HPHT Diamond Crystallization in the Mg-Si-C System: Effect of Mg/Si Composition
Abstract
:1. Introduction
2. Results
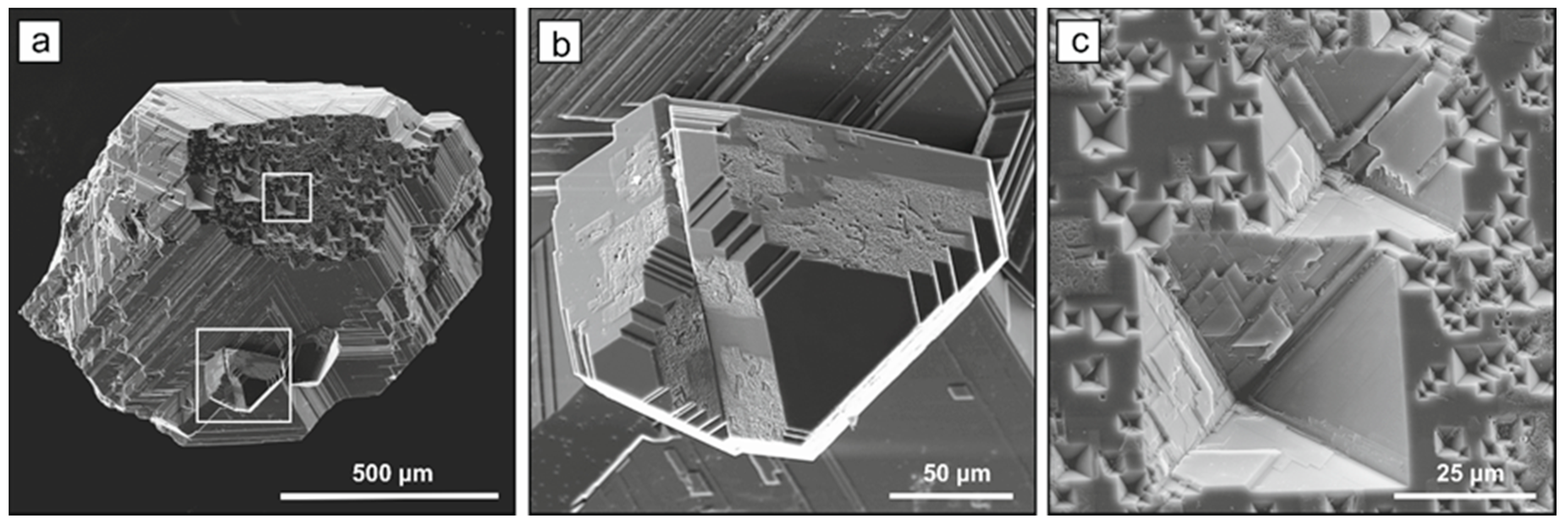
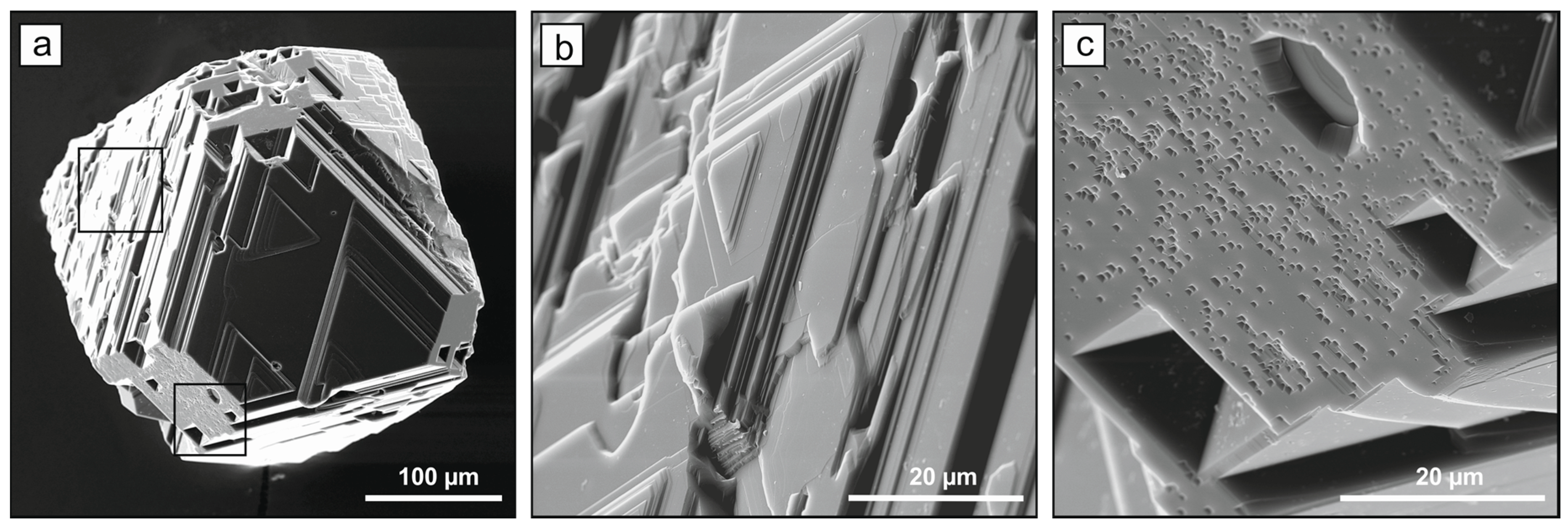
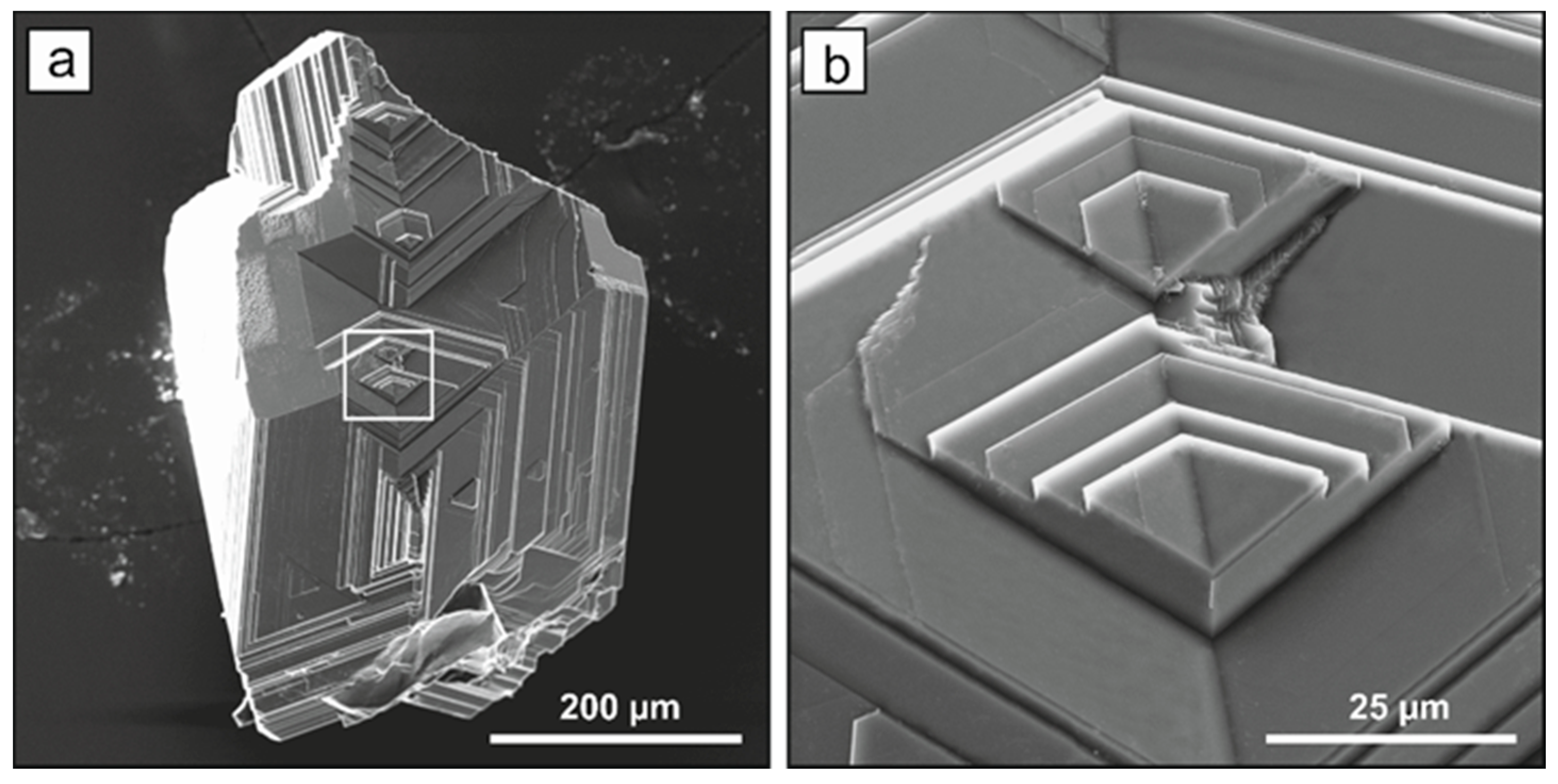
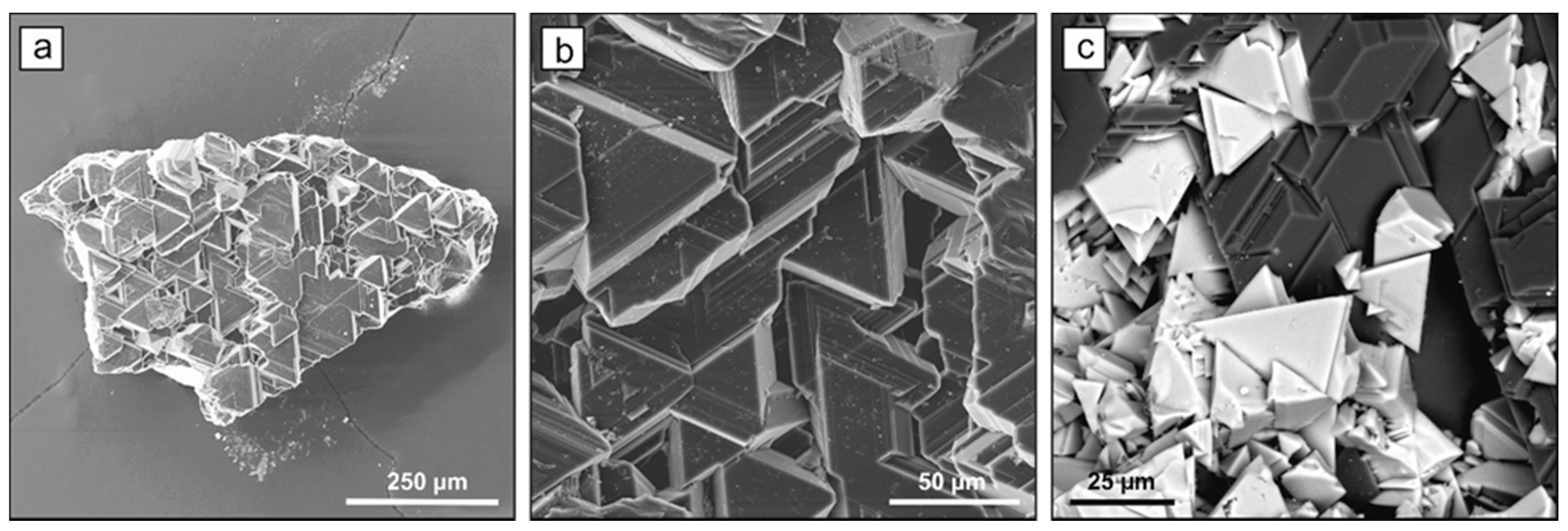
2.1. Diamond Crystallization
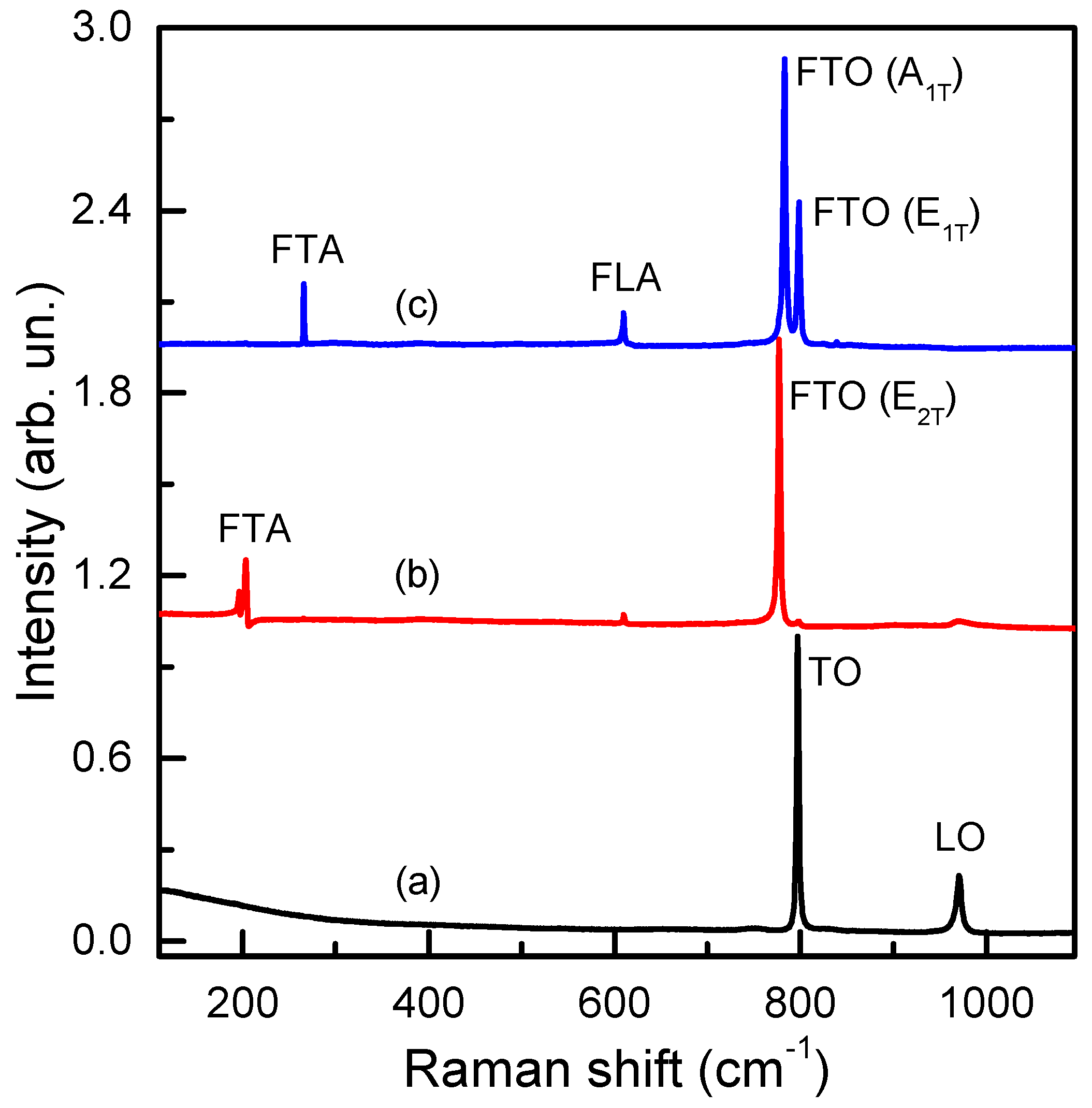
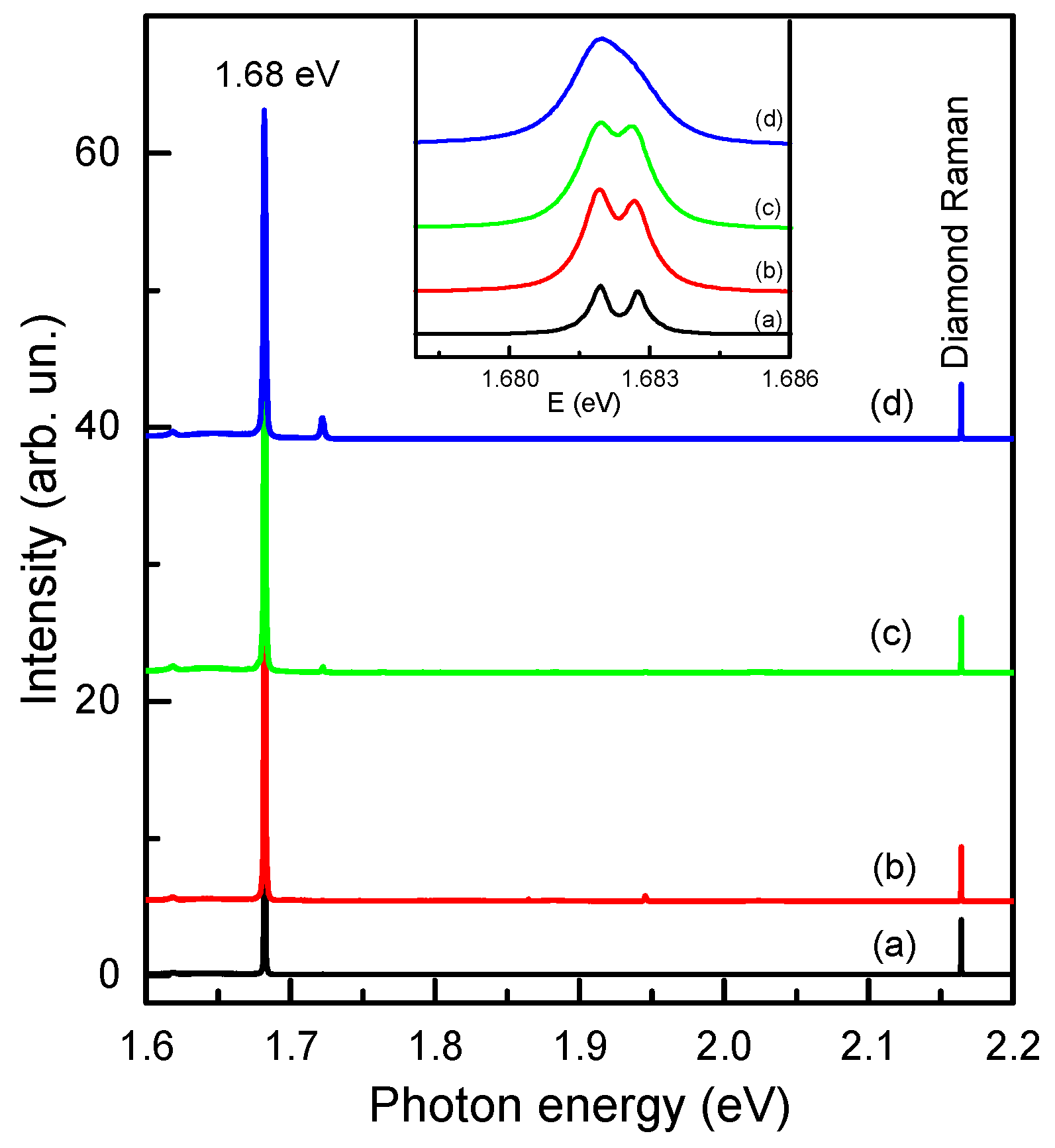
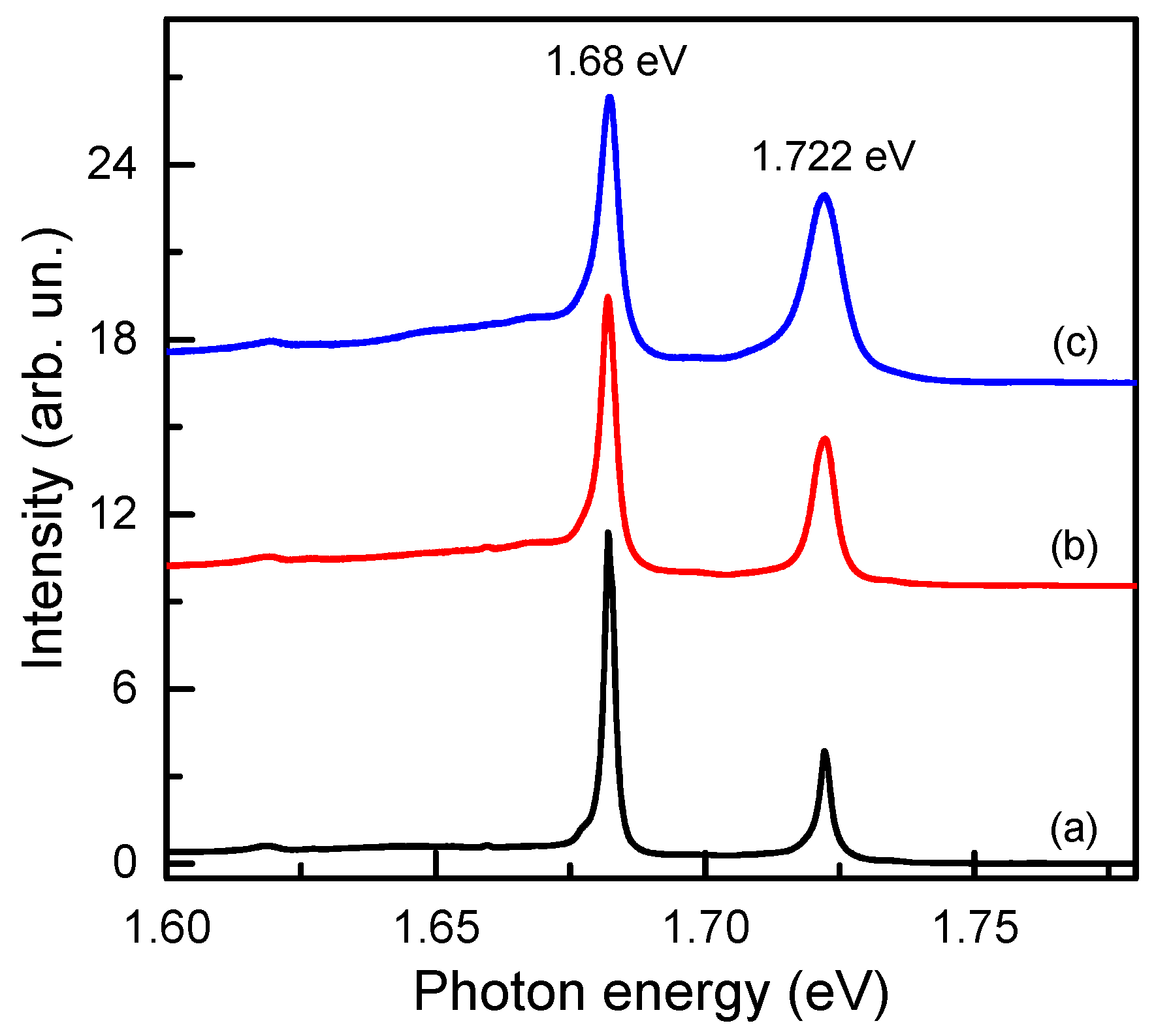
2.2. Spectroscopic Characterization
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bundy, F.P.; Hall, H.T.; Strong, H.M.; Wentorf, J.R. Man-made diamonds. Nature 1955, 176, 51–55. [Google Scholar] [CrossRef]
- Bovenkerk, H.P.; Bundy, F.P.; Hall, H.T.; Strong, H.M.; Wentorf, J.R. Preparation of diamond. Nature 1959, 184, 1094–1098. [Google Scholar] [CrossRef]
- Kanda, H. Classification of the catalysts for diamond growth. In Advances in New Diamond Science and Technology; Saito, S., Fujimori, N., Fukunaga, O., Kamo, M., Kobashi, K., Yoshikawa, M., Eds.; MYU: Tokyo, Japan, 1994; pp. 507–512. [Google Scholar]
- Wedlake, R.J. Technology of diamond growth. In The properties of diamond; Field, J.E., Ed.; Academic Press: London, UK, 1979; pp. 501–535. [Google Scholar]
- Burns, R.C.; Davies, G.J. Growth of synthetic diamond. In The Properties of Natural and Synthetic Diamond; Field, J.E., Ed.; Academic Press: London, UK, 1992; pp. 395–422. [Google Scholar]
- Palyanov, Y.; Kupriyanov, I.; Khokhryakov, A.; Ralchenko, V. Crystal Growth of Diamond. In Handbook of Crystal Growth, 2nd ed.; Nishinaga, T., Rudolph, P., Eds.; Elsevier: Amsterdam, Holland, 2015; Volume 2a, pp. 671–713. [Google Scholar]
- Ekimov, E.A.; Sidorov, V.A.; Bauer, E.D.; Mel’nik, N.N.; Curro, N.J.; Thompson, J.D.; Stishov, S.M. Superconductivity in diamond. Nature 2004, 428, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Akaishi, M.; Kanda, H.; Yamaoka, S. Phosphorous: An elemental catalyst for diamond synthesis and growth. Science 1993, 259, 1592–1593. [Google Scholar] [CrossRef] [PubMed]
- Palyanov, Y.N.; Kupriyanov, I.N.; Sokol, A.G.; Khokhryakov, A.F.; Borzdov, Y.M. Diamond growth from a phosphorus-carbon system at HPHT conditions. Cryst. Growth Des. 2011, 11, 2599–2605. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Kupriyanov, I.N.; Borzdov, Y.M.; Surovtsev, N.V. Germanium: A new catalyst for diamond synthesis and a new optically active impurity in diamond. Sci. Rep. 2015, 5, 14789. [Google Scholar] [CrossRef] [PubMed]
- Palyanov, Y.N.; Borzdov, Y.M.; Kupriyanov, I.N.; Khokhryakov, A.F.; Nechaev, D.V. Diamond crystallization from an Mg-C system at high pressure high temperature conditions. CrystEngComm 2015, 17, 4928–4936. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Kupriyanov, I.N.; Borzdov, Y.M.; Bataleva, Y.V. High-pressure synthesis and characterization of diamond from an Mg–Si–C system. CrystEngComm 2015, 17, 7323–7331. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Kupriyanov, I.N.; Borzdov, Y.M.; Khokhryakov, A.F.; Surovtsev, N.V. High-pressure synthesis and characterization of Ge-doped single crystal diamond. Cryst. Growth Des. 2016, 16, 3510–3518. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Sokol, A.G.; Borzdov, Y.M.; Palyanov, Y.N. Morphology of diamond crystals grown in magnesium-based systems at high temperatures and high pressures. J. Cryst. Growth 2015, 426, 276–282. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Nechaev, D.V.; Palyanov, Y.N. Unusual growth macrolayers on {100} faces of diamond crystals from magnesium-based systems. J. Cryst. Growth 2016, 455, 76–82. [Google Scholar] [CrossRef]
- Müller, T.; Hepp, C.; Pingault, B.; Neu, E.; Gsell, S.; Schreck, M.; Sternschulte, H.; Steinmüller-Nethl, D.; Becher, C.; Atatüre, M. Optical signatures of silicon-vacancy spins in diamond. Nat. Commun. 2014, 5, 3328. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Ishibashi, F.; Miyamoto, Y.; Doi, Y.; Kobayashi, S.; Miyazaki, T.; Tahara, K.; Jahnke, K.D.; Rogers, L.J.; Naydenov, B.; et al. Germanium-vacancy single color centers in diamond. Sci. Rep. 2015, 5, 12882. [Google Scholar] [CrossRef] [PubMed]
- Quantum Information Processing with Diamond; Prawer, S.; Aharonovich, I. (Eds.) Woodhead Publishing: Cambridge, UK, 2014; p. 330. [Google Scholar]
- Kovalenko, T.V.; Ivakhnenko, S.A. Properties of diamonds seed-grown in the magnesium-carbon system. J. Superhard Mater. 2013, 35, 131–136. [Google Scholar] [CrossRef]
- Collins, A.T.; Williams, A.W.S. The nature of the acceptor centre in semiconducting diamond. J. Phys. C Solid State Phys. 1971, 4, 1789–1800. [Google Scholar] [CrossRef]
- Goss, J.P.; Briddon, P.R.; Shaw, M.J. Density functional simulations of silicon-containing point defects in diamond. Phys. Rev. B 2007, 76, 075204. [Google Scholar] [CrossRef]
- Breeding, C.M.; Wang, W. Occurrence of the Si–V defect center in natural colorless gem diamonds. Diam. Relat. Mater. 2008, 17, 1335–1344. [Google Scholar] [CrossRef]
- Sugiyama, S.; Togaya, M. Phase relationship between 3C-and 6H-silicon carbide at high pressure and high temperature. J. Am. Ceram. Soc. 2001, 84, 3013–3016. [Google Scholar] [CrossRef]
- Jepps, N.W.; Page, T.F. The 6H→3C “reverse” transformation in silicon carbide compacts. J. Am. Ceram. Soc. 1981, 64, 2830–2833. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Khokhryakov, A.F. Fluid-bearing alkaline-carbonate melts as the medium for the formation of diamonds in the Earth’s mantle: an experimental study. Lithos 2002, 60, 145–159. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Khokhryakov, A.F.; Kupriyanov, I.N.; Sokol, A.G. Effect of nitrogen impurity on diamond crystal growth processes. Cryst. Growth Des. 2010, 10, 3169–3175. [Google Scholar] [CrossRef]
- Sokol, A.G.; Borzdov, Y.M.; Palyanov, Y.N.; Khokhryakov, A.F. High temperature calibration a multi-anvil high-pressure apparatus. High Press. Res. 2015, 35, 139–147. [Google Scholar] [CrossRef]
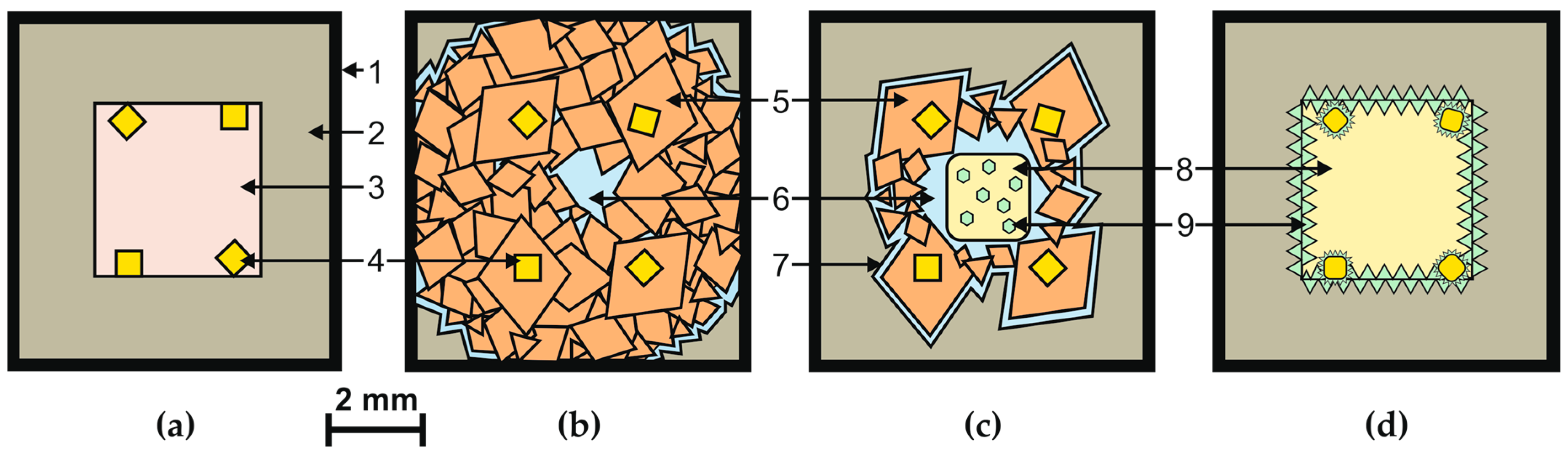



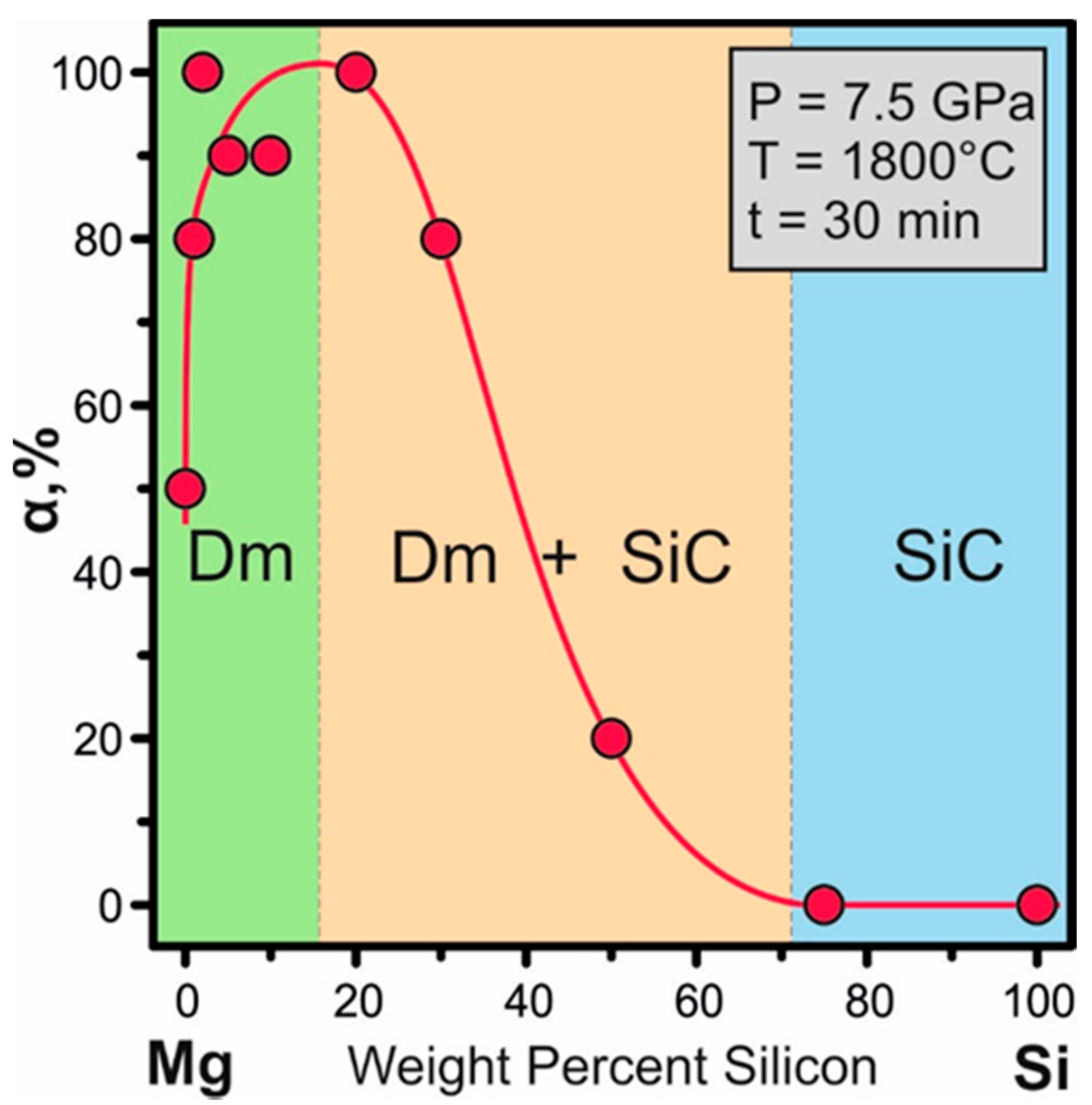
| Run N | P, GPa | T, °C | Time, min | Composition, wt % | α Gr → Dm Conversion | Diamond Morphology | SiC |
|---|---|---|---|---|---|---|---|
| MS-1 | 7.5 | 1800 | 30 | Mg | 50 | {100}, {100}>>{111} | - |
| MS-2 | 7.5 | 1800 | 30 | Mg99Si1 | 80 | {111}≈{100} | - |
| MS-3 | 7.5 | 1800 | 30 | Mg98Si2 | 100 | {111}>{100} | - |
| MS-4 | 7.5 | 1800 | 30 | Mg95Si5 | 90 | {111}>>{100} | - |
| MS-5 | 7.5 | 1800 | 30 | Mg90Si10 | 90 | {111}>>{100} | - |
| MS-6 | 7.5 | 1800 | 30 | Mg80Si20 | 100 | {111}>>{100} | 3C |
| MS-7 | 7.5 | 1800 | 30 | Mg70Si30 | 80 | {111} | 3C |
| MS-8 | 7.5 | 1800 | 30 | Mg50Si50 | 20 | {111} | 3C, 4H |
| MS-9 | 7.5 | 1800 | 30 | Mg25Si75 | 0 | - | 3C |
| MS-10 | 7.5 | 1800 | 30 | Si | 0 | - | 3C |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palyanov, Y.; Kupriyanov, I.; Borzdov, Y.; Nechaev, D.; Bataleva, Y. HPHT Diamond Crystallization in the Mg-Si-C System: Effect of Mg/Si Composition. Crystals 2017, 7, 119. https://doi.org/10.3390/cryst7050119
Palyanov Y, Kupriyanov I, Borzdov Y, Nechaev D, Bataleva Y. HPHT Diamond Crystallization in the Mg-Si-C System: Effect of Mg/Si Composition. Crystals. 2017; 7(5):119. https://doi.org/10.3390/cryst7050119
Chicago/Turabian StylePalyanov, Yuri, Igor Kupriyanov, Yuri Borzdov, Denis Nechaev, and Yuliya Bataleva. 2017. "HPHT Diamond Crystallization in the Mg-Si-C System: Effect of Mg/Si Composition" Crystals 7, no. 5: 119. https://doi.org/10.3390/cryst7050119





