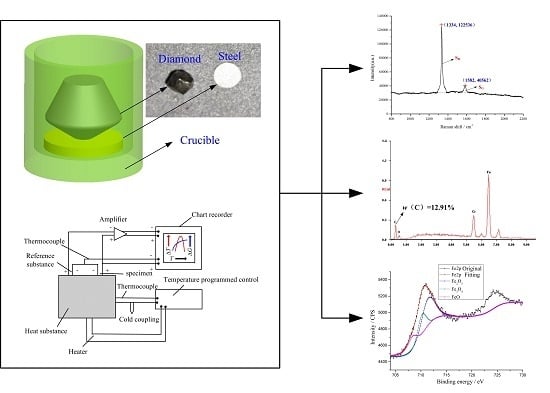
Thermochemical Wear of Single Crystal Diamond Catalyzed by Ferrous Materials at Elevated Temperature
Abstract
:1. Introduction
2. Materials and Methods
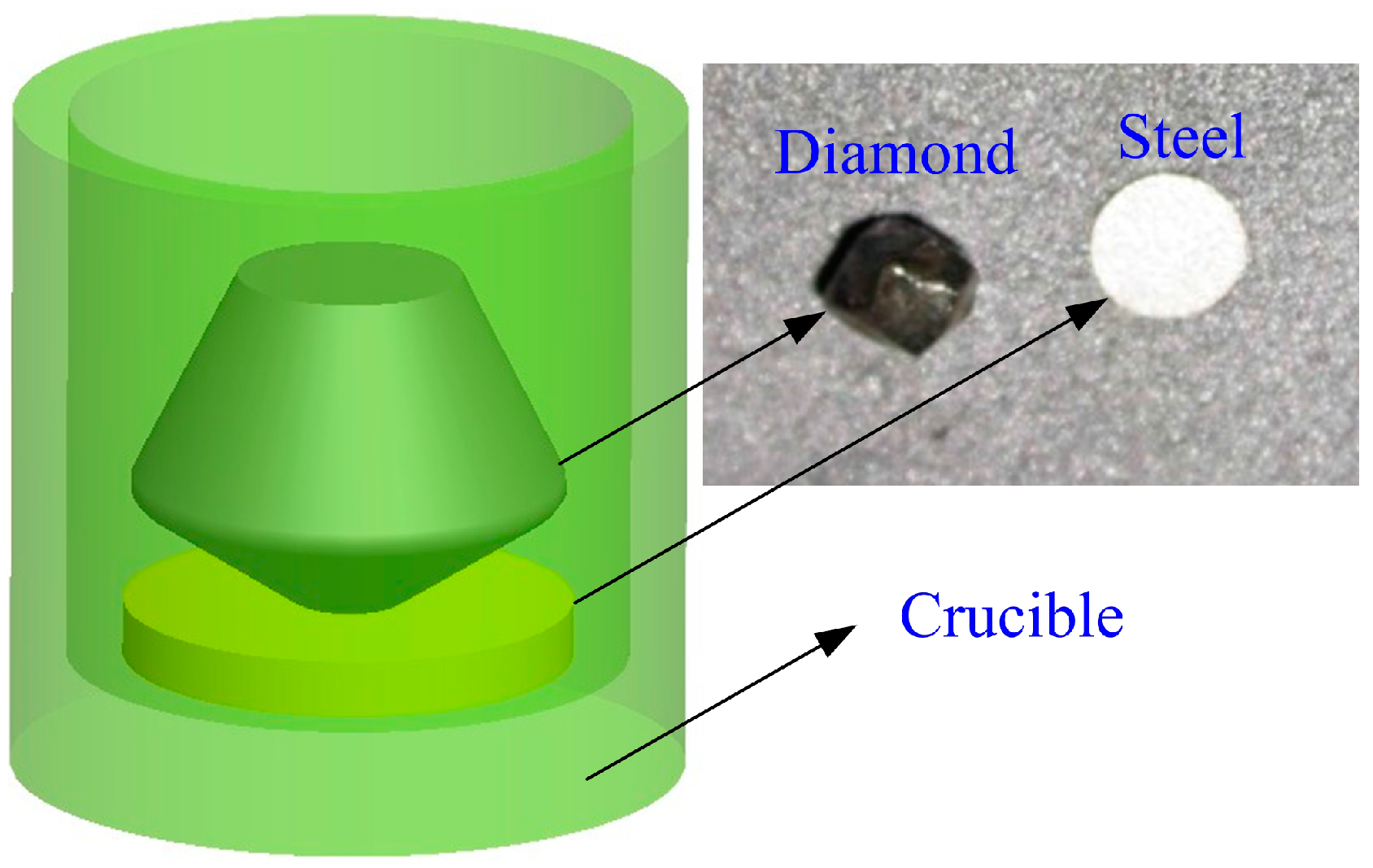
2.1. Sample Preparation
2.2. Characterization
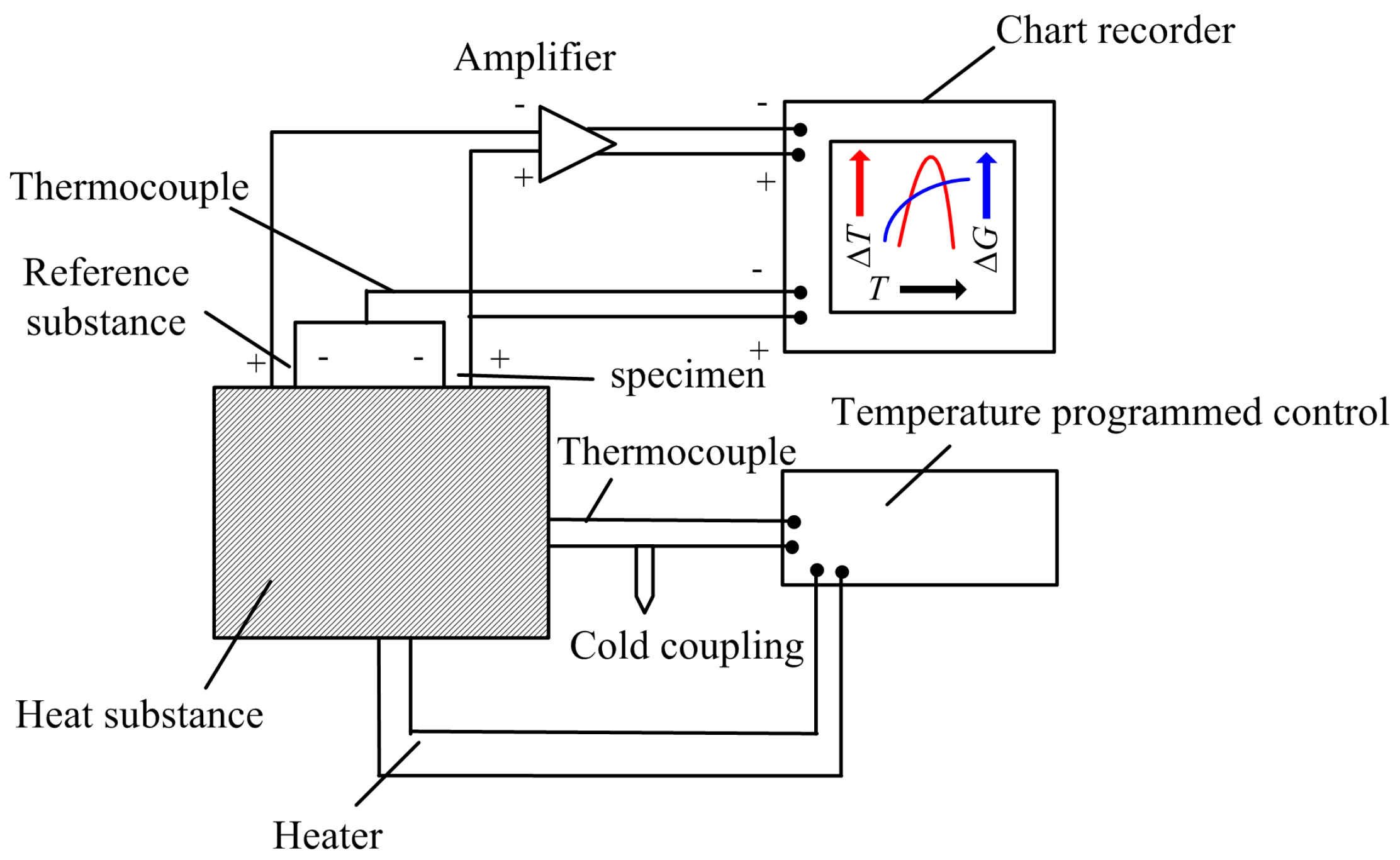
2.2.1. Experimental Procedure
2.2.2. Analytic Technique
3. Results and Discussion
3.1. Thermal Analysis Results
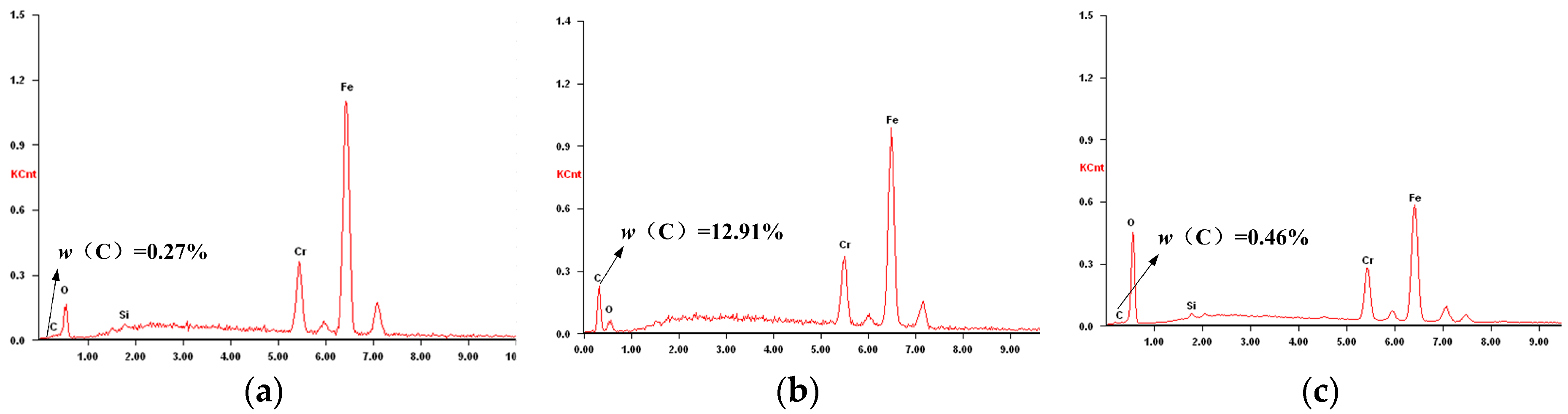
3.2. Thermochemical Wear Mechanisms
3.2.1. Graphitization Wear
3.2.2. Diffusion Wear
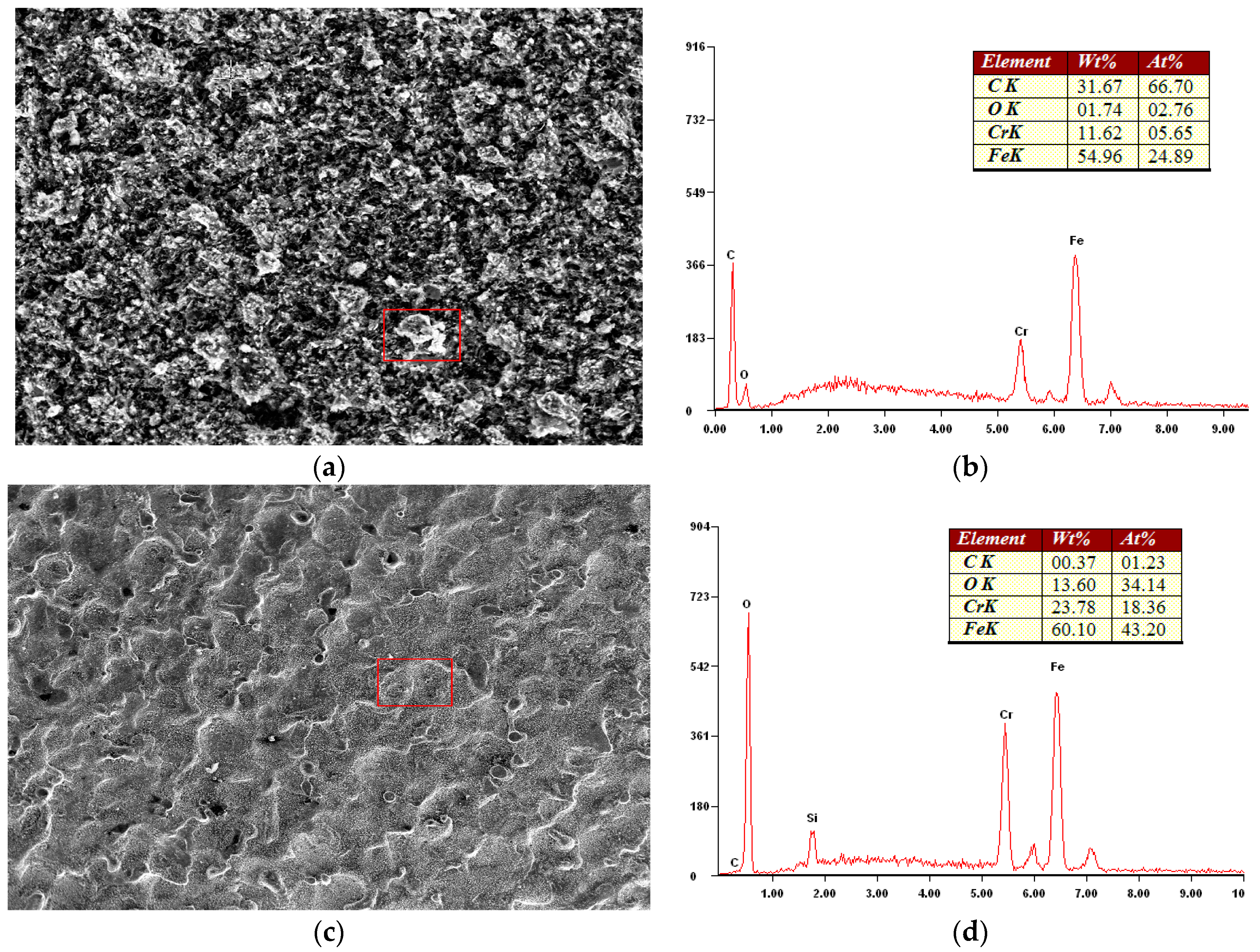
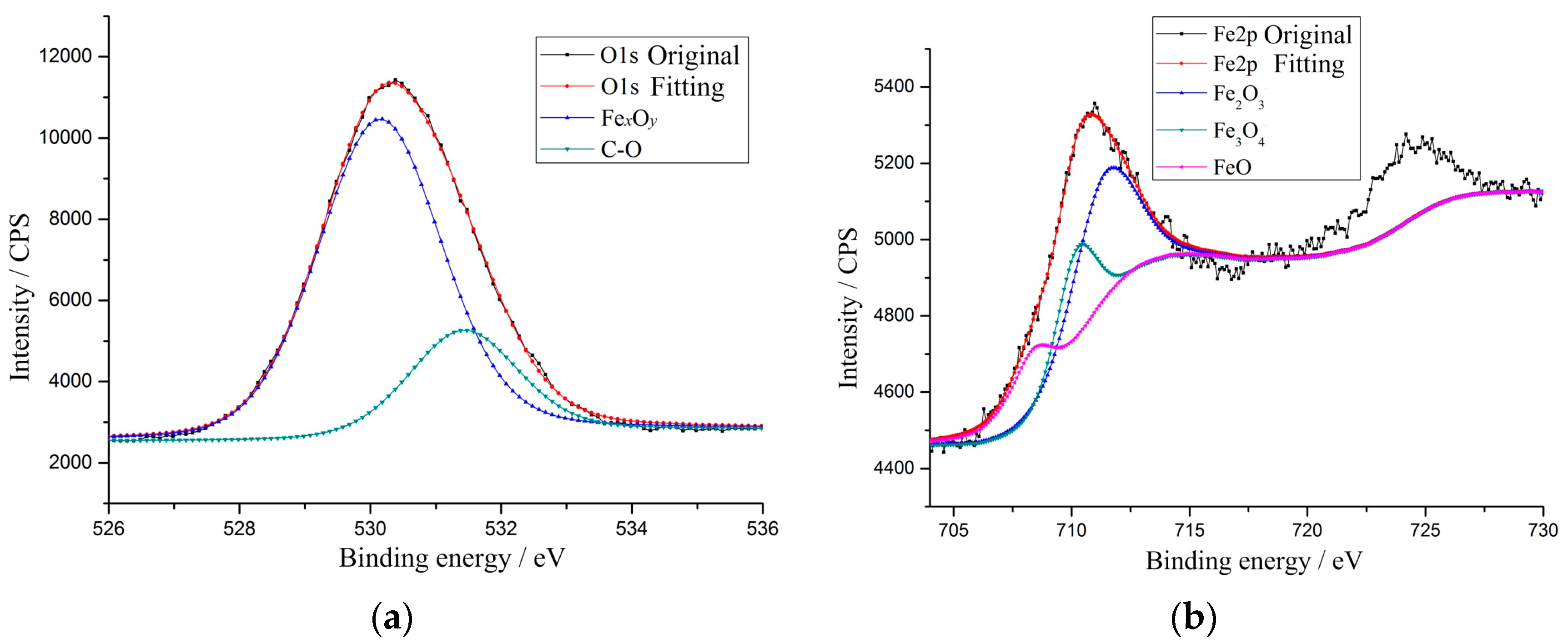
3.2.3. Oxidation Wear
4. Conclusions
- (1)
- Diamond thermochemical wear includes the graphitization, diffusion and oxidation, and graphitization is the main wear mechanism. Temperature should be considered as the key factor influencing these wear mechanisms.
- (2)
- The initial graphitization temperatures of diamond catalyzed by ferrous materials are obtained. The graphitized degree is observably intensified with the rising temperature, and it relies heavily on the diamond crystallographic plane while being insensitive to the work material.
- (3)
- The diffusion wear rule is preliminarily achieved by the established prediction model, and this can be used as fundamental research for further investigation of diamond diffusion wear in combination with the reasonable experimental studies. Additionally, more attention should be paid to reducing the temperature and designing a diffusion barrier at the interface to suppress it.
- (4)
- It is confirmed that the possibility of diamond reacting directly with iron to cause tool wear is relatively small by comparing with thermal analysis curves in different atmospheres. However, the presence of oxygen will lead to the occurrence of a large number of redox reactions at the interface, resulting in diamond oxidation wear.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheung, C.F.; Lee, W.B. Characterisation of nanosurface generation in single-point diamond turning. Int. J. Mach. Tools Manuf. 2001, 41, 851–875. [Google Scholar] [CrossRef]
- Fang, F.Z.; Venkatesh, V.C. Diamond turning of soft semiconductors to obtain nanometric mirror surfaces. Int. J. Adv. Manuf. Technol. 2002, 19, 637–641. [Google Scholar] [CrossRef]
- Ramanujachar, K.; Subramanian, S.V. Micromechanisms of tool wear in machining free cutting steels. Wear 1996, 197, 45–55. [Google Scholar] [CrossRef]
- Lane, B.M.; Dow, T.A.; Scattergood, R. Thermo-chemical wear model and worn tool shapes for single-crystal diamond tools cutting steel. Wear 2013, 300, 216–224. [Google Scholar] [CrossRef]
- Venkatesh, V.; Swain, N.; Srinivas, G.; Kumar, P.; Barshilia, H.C. Review on the machining characteristics and research prospects of conventional microscale machining operations. Mater. Manuf. Process. 2016. [Google Scholar] [CrossRef]
- Casstevens, J.M. Diamond turning of steel in carbon-saturated atmospheres. Precis. Eng. 1983, 5, 9–15. [Google Scholar] [CrossRef]
- Hitchiner, M.P. Factors affecting chemical wear during machining. Wear 1984, 93, 63–80. [Google Scholar] [CrossRef]
- Evans, C. Cryogenic diamond turning of stainless steel. CIRP Ann. Manuf. Technol. 1991, 40, 571–575. [Google Scholar] [CrossRef]
- Song, Y.C.; Nezu, K.; Park, C.H.; Moriwaki, T. Tool wear control in single-crystal diamond cutting of steel by using the ultra-intermittent cutting method. Int. J. Mach. Tools Manuf. 2009, 49, 339–343. [Google Scholar] [CrossRef]
- Shamoto, E.; Suzuki, N. Ultrasonic vibration diamond cutting and ultrasonic elliptical vibration cutting. Compr. Mater. Process. 2014, 11, 405–454. [Google Scholar]
- Brinksmeier, E.; Gläbe, R.; Osmer, J. Ultra-precision diamond cutting of steel molds. CIRP Ann. Manuf. Technol. 2006, 55, 551–554. [Google Scholar] [CrossRef]
- Inada, A.; Min, S.; Ohmori, H. Micro cutting of ferrous materials using diamond tool under ionized coolant with carbon particles. CIRP Ann. Manuf. Technol. 2011, 60, 97–100. [Google Scholar] [CrossRef]
- Thornton, A.G.; Wilks, J. The wear of diamond tools turning mild steel. Wear 1980, 65, 67–74. [Google Scholar] [CrossRef]
- Paul, E.; Evans, C.J. Chemical aspects of tool wear in single point diamond turning. Precis. Eng. 1996, 18, 4–19. [Google Scholar] [CrossRef]
- Tanaka, H.; Shimada, S.; Ikawa, N.; Yoshinaga, M. Wear mechanism of diamond cutting tool in machining of steel. Key Eng. Mater. 2001, 196, 69–78. [Google Scholar] [CrossRef]
- Shimada, S.; Tanaka, H.; Higuchi, M. Thermo-chemical wear mechanism of diamond tool in machining of ferrous metals. CIRP Ann. Manuf. Technol. 2004, 53, 57–60. [Google Scholar] [CrossRef]
- Narulkar, R.; Bukkapatnam, S.; Raff, L.M.; Komanduri, R. Graphitization as a precursor to wear of diamond in machining pure iron: A molecular dynamics investigation. Comput. Mater. Sci. 2009, 45, 358–366. [Google Scholar] [CrossRef]
- Zou, L.; Dong, G.J.; Zhou, M. Investigation on frictional wear of single crystal diamond against ferrous metals. Int. J. Refract. Met. Hard Mater. 2013, 41, 174–179. [Google Scholar] [CrossRef]
- Jen, F.L.; Jia, W.L.; Pal, J.W. Thermal analysis for graphitization and ablation depths of diamond films. Diam. Relat. Mater. 2006, 15, 1–9. [Google Scholar]
- Chen, Z.C.; Subhash, G.; Tulenko, J.S. Raman spectroscopic investigation of graphitization of diamond during spark plasma sintering UO2-diamond composite nuclear fuel. J. Nucl. Mater. 2016, 475, 1–5. [Google Scholar] [CrossRef]
- Berman, R. The diamond-graphite equilibrium calculation: The influence of a recent determination of the Gibbs energy difference. Solid State Commun. 1996, 99, 35–37. [Google Scholar] [CrossRef]
- Zou, L.; Zhou, M. Experimental investigation and numerical simulation on interfacial carbon diffusion of diamond tool and ferrous metals. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2016, 31, 307–314. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]



| Workpiece | Chemical Compositions (ω%, Balance Fe) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Mn | Si | S | P | Ni | Cr | Ti | Mo | |
| 0Cr18Ni9 | 0.045 | 1.13 | 0.35 | 0.003 | 0.033 | 8.04 | 17.10 | -- | 0.03 |
| 1Cr18Ni9Ti | 0.07 | 1.21 | 0.37 | 0.01 | 0.03 | 8.07 | 17.99 | 0.40 | -- |
| 2Cr13 | 0.27 | 0.39 | 0.56 | 0.01 | 0.02 | 0.14 | 12.40 | -- | -- |
| 3Cr13 | 0.29 | 0.21 | 0.89 | 0.004 | 0.021 | 0.21 | 12.47 | -- | -- |
| Parameters | Value |
|---|---|
| Heating range (K) | 293~1273/1573 |
| Heating rate (K/min) | 10 |
| Gas flow rate (mL/min) | 40 |
| Gas atmosphere | argon/air |
| Polished plane of diamond | (100)/(110) |
| Workpiece materials | 0Cr18Ni9/1Cr18Ni9Ti/2Cr13/3Cr13 |
| SG/SD (%) | 0Cr18Ni9 | 1Cr18Ni9Ti | 2Cr13 | 3Cr13 | |
|---|---|---|---|---|---|
| Argon | (100) | 24.6 | 24.3 | 23.8 | 24.1 |
| (110) | 18.9 | 17.7 | 18.5 | 17.8 | |
| Air | (100) | 45.8 | 45.1 | 44.2 | 44.5 |
| (110) | 36.5 | 37.4 | 36.8 | 36.2 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, L.; Huang, Y.; Zhou, M.; Xiao, G. Thermochemical Wear of Single Crystal Diamond Catalyzed by Ferrous Materials at Elevated Temperature. Crystals 2017, 7, 116. https://doi.org/10.3390/cryst7040116
Zou L, Huang Y, Zhou M, Xiao G. Thermochemical Wear of Single Crystal Diamond Catalyzed by Ferrous Materials at Elevated Temperature. Crystals. 2017; 7(4):116. https://doi.org/10.3390/cryst7040116
Chicago/Turabian StyleZou, Lai, Yun Huang, Ming Zhou, and Guijian Xiao. 2017. "Thermochemical Wear of Single Crystal Diamond Catalyzed by Ferrous Materials at Elevated Temperature" Crystals 7, no. 4: 116. https://doi.org/10.3390/cryst7040116