrossRef] [PubMed]
- Nabiev, S.S.; Sokolov, V.B.; Chaivanov, B.B. Structure of simple and complex noble gas fluorides. Crystallogr. Rep. 2011, 56, 774–791. [Google Scholar] [CrossRef]
- Brock, D.S.; Schrobilgen, G.J.; Žemva, B. Noble-Gas Chemistry. In Comprehensive Inorganic Chemistry II; Elsevier: Cambridge, MA, USA, 2013; pp. 755–822. [Google Scholar]
- Nabiev, S.S.; Sokolov, V.B.; Chaivanov, B.B. Molecular and crystal structures of noble gas compounds. Russ. Chem. Rev. 2014, 83, 1135–1180. [Google Scholar] [CrossRef]
- Haner, J.; Schrobilgen, G.J. The chemistry of Xenon(IV). Chem. Rev. 2015, 115, 1255–1295. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.F.; Mercier, H.P.A.; Schrobilgen, G.J. The chemistry of krypton. Coord. Chem. Rev. 2002, 233–234, 1–39. [Google Scholar] [CrossRef]
- Lozinšek, M.; Schrobilgen, G.J. The world of krypton revisited. Nat. Chem. 2016, 8, 732. [Google Scholar] [CrossRef] [PubMed]
- Lozinšek, M.; Mercier, H.P.A.; Brock, D.S.; Žemva, B.; Schrobilgen, G.J. Coordination of KrF2 to a Naked Metal Cation, Mg2+. Angew. Chem. Int. Ed. 2017, 56, 6251–6254. [Google Scholar] [CrossRef] [PubMed]
- Grosse, A.V.; Kirshenbaum, A.D.; Streng, A.G.; Streng, L.V. Krypton Tetrafluoride: Preparation and Some Properties. Science 1963, 139, 1047–1048. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.H.; Verdier, P.H. Krypton Tetrafluoride: 19F High-Resolution Magnetic Resonance Spectrum. J. Chem. Phys. 1964, 40, 2057. [Google Scholar] [CrossRef]
- Turner, J.J.; Pimentel, G.C. Krypton Fluoride: Preparation by the Matrix Isolation Technique. Science 1963, 140, 974–975. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, F.; Malm, J.G.; Hindman, J.C. The Preparation and Nuclear Magnetic Resonance of Krypton Difluoride. J. Am. Chem. Soc. 1965, 87, 25–28. [Google Scholar] [CrossRef]
- Bartlett, N. Xenon hexafluoroplatinate(V) Xe+[PtF6]−. Proc. Chem. Soc. 1962, 6, 218. [Google Scholar]
- Hargittai, I. Neil Bartlett and the first noble-gas compound. Struct. Chem. 2009, 20, 953–959. [Google Scholar] [CrossRef]
- Gunn, S.R. Heat of formation of krypton difluoride. J. Phys. Chem. 1967, 71, 2934–2937. [Google Scholar] [CrossRef]
- Chen, J.L.; Yang, C.Y.; Lin, H.J.; Hu, W.P. Theoretical prediction of new noble-gas molecules FNgBNR (Ng = Ar, Kr, and Xe; R = H, CH3, CCH, CHCH2, F, and OH). Phys. Chem. Chem. Phys. 2013, 15, 9701. [Google Scholar] [CrossRef] [PubMed]
- Gronowski, M.; Turowski, M.; Kołos, R. Quantum Chemical Study on HKrC5N, HXeC5N, and Related Rare Gas Compounds. J. Phys. Chem. A 2015, 119, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Manna, D.; Ghanty, T.K. Theoretical Prediction of Noble Gas Inserted Thioformyl Cations: HNgCS+ (Ng = He, Ne, Ar, Kr, and Xe). J. Phys. Chem. A 2015, 119, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.A.; McDowell, S.A.C. Comparative computational study of model halogen-bonded complexes of FKrCl. J. Phys. Chem. A 2015, 119, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, M.; Zhou, M.; Andrada, D.M.; Frenking, G. Experimental and theoretical studies of the infrared spectra and bonding properties of NgBeCO3 and a comparison with NgBeO (Ng = He, Ne, Ar, Kr, Xe). J. Phys. Chem. A 2015, 119, 2543–2552. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Pan, S.; Mandal, S.; Orozco, M.; Merino, G.; Chattaraj, P.K. Noble gas supported B3+ cluster: Formation of strong covalent noble gas–boron bonds. RSC Adv. 2016, 6, 78611–78620. [Google Scholar] [CrossRef]
- Chen, W.; Chen, G.H.; Wu, D.; Wang, Q. BNg3F3 : The first three noble gas atoms inserted into mono-centric neutral compounds—A theoretical study. Phys. Chem. Chem. Phys. 2016, 18, 17534–17545. [Google Scholar] [CrossRef] [PubMed]
- Khriachtchev, L.; Pettersson, M.; Runeberg, N.; Lundell, J.; Räsänen, M. A stable argon compound. Nature 2000, 406, 874–876. [Google Scholar] [PubMed]
- Evans, C.J.; Gerry, M.C.L. The microwave spectra and structures of Ar–AgX (X = F, Cl, Br). J. Chem. Phys. 2000, 112, 1321–1329. [Google Scholar] [CrossRef]
- Grochala, W.; Khriachtchev, L.; Räsänen, M. Noble-gas chemistry. In Physics & Chemistry at Low Temperatures; Khriachtchev, L., Ed.; Pan Stanford Publishing: Boca Raton, FL, USA, 2011; pp. 419–446. [Google Scholar]
- Grandinetti, F. Review: Gas-phase ion chemistry of the noble gases: Recent advances and future perspectives. Eur. J. Mass Spectrom. 2011, 17, 423–463. [Google Scholar] [CrossRef] [PubMed]
- Young, N.A. Main group coordination chemistry at low temperatures: A review of matrix isolated Group 12 to Group 18 complexes. Coord. Chem. Rev. 2013, 257, 956–1010. [Google Scholar] [CrossRef]
- Wang, X.; Andrews, L.; Brosi, F.; Riedel, S. Matrix Infrared Spectroscopy and Quantum-Chemical Calculations for the Coinage-Metal Fluorides: Comparisons of Ar-AuF, Ne-AuF, and Molecules MF2 and MF3. Chem. A Eur. J. 2013, 19, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Kurzydłowski, D.; Zaleski-Ejgierd, P. High-pressure stabilization of argon fluorides. Phys. Chem. Chem. Phys. 2016, 18, 2309–2313. [Google Scholar] [CrossRef] [PubMed]
- Somayazulu, M.; Dera, P.; Smith, J.; Hemley, R.J. Structure and stability of solid Xe(H2)n. J. Chem. Phys. 2015, 142, 104503. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, A.; Worth, N.; Pickard, C.J.; Needs, R.J.; Pascarelli, S.; Mathon, O.; Mezouar, M.; Irifune, T. Synthesis and stability of xenon oxides Xe2O5 and Xe3O2 under pressure. Nat. Chem. 2016, 8, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Howie, R.T.; Turnbull, R.; Binns, J.; Frost, M.; Dalladay-Simpson, P.; Gregoryanz, E. Formation of Xenon-nitrogen compounds at high pressure. Sci. Rep. 2016, 6, 34896. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, A.; Pépin, C.M.; Geneste, G.; Garbarino, G. Reaction between nickel or iron and xenon under high pressure. High Press. Res. 2017, 37, 137–146. [Google Scholar] [CrossRef]
- Dong, X.; Oganov, A.R.; Goncharov, A.F.; Stavrou, E.; Lobanov, S.; Saleh, G.; Qian, G.R.; Zhu, Q.; Gatti, C.; Deringer, V.L.; et al. A stable compound of helium and sodium at high pressure. Nat. Chem. 2017, 9, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Jung, D.Y.; Oganov, A.R.; Glass, C.W.; Gatti, C.; Lyakhov, A.O. Stability of xenon oxides at high pressures. Nat. Chem. 2013, 5, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Hermann, A.; Schwerdtfeger, P. Xenon Suboxides Stable under Pressure. J. Phys. Chem. Lett. 2014, 5, 4336–4342. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chen, Y.; Xiang, S.; Kuang, X.; Bi, Y.; Chen, H. High-temperature- and high-pressure-induced formation of the Laves-phase compound XeS2. Phys. Rev. B 2016, 93, 214112. [Google Scholar] [CrossRef]
- Zaleski-Ejgierd, P.; Lata, P.M. Krypton oxides under pressure. Sci. Rep. 2016, 6, 18938. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Wang, X.; Botana, J.; Miao, M. Noble gas bond and the behaviour of XeO3 under pressure. Phys. Chem. Chem. Phys. 2017, 19, 27463–27467. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Debessai, M.; Yoo, C.S. Two- and three-dimensional extended solids and metallization of compressed XeF2. Nat. Chem. 2010, 2, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Kurzydłowski, D.; Zaleski-Ejgierd, P.; Grochala, W.; Hoffmann, R. Freezing in resonance structures for better packing: XeF2 becomes (XeF+)(F−) at large compression. Inorg. Chem. 2011, 50, 3832–3840. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Huang, X.; Huang, Y.; Pan, L.; Li, F.; Li, X.; Liu, M.; Liu, B.; Cui, T. Confirmation of the Structural Phase Transitions in XeF2 under High Pressure. J. Phys. Chem. C 2017, 121, 6264–6271. [Google Scholar] [CrossRef]
- Peng, F.; Botana, J.; Wang, Y.; Ma, Y.; Miao, M. Unexpected trend in stability of Xe–F compounds under pressure driven by Xe–Xe covalent bonds. J. Phys. Chem. Lett. 2016, 4562–4567. [Google Scholar] [CrossRef] [PubMed]
- Braïda, B.; Hiberty, P.C. The essential role of charge-shift bonding in hypervalent prototype XeF2. Nat. Chem. 2013, 5, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Oganov, A.R.; Lyakhov, A.O.; Valle, M. How evolutionary crystal structure prediction works—And why. Acc. Chem. Res. 2011, 44, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Zurek, E.; Grochala, W. Predicting crystal structures and properties of matter under extreme conditions via quantum mechanics: The pressure is on. Phys. Chem. Chem. Phys. 2015, 17, 2917–2934. [Google Scholar] [CrossRef] [PubMed]
- Needs, R.J.; Pickard, C.J. Perspective: Role of structure prediction in materials discovery and design. APL Mater. 2016, 4, 53210. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Lv, J.; Ma, Y. Materials discovery at high pressures. Nat. Rev. Mater. 2017, 2, 17005. [Google Scholar] [CrossRef]
- Glass, C.W.; Oganov, A.R.; Hansen, N. USPEX—Evolutionary crystal structure prediction. Comput. Phys. Commun. 2006, 175, 713–720. [Google Scholar] [CrossRef]
- Oganov, A.R.; Glass, C.W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J. Chem. Phys. 2006, 124, 244704. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Hennig, R.G.; Ashcroft, N.W.; Hoffmann, R. Emergent reduction of electronic state dimensionality in dense ordered Li-Be alloys. Nature 2008, 451, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.F.; Dixon, D.A.; Schrobilgen, G.J. X-ray crystal structures of α-KrF2, [KrF][MF6] (M = As, Sb, Bi), [Kr2F3][SbF6]·KrF2, [Kr2F3]2[SbF6]2·KrF2, and [Kr2F3][AsF6]·[KrF][AsF6]; synthesis and characterization of [Kr2F3][PF6]·nKrF2 ; and theoretical studies of KrF2, KrF+, Kr2F3+, and the [KrF][MF6] (M = P, As, Sb, Bi) ion pairs. Inorg. Chem. 2001, 40, 3002–3017. [Google Scholar] [PubMed]
- Burbank, R.D.; Falconer, W.E.; Sunder, W.A. Crystal Structure of Krypton Difluoride at −80 °C. Science 1972, 178, 1285–1286. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.A.; Wang, T.H.; Grant, D.J.; Peterson, K.A.; Christe, K.O.; Schrobilgen, G.J. Heats of formation of krypton fluorides and stability predictions for KrF4 and KrF6 from high level electronic structure calculations. Inorg. Chem. 2007, 46, 10016–10021. [Google Scholar] [CrossRef] [PubMed]
- Savin, A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The electron localization function. Angew. Chem. Int. Ed. Engl. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Kurzydłowski, D.; Zaleski-Ejgierd, P. Hexacoordinated nitrogen(V) stabilized by high pressure. Sci. Rep. 2016, 6, 36049. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Galy, J.; Matar, S.F. ns2np4 (n = 4, 5) lone pair triplets whirling in M*F2E3 (M* = Kr, Xe): Stereochemistry and ab initio analyses. Solid State Sci. 2017, 64, 114–124. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA : A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Stokes, H.T.; Hatch, D.M. FINDSYM : Program for identifying the space-group symmetry of a crystal. J. Appl. Crystallogr. 2005, 38, 237–238. [Google Scholar] [CrossRef]
- Figgins, B.F.; Smith, B.L. Density and expansivity of solid krypton. Philos. Mag. 1960, 5, 186–188. [Google Scholar] [CrossRef]
- Pauling, L.; Keaveny, I.; Robinson, A.B. The crystal structure of α-fluorine. J. Solid State Chem. 1970, 2, 225–227. [Google Scholar] [CrossRef]
- Johannsen, P.G.; Holzapfel, W.B. Effect of pressure on Raman spectra of solid chlorine. J. Phys. C Solid State Phys. 1983, 16, L1177–L1179. [Google Scholar] [CrossRef]

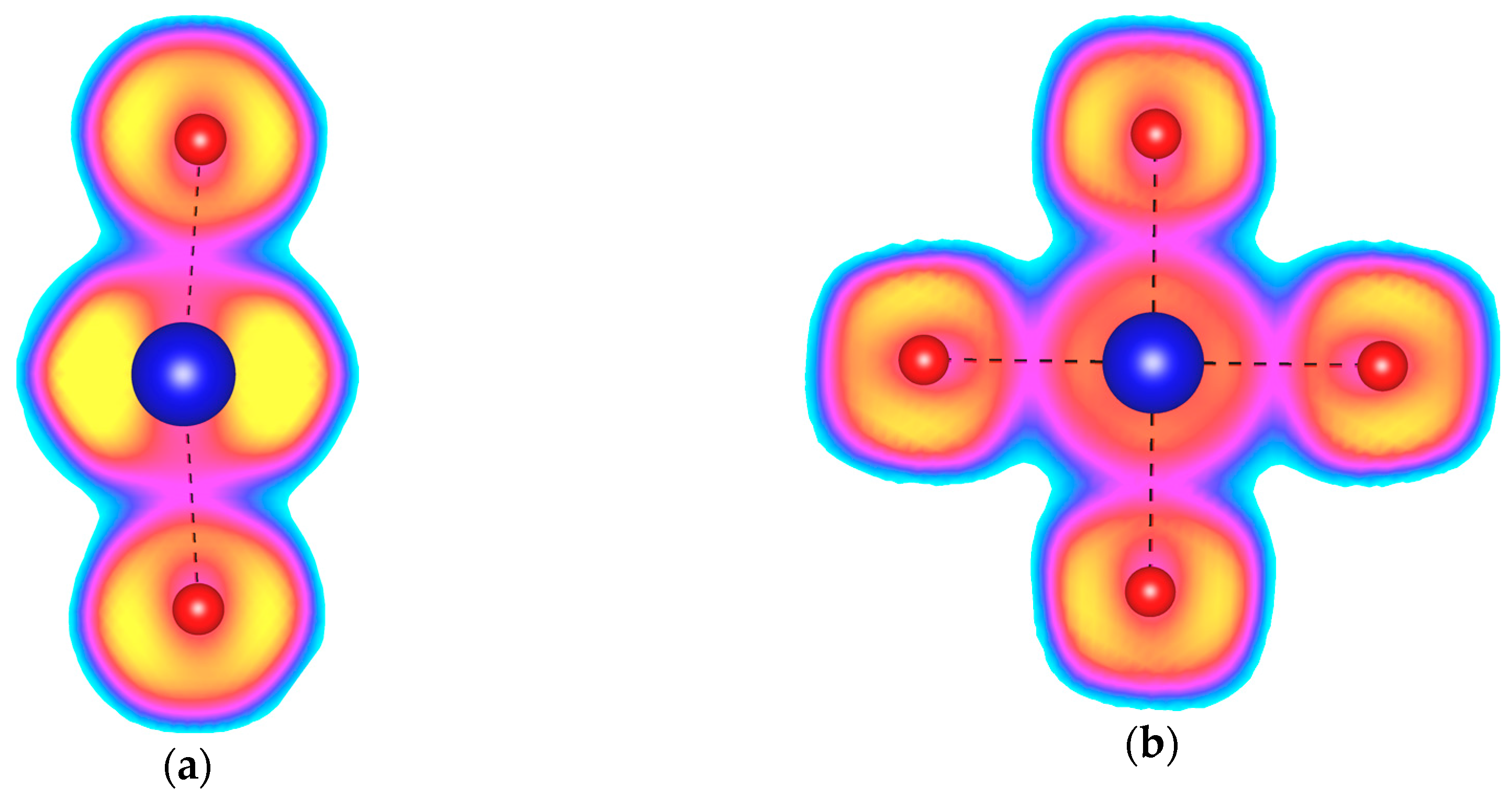
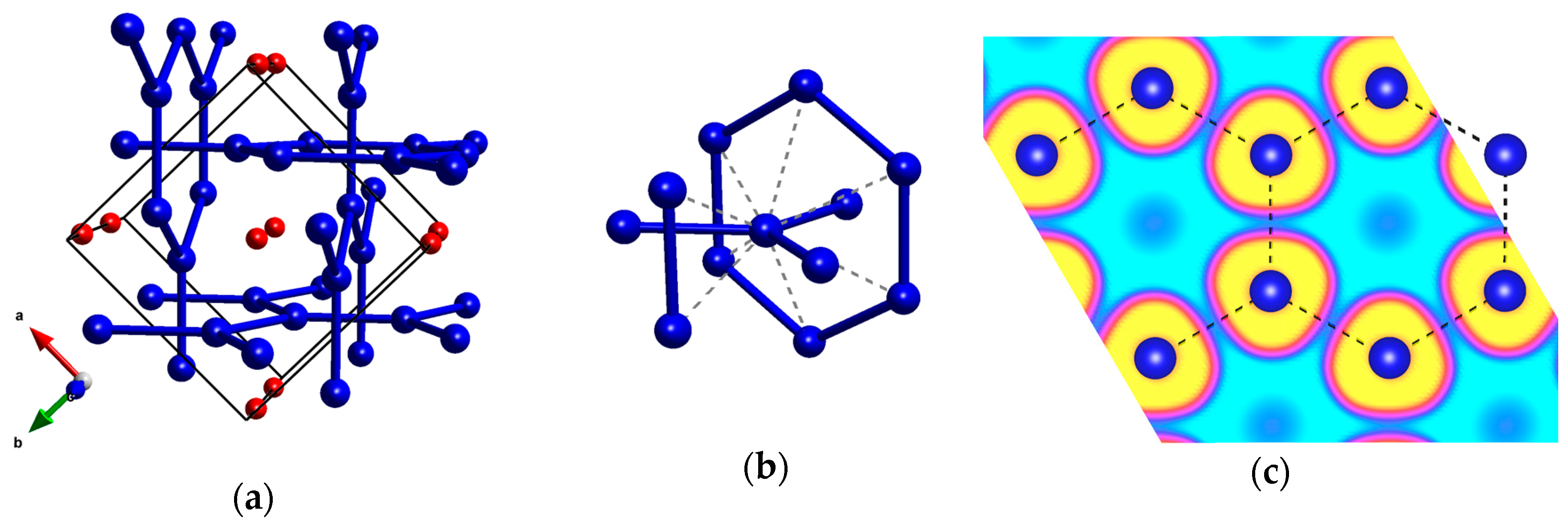

| Phase | Type | Kr–F | Kr–Kr | F–F |
|---|---|---|---|---|
| Kr | Atomic (3D) | - | 2.68–2.75 | - |
| Kr6F | 2.29–2.39 | 2.58–2.77 | 2.78 | |
| Kr4F | 2.31–2.34 | 2.62–2.70 | 2.68 | |
| Kr2F | 2D | 2.29 | 2.51–2.56; 2.83–2.84 | 2.21 |
| KrF | 1D | 2.27–2.33 | 2.29; 2.78–2.90 | 2.29 |
| KrF2 | Molecular (0D) | 1.80 | 2.66 | 2.19 |
| KrF4 | 1.80 | 3.57 | 2.00 | |
| F2 | - | - | 1.42 |
| Ng | NgF2 | NgF4 | NgF6 | Ref. |
|---|---|---|---|---|
| Ar | 58 (> 200) | > 200 | n.d. | [30] |
| Kr | amb. (180) | 15 | > 200 | this work |
| Xe | amb. (81) | amb. | amb. | [44] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzydłowski, D.; Sołtysiak, M.; Dżoleva, A.; Zaleski-Ejgierd, P. High-Pressure Reactivity of Kr and F2—Stabilization of Krypton in the +4 Oxidation State. Crystals 2017, 7, 329. https://doi.org/10.3390/cryst7110329
Kurzydłowski D, Sołtysiak M, Dżoleva A, Zaleski-Ejgierd P. High-Pressure Reactivity of Kr and F2—Stabilization of Krypton in the +4 Oxidation State. Crystals. 2017; 7(11):329. https://doi.org/10.3390/cryst7110329
Chicago/Turabian StyleKurzydłowski, Dominik, Magdalena Sołtysiak, Aleksandra Dżoleva, and Patryk Zaleski-Ejgierd. 2017. "High-Pressure Reactivity of Kr and F2—Stabilization of Krypton in the +4 Oxidation State" Crystals 7, no. 11: 329. https://doi.org/10.3390/cryst7110329





