Hydrothermal Crystal Growth of Piezoelectric α-Quartz Phase of AO2 (A = Ge, Si) and MXO4 (M = Al, Ga, Fe and X = P, As): A Historical Overview
Abstract
:1. Introduction
2. Hydrothermal Crystal Growth
2.1. Hydrothermal Growth of AIVO2 (A = Si, Ge)
2.2. Hydrothermal Growth of MIIIXVO4 (M = Al, Ga, Fe; X = P, As)
2.2.1. AlPO4 Single Crystals
2.2.2. GaPO4 Single Crystals
2.2.3. GaAsO4 Single Crystals
2.2.4. α-quartz FePO4
2.2.5. M’(1−x)MxPO4 Solid Solutions
Al1−xGaxPO4
Ga1−xFexPO4
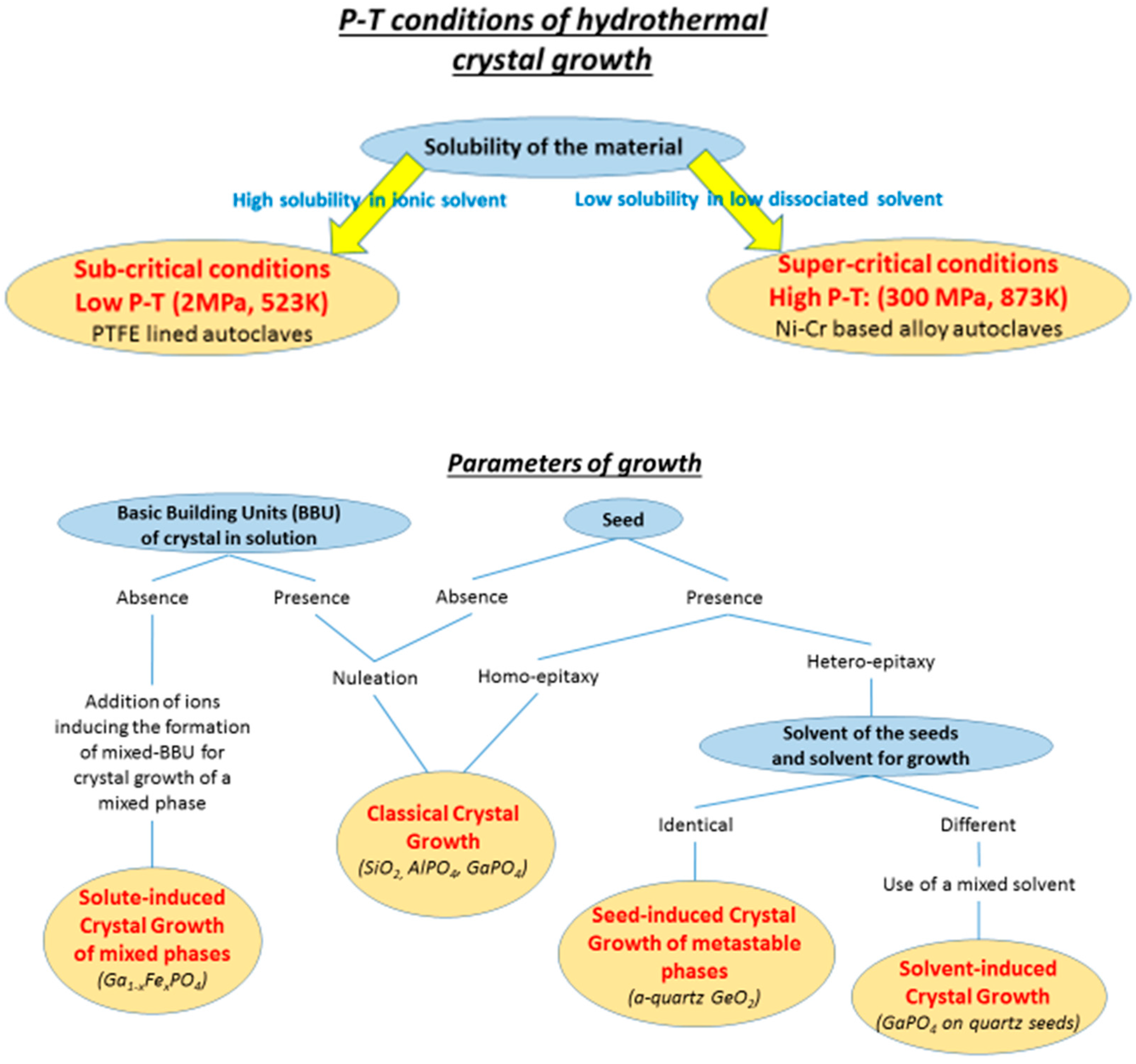
3. General Discussion about Hydrothermal Crystal Growth Conditions
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Schafthault, K.F.E. Gelehrte Anzeigen Bayer. Akad 1845, 20, 557. [Google Scholar]
- Spezia, G. Sull accresimento del quarzo. Atti Accad. Sci. Torino 1900, 35, 95–107. [Google Scholar]
- Kinloch, D.R.; Belt, R.F.; Puttbach, R.C. Hydrothermal growth of calcite in large autoclaves. J. Cryst. Growth 1974, 24, 610–613. [Google Scholar] [CrossRef]
- Kikuta, K.; Hirano, S. Hydrothermal growth and dissolution behavior of calcite single-crystal in nitrate solutions. J. Cryst. Growth 1990, 99, 895–899. [Google Scholar] [CrossRef]
- Shigley, J.E.; McClure, S.F.; Cole, J.E.; Koivula, J.I.; Lu, T.J.; Elen, S.; Demianets, L.N. Hydrothermal synthetic red beryl from institute of crystallography, Moscow. Gems Gemol. 2001, 37, 42–55. [Google Scholar] [CrossRef]
- Demianets, L.N.; Ivanov-Shitz, A.K.; Gainutdinov, R.V. Hydrothermal growth of beryl single crystals and morphology of their singular faces. Inorg. Mater. 2006, 42, 989–995. [Google Scholar] [CrossRef]
- Gibbs, J.W. Collected Works; Longmans Greenand Co.: London, UK, 1878. [Google Scholar]
- Frenkel, J. Importance of steps and kink sites in crystal growth. J. Phys. USSR 1945, 9, 302. [Google Scholar]
- Burton, W.K.; Cabrera, N. Crystal growth and surface structure. Discuss. Faraday Soc. 1949, 5, 33. [Google Scholar] [CrossRef]
- Burton, W.K.; Cabrera, N.; Frank, F.C. The growth of crystals and the equilibrium structure of their surfaces. Philos. Trans. Soc. Lond. 1951, A243, 299–358. [Google Scholar] [CrossRef]
- Bennema, P. Analysis of crystal growth models for slightly supersaturated solutions. J. Cryst. Growth 1967, 1, 278–286. [Google Scholar] [CrossRef]
- Gilmer, G.H.; Bennema, P. Simulation of crystal growth with surface diffusion. J. Appl. Phys. 1972, 43, 1347. [Google Scholar] [CrossRef]
- Sunagawa, I.; Bennema, P. Morphology of growth spirals, theoretical and experimental. In Preparation and Properties of Solid State Materials; Wilcox, W.A., Ed.; Marcel Dekker: New York, NY, USA, 1982; Volume 7, pp. 1–129. [Google Scholar]
- Cabrera, N.; Levine, M.M. On the dislocation theory of evaporation of crystals. Philos. Mag. 1955, 1, 450–458. [Google Scholar] [CrossRef]
- Jonhston, W.G. Dislocation etch pits in non-metallic crystals. Prog. Ceram. Sci. 1962, 2, 1–75. [Google Scholar]
- Marshall, W.L.; Franck, E.U. Ion product of water substance, 0–1000 °C, 1–10,000 bars new international formulation and its background. J. Phys. Chem. Ref. Data 1981, 10, 295–304. [Google Scholar] [CrossRef]
- Buisson, X.; Arnaud, R. Hydrothermal growth of quartz crystals in industry. Present status and evolution. J. Phys. IV 1994, 4, 25–32. [Google Scholar] [CrossRef]
- Sawyer, B. Q capablity indications from infrared-absorption measuremets for Na2CO3 process cultured quartz. IEEE Trans. Sonics Ultrason. 1972, 19, 41–44. [Google Scholar] [CrossRef]
- Nacken, R. Hydrothermal syntheses als grundlage für zuchtung von quartzkristallen. Chemiker Zeitung 1950, 74, 745–749. [Google Scholar]
- Laudise, R.A. Kinetics of hydrothermal quartz crystallization. J. Am. Chem. Soc. 1959, 81, 562–566. [Google Scholar] [CrossRef]
- Laudise, R.A.; Ballman, A.A. Solubility of quartz under hydrothermal conditions. J. Phys. Chem. 1961, 65, 1396–1400. [Google Scholar] [CrossRef]
- King, J.C.; Laudise, R.A.; Ballman, A.A. Improvement of mechanical Q of quartz by addition of impurities to growth solution. J. Phys. Chem. Solids 1962, 23, 1019–1921. [Google Scholar] [CrossRef]
- Laudise, R.A.; Kolb, E.D. Hydrothermal synthesis of single crystals. Endeavour 1969, 28, 114–117. [Google Scholar]
- Barns, R.L.; Freeland, P.E.; Kolb, E.D.; Laudise, R.A.; Patel, J.R. Dislocation-free and low-dislocation quartz prepared by hydrothermal crystallization. J. Cryst. Growth 1978, 43, 676–686. [Google Scholar] [CrossRef]
- Laudise, R.A.; Barns, R.L. Perfection of quartz and its connection to crystal-growth. IEEE Trans. Ultrason. Ferroelectr. Freq.Control 1988, 35, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Zarka, A.; Lin, L.; Buisson, M. Influence of seeds on the density of dislocations produced during the growth of synthetic quartz. J. Cryst. Growth 1982, 57, 466–467. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal processing of materials: Past, present and future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Demazeau, G. Solvothermal Processes: Definition, key factors governing the involved chemical reactions and new trends. Z. Naturforsch. B 2010, 65, 999–1006. [Google Scholar] [CrossRef]
- McMillen, C.D.; Kolis, J.W. Bulk single crystal growth from hydrothermal solutions. Philos. Mag. 2012, 92, 2686–2711. [Google Scholar] [CrossRef]
- Demazeau, G.; Largeteau, A. Hydrothermal/Solvothermal crystal growth: An old but adaptable process. Z. Anorg. Allg. Chem. 2015, 641, 159–163. [Google Scholar] [CrossRef]
- McMillen, C.D.; Kolis, J.W. Hydrothermal synthesis as a route to mineralogically-inspired structures. Dalton Trans. 2016, 45, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Haines, J.; Cambon, O.; Keen, D.A.; Tucker, M.G.; Dove, M.T. Structural disorder and loss of piezoelectric properties in alpha-quartz at high temperature. Appl. Phys. Lett. 2002, 81, 2968–2970. [Google Scholar] [CrossRef]
- Philippot, E.; Goiffon, A.; Ibanez, A.; Pintard, M. Structure Deformations and Existence of the α-β Transition in MXO4 Quartz-like Materials. J. Solid State Chem. 1994, 110, 356. [Google Scholar] [CrossRef]
- Philippot, E.; Palmier, D.; Pintard, M.; Goiffon, A. A general survey of quartz and quartz-like materials: Packing distortions, temperature, and pressure effects. J. Solid State Chem. 1996, 123, 1–13. [Google Scholar] [CrossRef]
- Haines, J.; Chateau, C.; Leger, J.M.; Marchand, R. The use of composition and high pressure to extend the range of alpha-quartz isotypes. Ann. Chim. Sci. Mater. 2001, 26, 209–216. [Google Scholar] [CrossRef]
- Haines, J.; Cambon, O.; Philippot, E.; Chapon, L.; Hull, S. A neutron diffraction study of the thermal stability of the alpha-quartz-type structure in germanium dioxide. J. Solid State Chem. 2002, 166, 434–441. [Google Scholar] [CrossRef]
- Hermet, P. Piezoelectric response in alpha-quartz-Type GeO2. J. Phys. Chem. C 2016, 120, 126–132. [Google Scholar] [CrossRef]
- Hermet, P.; Aubry, J.P.; Haines, J.; Cambon, O. Origin and mechanism of the piezoelectricity in a-quartz-type MIIIXVO4 compounds (M = B, Al, Ga; X = P, As). J. Phys. Chem. C 2016, 120, 26645–26651. [Google Scholar] [CrossRef]
- Passaret, M.; Regreny, A.; Bayon, J.F.; Aumont, R.; Toudic, Y. Recrystallization of TiO2, GeO2, SiO2, Si(1-X)Ge(X)O2 in fluorinated hydrothermal solutions. J. Cryst. Growth 1971, 13, 524. [Google Scholar]
- Balitsky, V.S.; Balitsky, D.V.; Nekrasov, A.N.; Balitskaya, L.V. Growth and characterization of SixGe1-xO2 solid solution single crystals with quartz structure. J. Cryst. Growth 2005, 275, E807–E811. [Google Scholar] [CrossRef]
- Miller, W.S.; Roy, R.; Shafer, E.C.; Dachille, F. System GeO2-SiO2. Am. Mineral. 1963, 48, 1024. [Google Scholar]
- Ranieri, V.; Darracq, S.; Cambon, M.; Haines, J.; Cambon, O.; Largeteau, A.; Demazeau, G. Hydrothermal growth and structural studies of Si(1-x)Ge(x)O2 single crystals. Inorg. Chem. 2011, 50, 4632–4639. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.; Haines, J.; Cambon, O.; Levelut, C.; Le Parc, R.; Cambon, M.; Hazemann, J.L. In Situ X-ray absorption spectroscopy study of Si1-xGexO2 dissolution and Germanium aqueous speciation under hydrothermal conditions. Inorg. Chem. 2012, 51, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.; Bourgogne, D.; Darracq, S.; Cambon, M.; Haines, J.; Cambon, O.; Leparc, R.; Levelut, C.; Largeteau, A.; Demazeau, G. Raman scattering study of alpha-quartz and Si1-xGexO2 solid solutions. Phys. Rev. B 2009, 79, 224304. [Google Scholar] [CrossRef]
- Clavier, D.; Prakasam, M.; Largeteau, A.; Boy, J.J.; Hehlen, B.; Cambon, M.; Hermet, P.; Haines, J.; Cambon, O. Piezoelectric and non-linear optical properties of alpha-quartz type Si1-xGexO2 single crystals. Crystengcomm 2016, 18, 2500–2508. [Google Scholar] [CrossRef]
- Kosova, T.B.; Demyanets, L.N.; Uvarova, T.G. Study of germanium dioxide solubility in water at the 25-300-Degrees-C. Zhurnal Neorganicheskoi Khimii 1987, 32, 768–772. [Google Scholar]
- Demianets, L.N. Hydrothermal synthesis of new compounds. Prog. Cryst. Growth Charact. 1990, 21, 299–355. [Google Scholar] [CrossRef]
- Roy, R.; Theokrit, S. Crystal growth of metastable phases. J. Cryst. Growth 1972, 12, 69–72. [Google Scholar] [CrossRef]
- Balitsky, V.S.; Mahina, I.B. Patent N 461551, 1974. (In Russian)
- Kosova, T.B.; Demianets, L.N. Hydrothermal chemistry and growth of hexagonal germanium dioxide. In Growth of Crystals; Springer: New York, NY, USA, 1991; Volume 16, pp. 81–94. [Google Scholar]
- Balitsky, D.V.; Balitsky, V.S.; Pisarevsky, Y.V.; Philippot, E.; Silvestrova, O.Y.; Pushcharovsky, D.Y. Growth of germanium dioxide single crystals with alpha-quartz structure and investigation of their crystal structure, optical, elastic, piezoelectric, dielectric and mechanical properties. Ann. Chim. Sci. Mater. 2001, 26, 183–192. [Google Scholar] [CrossRef]
- Blomstrand, C.W. Om Westana Mineralier, Öfversigt at Kongliga Vetenskaps-Akademiens Handlingar; P. A. Norstedt & Söner: Stockholm, Sweden, 1868. [Google Scholar]
- Onac, B.P.; White, W.B. First reported sedimentary occurrence of berlinite (AlPO4) in phosphate-bearing sediments from Cioclovina Cave, Romania. Am. Mineral. 2003, 88, 1395–1397. [Google Scholar] [CrossRef]
- Jahn, V.W.; Kordes, E. Hydrothermal synthesis of large aluminum phosphate crystals. Chem. Earth 1953, 70, 75. [Google Scholar]
- Stanley, J.M. Hydrothermal synthesis of large aluminum phosphate crystals. Ind. Eng. Chem. 1954, 468, 1684–1689. [Google Scholar] [CrossRef]
- Chang, Z.P.; Barsch, G.R. Elastic constants and thermal expansion of berlinite. IEEE Trans. Sonics Ultrason. 1976, 23, 127–130. [Google Scholar] [CrossRef]
- Kolb, E.D.; Grenier, J.C.; Laudise, R.A. Solubility and Growth of AlPO4 in a hydrothermal solvent: HCl. J. Cryst. Growth 1981, 51, 178–182. [Google Scholar] [CrossRef]
- Kolb, E.D.; Laudise, R.A. Pressure volume temperature behavior in the system H2O-H3PO4-AlPO4 and its relationship to the hydrothermal growth of AlPO4. J. Cryst. Growth 1982, 56, 83–92. [Google Scholar] [CrossRef]
- Byrappa, K.; Venkatachalapathy, V.; Puttaraj, B. Crystallization of aluminum ortho-phosphate. J. Mater. Sci. 1984, 19, 2855–2862. [Google Scholar] [CrossRef]
- Laudise, R.A. Hydrothermal synthesis of inorganic solids. Abstr. Pap. Am. Chem. Soc. 1985, 189, 48. [Google Scholar]
- Byrappa, K. Recent progress inthe growth and characterization of aluminum orthophophate crystals. Prog. Cryst. Growth Charact. 1990, 21, 199–254. [Google Scholar] [CrossRef]
- Chai, B.H.T. Hydrothermal crystal growing process and apparatus. Patent US 4382840, 10 May 1983. [Google Scholar]
- Goiffon, A.; Jumas, J.C.; Avinens, C.; Philippot, E. Improving Berlinite crystal quality. Solubility and growth of Berlinite in sulfuric-acid. Rev. Chim. Miner. 1987, 24, 593–604. [Google Scholar]
- Jumas, J.C.; Goiffon, A.; Capelle, B.; Zarka, A.; Doukhan, J.C.; Schwartzel, J.; Detaint, J.; Philippot, E. Crystal growth of Berlinite, AlPO4—Physical characterization and comparison with Quartz. J. Cryst. Growth 1987, 80, 133–148. [Google Scholar] [CrossRef]
- Philippot, E.; Goiffon, A.; Maurin, M.; Detaint, J.; Schwartzel, J.C.; Toudic, Y.; Capelle, B.; Zarka, A. Evaluation of high-quality Berlinite crystals grown in sulfuric-acid medium. J. Cryst. Growth 1990, 104, 713–726. [Google Scholar] [CrossRef]
- Detaint, J.; Poignan, H.; Toudic, Y. Experimental thermal behavior of berlinite resonators. In 34th Symposium on Frequency Control; IEEE: Munchen, Germany, 1980; pp. 28–30. [Google Scholar]
- Detaint, J.; Philippot, E.; Jumas, J.C.; Schwartzel, J.; Zarka, A.; Capelle, B.; Doukhan, J.C. Crystal growth, physical characterization and BAW devices applications of berlinite. In Proceedings of the 3rd Annual Frequency Control Symposium, Philadelphia, PA, USA, 29–31 May 1985.
- Philippot, E.; Goiffon, A.; Maurin, M.; Cambon, O.; Ibanez, A.; Aubry, J.P. Procédé de Dissolution d’un Matériau Cristallin. Patent WO 1992009110 A1, 29 May 1992. [Google Scholar]
- Cambon, O.; Goiffon, A.; Ibanez, A.; Philippot, E. Kinetics and dissolution mechanism of berlinite, AlPO4, in acid medium. Eur. J. Sol. State Inorg. 1992, 29, 547–561. [Google Scholar]
- Cambon, O.; Goiffon, A.; Ibanez, A.; Philippot, E. Controlled dissolution of crystals: Application to Berlinite (α-AlPO4), a piezoelectric material. J. Solid State Chem. 1993, 103, 240–252. [Google Scholar] [CrossRef]
- Hirano, S.; Miwa, K.; Naka, S. Hydrothermal synthesis of gallium ortho-phosphate crystals. J. Cryst. Growth 1986, 79, 215–218. [Google Scholar] [CrossRef]
- Philippot, E.; Ibanez, A.; Goiffon, A.; Cochez, M.; Zarka, A.; Capelle, B.; Schwartzel, J.; Detaint, J. A quartz-like material—Gallium Phosphate (GaPO4): Crystal growth and characterization. J. Cryst. Growth 1993, 130, 195–208. [Google Scholar] [CrossRef]
- Cochez, M.; Foulon, J.D.; Ibanez, A.; Goiffon, A.; Philippot, E.; Capelle, B.; Zarka, A.; Schwartzel, J.; Detaint, J. Crystal growth and characterizations of α-quartz like material: GaPO4. J. Phys. IV 1994, 4, 183–188. [Google Scholar] [CrossRef]
- Cochez, M.; Ibanez, A.; Goiffon, A.; Philippot, E. Crystal growth and infrared characterization of GaPO4 in phospho-sulfuric media. Eur. J. Sol. State Inorg. 1993, 30, 509–519. [Google Scholar]
- Palmier, D.; Goiffon, A.; Capelle, B.; Detaint, J.; Philippot, E. Crystal growth and characterizations of quartz-like material: Gallium phosphate (GaPO4). J. Cryst. Growth 1996, 166, 347–353. [Google Scholar] [CrossRef]
- Yot, P.; Palmier, D.; Cambon, O.; Goiffon, A.; Pintard, M.; Philippot, E. Crystal growth and characterization of an alpha-quartz-like piezoelectric material, gallium orthophosphate. Ann. Chim. Sci. Mater. 1997, 22, 679–682. [Google Scholar]
- Cambon, O.; Yot, P.; Balitsky, D.; Goiffon, A.; Philippot, E.; Capelle, B.; Detaint, J. Crystal growth of GaPO4, a very promising material for manufacturing BAW devices. Ann. Chim. Sci Mater. 2001, 26, 79–84. [Google Scholar] [CrossRef]
- Yot, P.; Cambon, O.; Balitsky, D.; Goiffon, A.; Philippot, E.; Capelle, B.; Detaint, J. Advances in crystal growth and characterizations of gallium orthophosphate, GaPO4. J. Cryst. Growth 2001, 224, 294–302. [Google Scholar] [CrossRef]
- Yot, P. Matériaux piezoelectriques MIIIXVO4 avec M = Al, Ga, Fe et X = P, As isotypes du quartz alpha: Etude structurale comparée et prévision des propriétés—Maîtrise de la croissance cristalline de l’orthophosphate de gallium, Université de Montpellier, Montpellier, France, 1999.
- Capelle, B.; Zarka, A.; Schwartzel, J.; Detaint, J.; Philippot, E.; Denis, J.P. Characterization of piezoelectric materials—old and new Crystals. J. Phys. IV 1994, 4, 123–134. [Google Scholar] [CrossRef]
- Detaint, J.; Capelle, B.; Cambon, O.; Philippot, E. Gallium phosphate plane resonators and filters. In Proceedings of the 2003 IEEE International Frequency Control Symposium & Pda Exhibition Jointly with 17th European Frequency and Time Forum, Tampa, FL, USA, 4–8 May 2003; pp. 679–687.
- Prudhomme, N.; Flaud, V.; Papet, P.; Cambon, O.; Zaccaro, J.; Ibanez, A. Design of high frequency GaPO4 BAW resonators by chemical etching. Sens. Actuators B Chem. 2008, 131, 270–278. [Google Scholar] [CrossRef]
- Cambon, O.; Haines, J.; Fraysse, G.; Keen, D.A.; Tucker, M.G. Piezoelectric properties at high temperature in alpha-quartz materials. J. Phys. V 2005, 126, 27–30. [Google Scholar]
- Haines, J.; Cambon, O.; Prudhomme, N.; Fraysse, G.; Keen, D.A.; Chapon, L.C.; Tucker, M.G. High-temperature, structural disorder, phase transitions, and piezoelectric properties of GaPO4. Phys. Rev. B 2006, 73, 014103. [Google Scholar] [CrossRef]
- Krempl, P.W.; Krispel, F.; Wallnofer, W. Industrial development and prospects of GaPO4. Ann. Chim.Sci. Mater. 1997, 22, 623–626. [Google Scholar]
- Reiter, C.; Thanner, H.; Wallnofer, W.; Krempl, P.W. Properties of GaPO4 thickness shear resonators. Ann. Chim. Sci. Mater. 1997, 22, 633–636. [Google Scholar]
- Krempl, P.; Voborsky, G.; Posch, U.; Wallnofer, W. Hydrothermal Process for Growing Large Crystals or Crystal Layers. U.S. Patent 5375556, 27 December 1994. [Google Scholar]
- Philippot, E.; Armand, P.; Yot, P.; Cambon, O.; Goiffon, A.; McIntyre, G.J.; Bordet, P. Neutron and X-ray structure refinements between 15 and 1073 K of piezoelectric gallium arsenate, GaAsO4: Temperature and pressure behavior compared with other alpha-quartz materials. J. Solid State Chem. 1999, 146, 114–123. [Google Scholar] [CrossRef]
- Cambon, O.; Yot, P.; Rul, S.; Haines, J.; Philippot, E. Growth and dielectric characterization of large single crystals of GaAsO4, a novel piezoelectric material. Solid State Sci. 2003, 5, 469–472. [Google Scholar] [CrossRef]
- Cambon, O.; Haines, J.; Fraysse, G.; Detaint, J.; Capelle, B.; Van der Lee, A. Piezoelectric characterization and thermal stability of a high-performance alpha-quartz-type material, gallium arsenate. J. Appl. Phys. 2005, 97, 074110. [Google Scholar] [CrossRef]
- Cambon, O.; Bhalerao, G.M.; Bourgogne, D.; Haines, J.; Hermet, P.; Keen, D.A.; Tucker, M.G. Vibrational Origin of the Thermal Stability in the High-Performance Piezoelectric Material GaAsO4. J. Am. Chem. Soc. 2011, 133, 8048–8056. [Google Scholar] [CrossRef] [PubMed]
- Bhalerao, G.M.; Cambon, O.; Haines, J.; Levelut, C.; Mermet, A.; Sirotkin, S.; Menaert, B.; Debray, J.; Baraille, I.; Darrigan, C.; Rerat, M. Brillouin spectroscopy, calculated elastic and bond properties of GaAsO4. Inorg. Chem. 2010, 49, 9470–9478. [Google Scholar] [CrossRef] [PubMed]
- Souleiman, M.; Bhalerao, G.M.; Guillet, T.; Haidoux, A.; Cambon, M.; Levelut, C.; Haines, J.; Cambon, O. Hydrothermal growth of large piezoelectric single crystals of GaAsO4. J. Cryst. Growth 2014, 397, 29–38. [Google Scholar] [CrossRef]
- Hermet, P.; Souleiman, M.; Clavier, D.; Hehlen, B.; Levelut, C.; Sans, P.; Haines, J.; Cambon, O. GaAsO4: A bifunctional material for piezoelectricity and second harmonic generation. J. Phys. Chem. C 2015, 119, 8459–8464. [Google Scholar] [CrossRef]
- Roncal-Herrero, T.; Rodriguez-Blanco, J.D.; Benning, L.G.; Oelkers, E.H. Precipitation of iron and aluminum phosphates directly from aqueous solution as a function of temperature from 50 to 200 °C. Cryst. Growth. Des. 2009, 9, 5197–5205. [Google Scholar] [CrossRef]
- Zaghib, K.; Julien, C.M. Structure and electrochemistry of FePO4 center dot 2H2O hydrate. J. Power Sources 2005, 142, 279–284. [Google Scholar] [CrossRef]
- Smirnov, M.; Mazhenov, N.; Aliouane, N.; Saint-Gregoire, P. Novel features of the alpha-beta phase transition in quartz-type FePO4 as evidenced by X-ray diffraction and lattice dynamics. J. Phys. Condens. Mater. 2010, 22, 225403. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W. Synthesis of nanorods FePO4 via a facile route. J. Nanopart. Res. 2010, 12, 2003–2006. [Google Scholar] [CrossRef]
- Song, Y.N.; Yang, S.F.; Zavalij, P.Y.; Whittingham, M.S. Temperature-dependent properties of FePO4 cathode materials. Mater. Res. Bull. 2002, 37, 1249–1257. [Google Scholar] [CrossRef]
- Souleiman, M.; Hermet, P.; Haidoux, A.; Levelut, C.; Haines, J.; Cambon, O. Combined experimental and theoretical Raman scattering studies of alpha-quartz-type FePO4 and GaPO4 end members and Ga1-xFexPO4 solid solutions. RSC Adv. 2013, 3, 22078–22086. [Google Scholar] [CrossRef]
- Cachau-Herreillat, D.; Bennazha, J.; Goiffon, A.; Ibanez, A.; Philippot, E. X-ray, DTA and crystal growth investigation on AlPO4-GaPO4 and AlPO4-AlAsO4 systems. Eur. J. Solid State Inorg. Chem. 1992, 29, 1295. [Google Scholar]
- Xia, H. R.; Qin, Z.K.; Yuan, W.; Liu, S.F.; Zou, Z.Q.; Han, J.R. Growth and properties of trigonal aluminium gallium orthophosphate single crystals Al1-xGaxPO4. Cryst. Res. Technol. 1997, 32, 783–788. [Google Scholar] [CrossRef]
- Xia, H.R.; Wang, J.Y.; Li, L.X.; Zou, Z.Q. Growth and Raman scattering of aluminium gallium orthophosphate piezoelectric crystals. Prog. Cryst. Growth Charact. Mater. 2000, 40, 253–261. [Google Scholar] [CrossRef]
- Barz, R.U.; David, F.; Schneider, J.; Gille, P. Polymorph stability and phase transitions of trigonal Al1-xGaxPO4 mixed crystals. Z. Kristallogr. 2001, 216, 501–508. [Google Scholar] [CrossRef]
- Haines, J.; Cambon, O.; Fraysse, G.; van der Lee, A. An X-ray powder diffraction study of the high temperature phase transitions in alpha-quartz-type AlPO4-GaPO4 solid solutions. J. Phys. Condens. Mater. 2005, 17, 4463–4474. [Google Scholar] [CrossRef]
- Cambon, O.; Haines, J.; Cambon, M.; Keen, D.A.; Tucker, M.G.; Chapon, L.; Hansen, N.K.; Souhassou, M.; Porcher, F. Effect of Ga content on the instantaneous structure of Al(1-x)GaxPO4 solid solutions at high temperature. Chem. Mater. 2009, 21, 237–246. [Google Scholar] [CrossRef]
- Souleiman, M. Studies of M(1-x)M’xXO4 Solid Solutions of α-Quartz Homeotypes and Crystal Growth of GaAsO4, a Bifunctional Material with Piezoelectric and Non-Linear Optics Properties. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 2013. [Google Scholar]
- Souleiman, M.; Cambon, O.; Haidoux, A.; Haines, J.; Levelut, C.; Ranieri, V.; Hazemann, J.L. Study of Ga3+-induced hydrothermal crystallization of an alpha-quartz type Ga1-xFexPO4 single crystal by in-situ X-ray absorption spectroscopy (XAS). Inorg. Chem. 2012, 51, 11811–11819. [Google Scholar] [CrossRef] [PubMed]
- Clavier, D. Croissance hydrothermale de monocristaux isotypes du quartz-α, étude des propriétés physiques et recherche de nouvelles solutions solides avec des oxydes du bloc p (Ge, Sn) et du bloc d (Mn, V, Ti). Ph.D. Thesis, Université de Montpellier, Montpellier, France, 2015. [Google Scholar]

| Compound | Phase Transitions |
|---|---|
| SiO2 | α-Quartz β-Quartz Tridymite β-Cristobalite liquid |
| GeO2 | β-Rutile α-Quartz Liquid |
| AlPO4 | α-Quartz β-Quartz Tridymite β-Cristobalite liquid |
| FePO4 | α-Quartz β-Quartz Liquid |
| GaPO4 | α-Quartz β-Cristobalite Liquid |
| GaAsO4 | α-Quartz Chemical decomposition |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cambon, O.; Haines, J. Hydrothermal Crystal Growth of Piezoelectric α-Quartz Phase of AO2 (A = Ge, Si) and MXO4 (M = Al, Ga, Fe and X = P, As): A Historical Overview. Crystals 2017, 7, 38. https://doi.org/10.3390/cryst7020038
Cambon O, Haines J. Hydrothermal Crystal Growth of Piezoelectric α-Quartz Phase of AO2 (A = Ge, Si) and MXO4 (M = Al, Ga, Fe and X = P, As): A Historical Overview. Crystals. 2017; 7(2):38. https://doi.org/10.3390/cryst7020038
Chicago/Turabian StyleCambon, Olivier, and Julien Haines. 2017. "Hydrothermal Crystal Growth of Piezoelectric α-Quartz Phase of AO2 (A = Ge, Si) and MXO4 (M = Al, Ga, Fe and X = P, As): A Historical Overview" Crystals 7, no. 2: 38. https://doi.org/10.3390/cryst7020038







