Crystallography and Growth of Epitaxial Oxide Films for Fundamental Studies of Cathode Materials Used in Advanced Li-Ion Batteries
Abstract
:1. Introduction
2. Crystallographic Information on the Structure of Oxide Cathode Materials
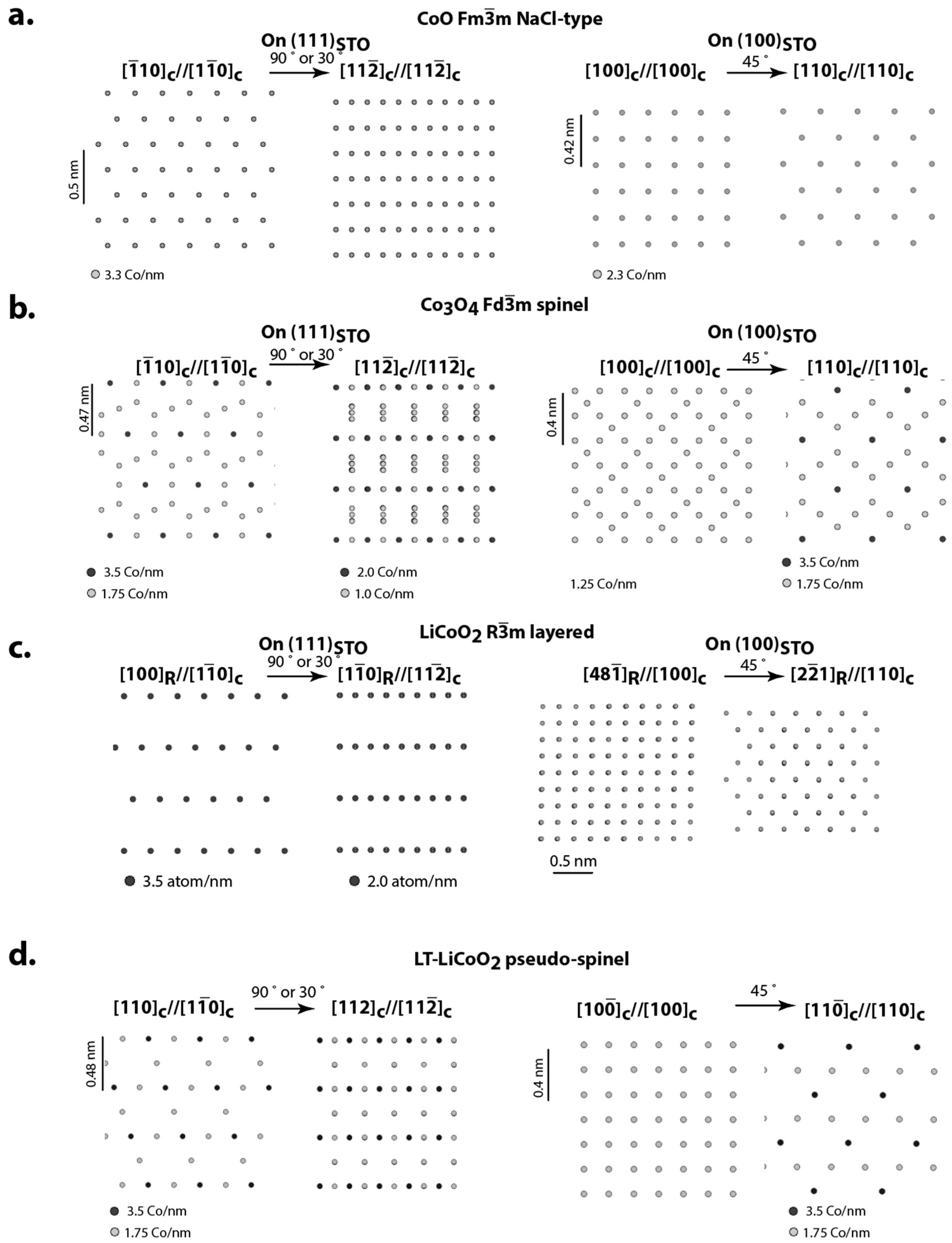
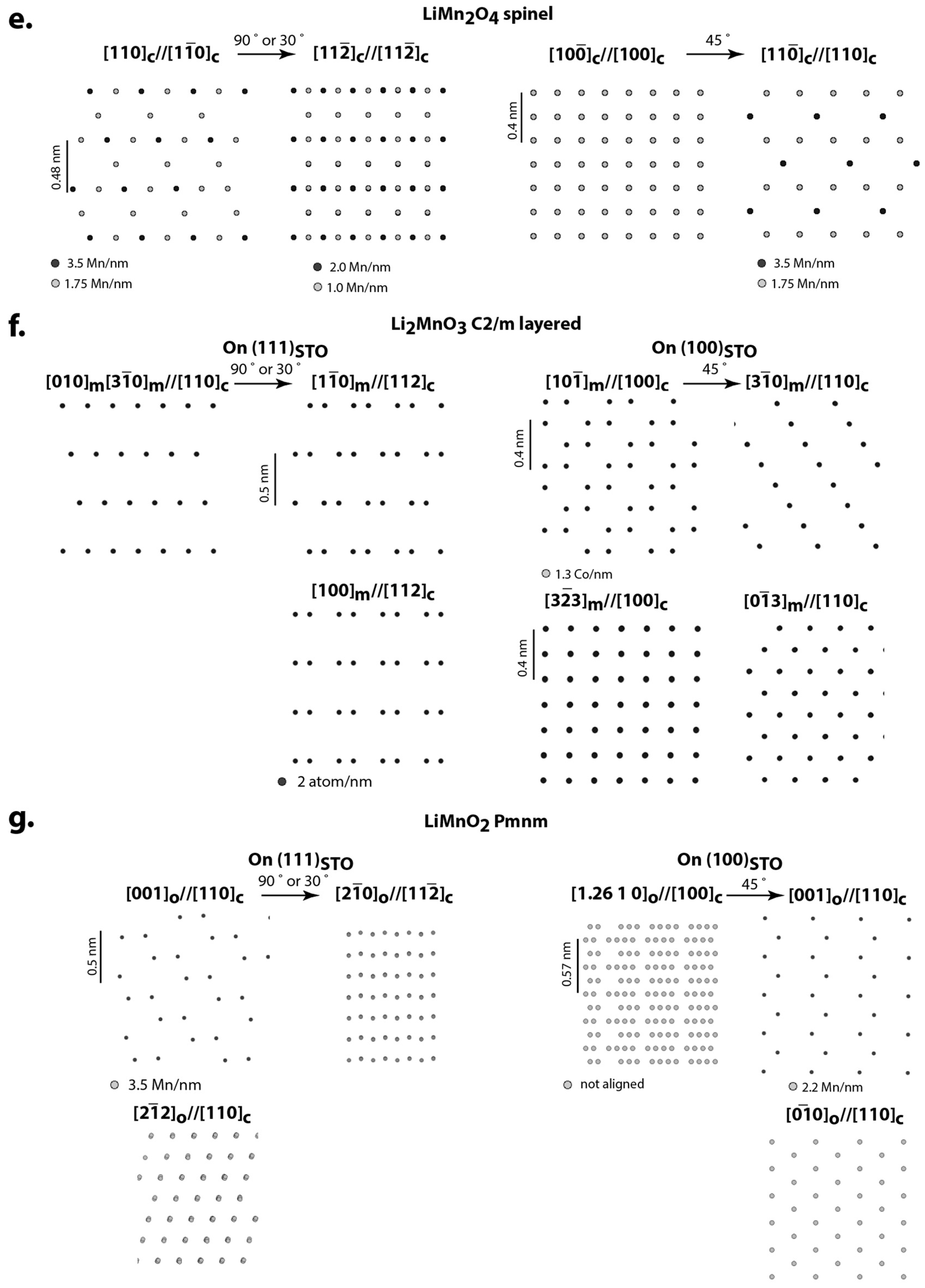
3. Expected Orientation Relationship and Structural Variants for Oxide Cathode Materials Deposited on Perovskite Oxide Substrates
4. Experimental Results from Transmission Electron Microscopy Studies
4.1. LiCoO2 on STO and SRO/STO
4.2. Li2MnO3 and LiMnO2 on SRO/STO
4.3. Li1.2Mn0.55Ni0.15Co0.1O2 on SRO/STO
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bates, J.B.; Dudney, N.J.; Lubben, D.C.; Gruzalski, G.R.; Kwak, B.S.; Yu, X.; Zuhr, R.A. Thin-film rechargeable lithium batteries. J. Power Sources 1995, 54, 1–5. [Google Scholar] [CrossRef]
- Wang, B.; Bates, J.B.; Hart, F.X.; Sales, B.C.; Zuhr, R.A.; Robertson, J.D. Characterization of thin-film rechargeable lithium batteries with lithium cobalt oxide cathodes. J. Electrochem. Soc. 1996, 143, 3203. [Google Scholar] [CrossRef]
- Dudney, N.J. Solid-state thin-film rechargeable batteries. Mater. Sci. Eng. B 2005, 116, 245. [Google Scholar] [CrossRef]
- Long, J.W.; Dunn, B.; Rolison, D.R.; White, H.S. Three-dimensional battery architectures. Chem. Rev. 2004, 104, 4463. [Google Scholar] [CrossRef] [PubMed]
- Baggetto, L.; Oudenhoven, J.F.M.; van Dongen, T.; Klootwijk, J.H.; Mulder, M.; Niessen, R.A.H.; de Croon, M.H.J.M.; Notten, P.H.L. On the electrochemistry of an anode stack for all-solid-state 3D-integrated batteries. J. Power Sources 2009, 189, 402–410. [Google Scholar] [CrossRef]
- Arthur, T.S.; Bates, D.J.; Cirigliano, N.; Johnson, D.C.; Malati, P.; Mosby, J.M.; Perre, E.; Rawls, M.T.; Prieto, A.L.; Dunn, B. Three-dimensional electrodes and battery architectures. Mater. Res. Bull. 2011, 36, 523–531. [Google Scholar] [CrossRef]
- Hirayama, M.; Sonoyama, N.; Abe, T.; Minoura, M. Characterization of electrode/electrolyte interface for lithium batteries using in situ synchrotron X-ray reflectometry—A new experimental technique for LiCoO2 model electrode. J. Power Sources 2007, 168, 493. [Google Scholar] [CrossRef]
- Tsuruhama, T.; Hitosugi, T.; Oki, H.; Hirose, Y.; Hasegawa, T. Preparation of layered-rhombohedral LiCoO2 epitaxial thin films using pulsed laser deposition. Appl. Phys. Express 2009, 2, 085502. [Google Scholar] [CrossRef]
- Hang, B.T.; Xu, X.; Osada, M.; Takada, K. Quality control of epitaxial LiCoO2 thin films grown by pulsed laser deposition. J. Mater. Res. 2010, 25, 1886–1889. [Google Scholar]
- Ohnishi, T.; Takada, K. High-rate growth of high-crystallinity LiCoO2 epitaxial thin films by pulsed laser deposition. Appl. Phys. Express 2012, 5, 055502. [Google Scholar] [CrossRef]
- Nishio, K.; Ohnishi, T.; Akatsuka, K.; Takada, K. Crystal orientation of epitaxial LiCoO2 films grown on SrTiO3 substrates. J. Power Sources 2014, 247, 687–691. [Google Scholar] [CrossRef]
- Takeuchi, S.; Tan, H.; Bharathi, K.K.; Stafford, G.R.; Shin, J.; Yasui, S.; Takeuchi, I.; Bendersky, L.A. Epitaxial LiCoO2 films as a model system for fundamental electrochemical studies of positive electrodes. ACS Appl. Mater. Interfaces 2015, 7, 7901–7911. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Takeuchi, S.; Bharathi, K.K.; Takeuchi, I.; Bendersky, L.A. Microscopy study of structural evolution in epitaxial LiCoO2 positive electrode films during electrochemical cycling. ACS Appl. Mater. Interfaces 2016, 8, 6727–6735. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yasui, S.; Takeuchi, S.; Creuziger, A.; Maruyama, S.; Herzing, A.A.; Takeuchi, I.; Bendersky, L.A. Structural study of epitaxial LiCoO2 films grown by PLD on single crystal SrTiO3 substrates. Thin Solid Films 2016, 612, 472. [Google Scholar] [CrossRef]
- Tan, T.; Bharathi, K.K.; Takeuchi, I.; Bendersky, L.A. Transmission electron microscopy study of epitaxial Li-Mn-O films grown by PLD: The effect of temperature on formation of phases. Thin Solid Films 2017, in press. [Google Scholar]
- Sonoyama, N.; Iwase, K.; Takatsuka, H.; Matsumura, T.; Imanishi, N.; Takeda, Y.; Kanno, R. Electrochemistry of LiMn2O4 epitaxial films deposited on various single crystal substrates. J. Power Sources 2009, 189, 561–565. [Google Scholar] [CrossRef]
- Suzuki, K.; Kim, K.; Taminato, S.; Hirayama, M.; Kanno, R. Fabrication and electrochemical properties of LiMn2O4/SrRuO3 multi-layer epitaxial thin film electrodes. J. Power Sources 2013, 226, 340–345. [Google Scholar] [CrossRef]
- Gao, X.; Ikuhara, Y.H.; Fisher, C.A.J.; Moriwake, H.; Kuwabara, A.; Oki, H.; Kohama, K.; Yoshida, R.; Huang, R.; Ikuhara, Y. Structural distortion and compositional gradients adjacent to epitaxial LiMn2O4 thin film interfaces. Adv. Mater. Interfaces 2014, 1, 140014. [Google Scholar] [CrossRef]
- Taminato, S.; Hirayama, M.; Suzuki, K.; Yamada, N.L.; Yonemura, M.; Son, J.Y.; Kanno, R. Highly reversible capacity at the surface of a lithium-rich manganese oxide: A model study using an epitaxial film system. Chem. Commun. 2015, 51, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Sonoyama, N.; Ito, M.; Minoura, M.; Mori, D.; Yamada, A.; Tamura, K.; Mizuki, J.; Kanno, R. Characterization of electrode electrolyte interface with X-ray reflectometry and epitaxial-film LiMn2O4 electrode. J. Electrochem. Soc. 2007, 154, A1065. [Google Scholar] [CrossRef]
- Sakamoto, K.; Konishi, H.; Sonoyama, N.; Yamada, A.; Tamura, K.; Mizuki, J.; Kanno, R. Mechanistic study on lithium intercalation using a restricted reaction field in LiNi0.5Mn0.5O2. J. Power Sources 2007, 174, 678–682. [Google Scholar] [CrossRef]
- Sakamoto, K.; Hirayama, M.; Konishi, H.; Sonoyama, N.; Dupre, N.; Guyomard, D.; Tamura, K.; Mizuki, J.; Kanno, R. Structural changes in surface and bulk LiNi0.5Mn0.5O2 during electrochemical reaction on epitaxial thin-film electrodes characterized by in situ X-ray scattering. Phys. Chem. Chem. Phys. 2010, 12, 3815–3823. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Suzuki, K.; Taminato, S.; Kim, K.; Kim, S.; Lim, J.; Hirayama, M.; Kanno, R. Structure and electrochemical properties of LiNi0.5Mn1.5O4 epitaxial thin film electrodes. J. Power Sources 2014, 246, 365–370. [Google Scholar] [CrossRef]
- Lim, J.; Lee, S.; Suzuki, K.; Kim, K.; Kim, S.; Taminato, S.; Hirayama, M.; Oshima, Y.; Takayanagi, K.; Kanno, R. Synthesis, structure and electrochemical properties of novel Li–Co–Mn–O epitaxial thin-film electrode using layer-by-layer deposition process. J. Power Sources 2015, 279, 502–509. [Google Scholar] [CrossRef]
- Redman, M.J.; Steward, E.G. Cobaltous Oxide with the Zinc Blende/Wurtzite-type Crystal Structure. Nature 1962, 193, 867. [Google Scholar] [CrossRef]
- Zabdyr, L.A.; Fabrichnaya, O.B. Phase equilibria in the cobalt oxide-copper oxide system. J. Phase Eqil. Diff. 2002, 23, 149–155. [Google Scholar] [CrossRef]
- Johnston, W.D.; Heikes, R.R.; Sestrich, D. The preparation, crystallography, and magnetic properties of the LixCo(1-x)O system. J. Phys. Chem. Solids 1958, 7, 1–13. [Google Scholar] [CrossRef]
- Orman, H.J.; Wiseman, P.J. Cobalt (III) lithium oxide, CoLiO2: Structure refinement by powder neutron diffraction. Acta Cryst. C 1984, C40, 2–14. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Ohzuku, T. Novel lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries. J. Power Sources 2003, 119–121, 171–174. [Google Scholar] [CrossRef]
- Koyama, Y.; Tanaka, I.; Adachi, H.; Makimura, Y.; Ohzuku, T. Crystal and electronic structures of superstructural Li1−x[Co1/3Ni1/3Mn1/3]O2 (0≤ x ≤1). J. Power Sources 2003, 119–121, 644–648. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, J.; Yu, X.; Kovarik, L.; Gu, M.; Omenya, F.; Chen, X.; Yang, X.-Q.; Liu, J.; Graff, G.L.; et al. Enhanced Li+ ion transport in LiNi0.5Mn1.5O4 through control of site disorder. Phys. Chem. Chem. Phys. 2012, 14, 13515–13521. [Google Scholar] [CrossRef] [PubMed]
- Makimura, Y.; Ohzuku, T. Lithium insertion material of LiNi1/2Mn1/2O2 for advanced lithium-ion batteries. J. Power Sources 2003, 119–121, 156–160. [Google Scholar] [CrossRef]
- Lee, B.R.; Noh, H.J.; Myung, S.T.; Amine, K.; Sun, Y.K. High-voltage performance of Li[Ni0.55Co0.15Mn0.3]O2 positive electrode material for rechargeable Li-ion batteries. J. Electrochem. Soc. 2011, 158, A180. [Google Scholar]
- Li, Z.; Chernova, N.A.; Roppolo, M.; Upreti, S.; Petersburg, C.; Alamgir, F.M.; Whittingham, M.S. Comparative study of the capacity and rate capability of LiNiyMnyCo1–2yO2 (y = 0.5, 0.45, 0.4, 0.33). J. Electrochem. Soc. 2011, 158, A516. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Holzapfel, M.; Novak, P.; Johnson, C.S.; Kang, S.-H.; Thackeray, M.M.; Bruce, P.G. Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J. Am. Chem. Soc. 2006, 128, 8694–8698. [Google Scholar] [CrossRef] [PubMed]
- Julien Breger, J.; Dupre, N.; Chupas, P.J.; Lee, P.; Proffen, T.; Parise, J.B.; Grey, C.P. Short- and long-range order in the positive electrode material, Li(NiMn)0.5O2: A joint X-ray and neutron diffraction, pair distribution function analysis and NMR study. J. Am. Chem. Soc. 2005, 127, 7529–7537. [Google Scholar] [CrossRef] [PubMed]
- Rossen, E.; Reimers, J.N.; Dahn, J.R. Synthesis and electrochemistry of spinel LT-LiCoO2. Solid State Ion. 1993, 62, 53–60. [Google Scholar] [CrossRef]
- Gummow, R.J.; Liles, D.C.; Thackeray, M.M.; David, W.I.F. A reinvestigation of the structures of lithium-cobalt- oxides with neutron-diffraction data. Mat. Res. Bull. 1993, 28, 1177–1184. [Google Scholar] [CrossRef]
- Reimers, J.N.; Dahn, J.R. Electrochemical and in situ-ray diffraction studies of lithium intercalation in LixCoO2. J. Electrochem. Soc. 1992, 139, 2091–2097. [Google Scholar] [CrossRef]
- Thackeray, M.M.; David, W.I.F.; Bruce, P.G.; Goodenough, J.B. Lithium insertion into manganese spinels. Mat. Res. Bull. 1983, 18, 461–472. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Johnson, P.J.; De Picciotto, L.A.; Bruce, P.G.; Goodenough, J.B. Electrochemical extraction of lithium from LiMn2O4. Mater. Res. Bull. 1984, 19, 179–187. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Wang, E.; Shokoohi, F.K.; McKinnon, W.R.; Colson, S. The spinel phase of LiMn2O4 as a cathode in secondary lithium cells. J. Electrochem. Soc. 1991, 138, 2859. [Google Scholar] [CrossRef]
- Feng, L.; Chang, Y.; Wu, L.; Lu, T. Electrochemical behavior of spinel LiMn2O4 as positive electrode in rechargeable lithium cells. J. Power Sources 1996, 63, 149. [Google Scholar] [CrossRef]
- Choa, J.; Thackeray, M.M. Structural changes of LiMn2O4 spinel electrodes during electrochemical cycling. J. Electrochem. Soc. 1999, 146, 3577. [Google Scholar] [CrossRef]
- Takahashi, Y.; Akimoto, J.; Gotoh, Y.; Dokko, K.; Nishizawa, M.; Uchida, I. Structure and electron density analysis of lithium manganese oxides by single-crystal X-ray diffraction. J. Phys. Soc. Jpn. 2003, 72, 1483–1490. [Google Scholar] [CrossRef]
- Gummow, R.J.; Liles, D.C.; Thackeray, M.M. Lithium extraction from orthorhombic lithium manganese oxide and the phase transformation to spinel. Mat. Res. Bull. 1993, 28, 1249–1256. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Bruce, R.G. Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature 1996, 381, 499–500. [Google Scholar] [CrossRef]
- Davidson, I.J.; McMillan, R.B.; Murray, R.S.; Greedan, J.E. Lithium-ion cell based on orthorhombic LiMnO2. J. Power Sources 1995, 54, 232–235. [Google Scholar] [CrossRef]
- Dittrich, G.; Hopp, R. Zur Kristallstruktur von LiMnO2. Z. Anorg. Allg. Chem. 1969, 368, 262. [Google Scholar] [CrossRef]
- Hoppe, R.; Brachtel, G.; Jansen, M. Uber LiMnO2, und β-NaMnO2. Z. Anorg. Allg. Chem. 1975, 417, 1–10. [Google Scholar] [CrossRef]
- Jang, Y.-I.; Huang, B.; Chiang, Y.-M.; Sadoway, D.R. Stabilization of LiMnO2 in the α-NaFeO2 structure type by LiAlO2 addition. Electrochem. Solid-State Lett. 1998, 1, 13–16. [Google Scholar] [CrossRef]
- Kim, J.-S.; Johnson, C.S.; Thackeray, M.M. Layered xLiMO2·(1−x)Li2MO3 electrodes for lithium batteries: A study of 0.95LiMn0.5Ni0.5O2·0.05Li2TiO3. Electrochem. Commun. 2002, 4, 205–209. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.H.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112–3125. [Google Scholar] [CrossRef]
- Strobel, P.; Lambert-Andron, B. Crystallographic and magnetic structure of Li2MnO3. J. Solid State Chem. 1988, 75, 90. [Google Scholar] [CrossRef]
- Massarotti, V.; Bini, M.; Capsoni, D.; Atomare, A.; Moliterni, A.G.G. Ab Initio structure determination of Li2MnO3 from X-ray powder diffraction data. J. Appl. Cryst. 1997, 30, 123. [Google Scholar] [CrossRef]
- Krivanek, O.L.; Chisholm, M.F.; Nicolosi, V.; Pennycook, T.J.; Corbin, G.J.; Dellby, N.; Murfitt, M.F.; Own, C.S.; Szilagyi, Z.S.; Oxley, M.P.; et al. Atom-by-atom structural and chemical analysis by annular dark-field electron microscopy. Nature 2010, 464, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Molina, S.I.; Sales, D.L.; Galindo, P.L.; Fuster, D.; Gonzalez, Y.; Alen, B.; Gonzalez, L.; Varela, M.; Pennycook, S.J. Column-by-column compositional mapping by Z-contrast imaging. Ultramicroscopy 2009, 109, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Eason, R. Pulsed Laser Deposition of Thin Films: Applications-Led Growth of Functional Materials; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Johnston-Peck, A.C.; Levin, I.; Herzing, A.A.; Bendersky, L.A. Structural studies of Li1.2Mn0.55Ni0.15Co0.1O2 electrode material. Mater. Charact. 2016, 119, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Ramasse, Q.M.; Ophus, C.; Duncan, H.; Hage, F.; Chen, G. Unravelling structural ambiguities in lithium- and manganese-rich transition metal oxides. Nat. Commun. 2015, 6, 8711. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Ophus, C.; Gammer, C.; Ramasse, Q. Study of structure of Li- and Mn-rich transition metal oxides using 4D-STEM. Microsc. Microanal. 2016, 22, 494–495. [Google Scholar] [CrossRef]
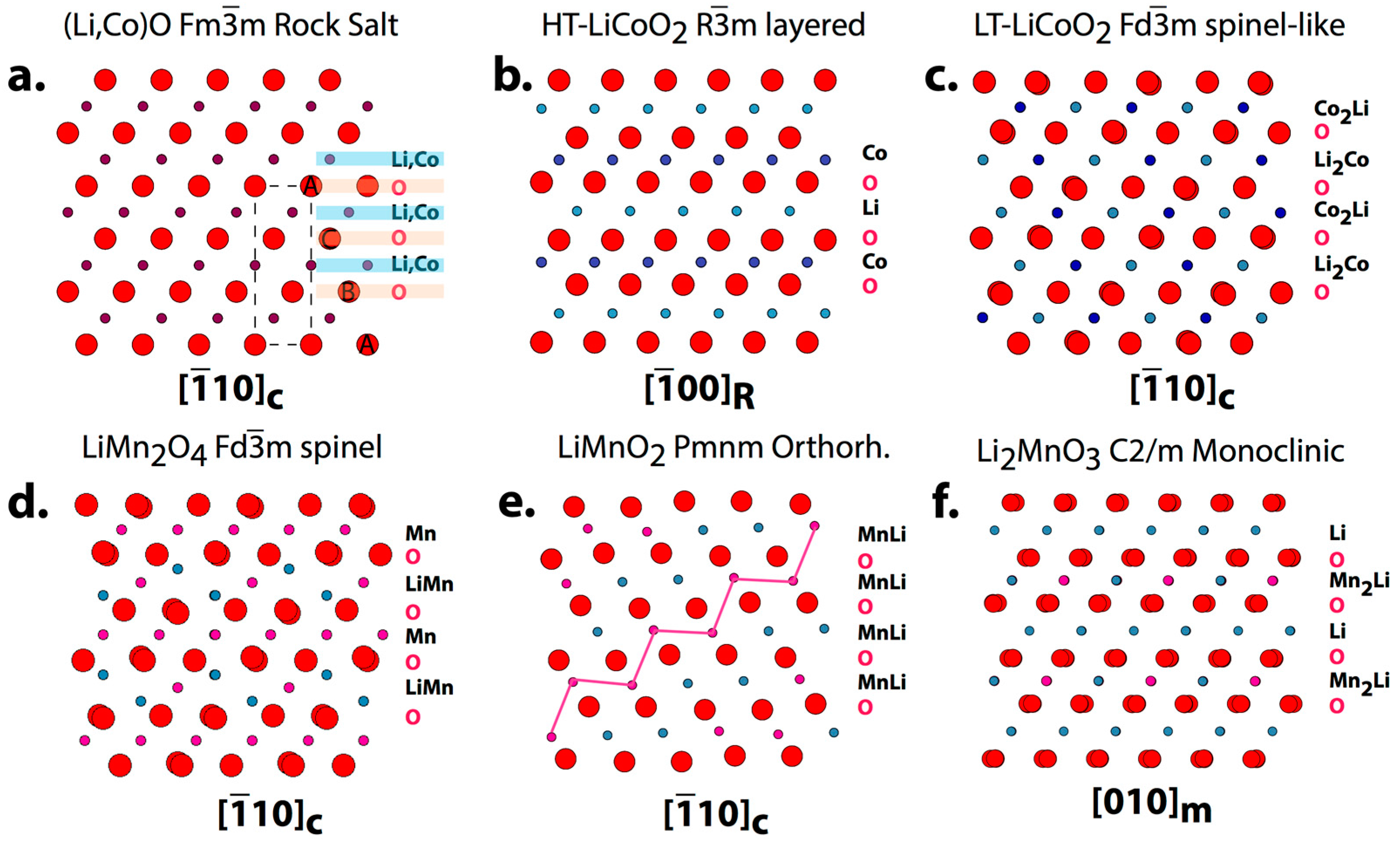
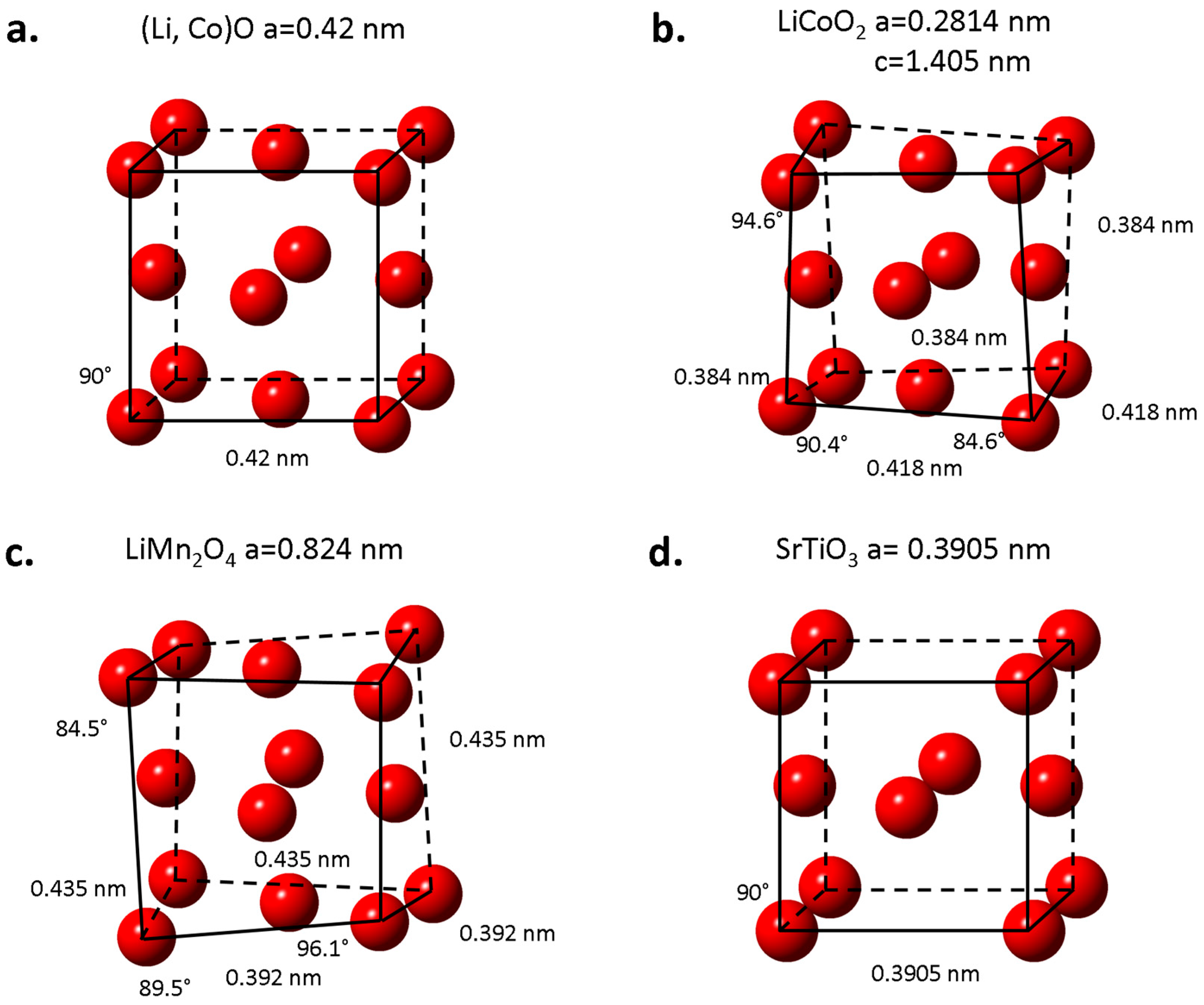
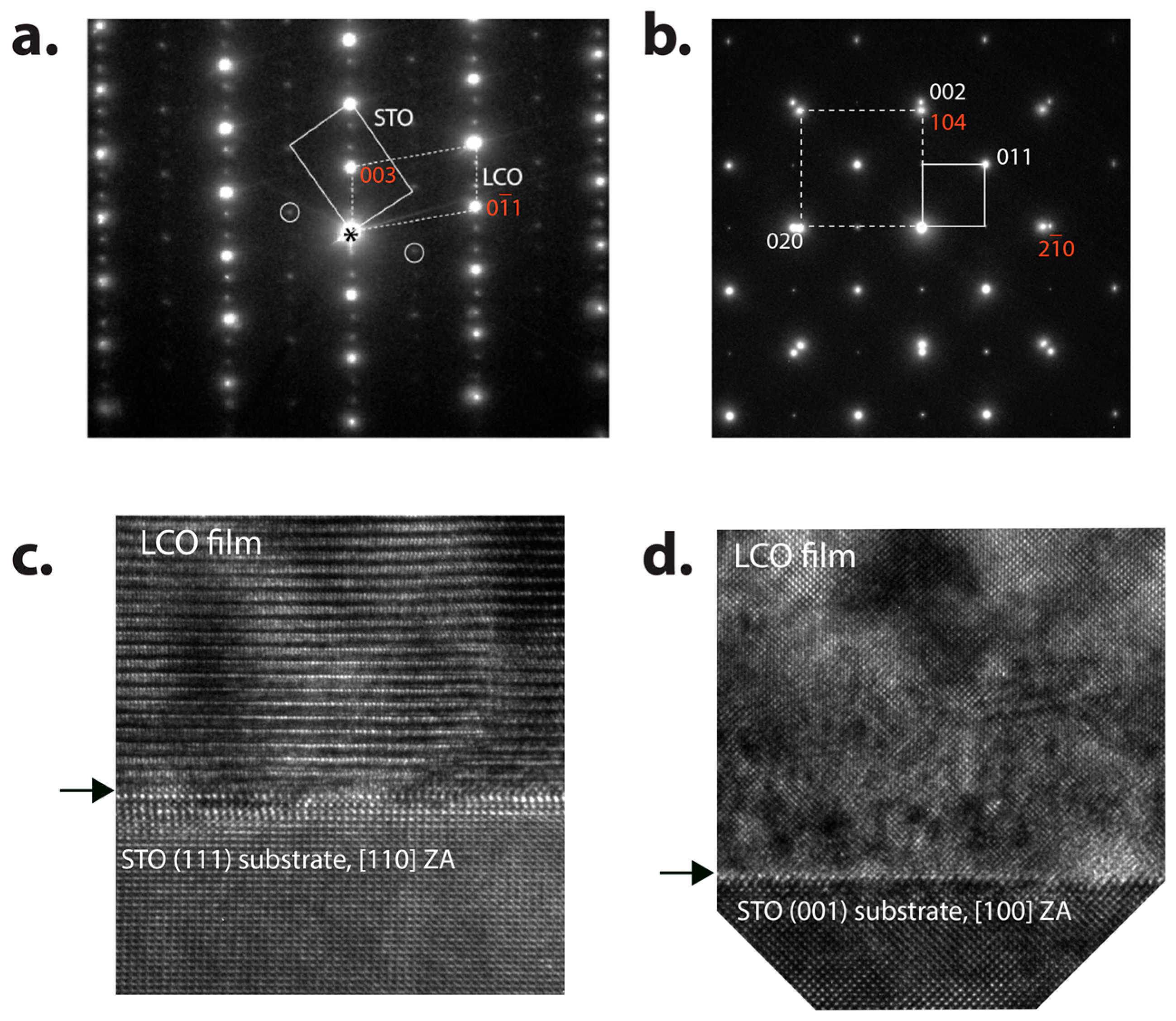
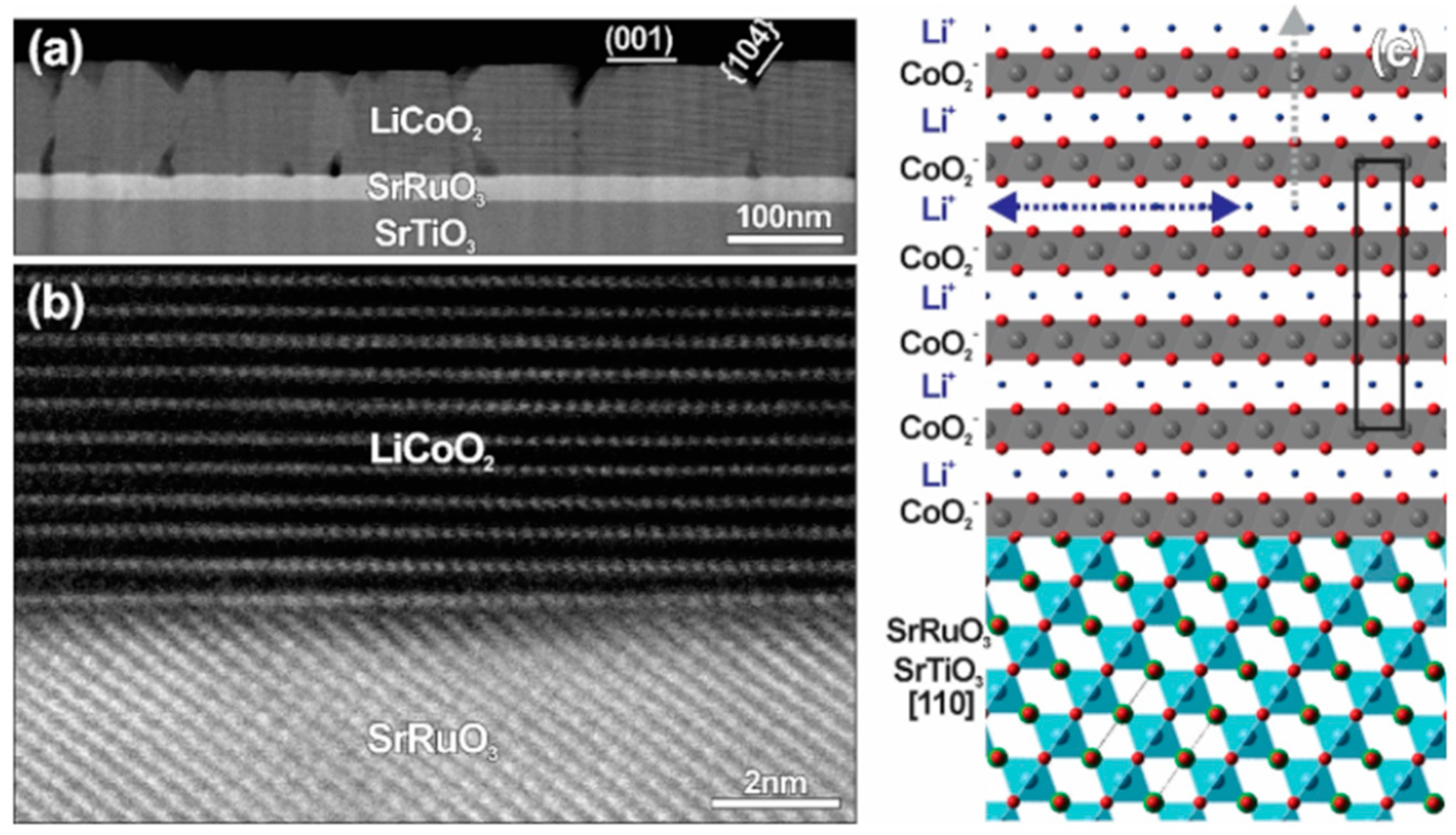

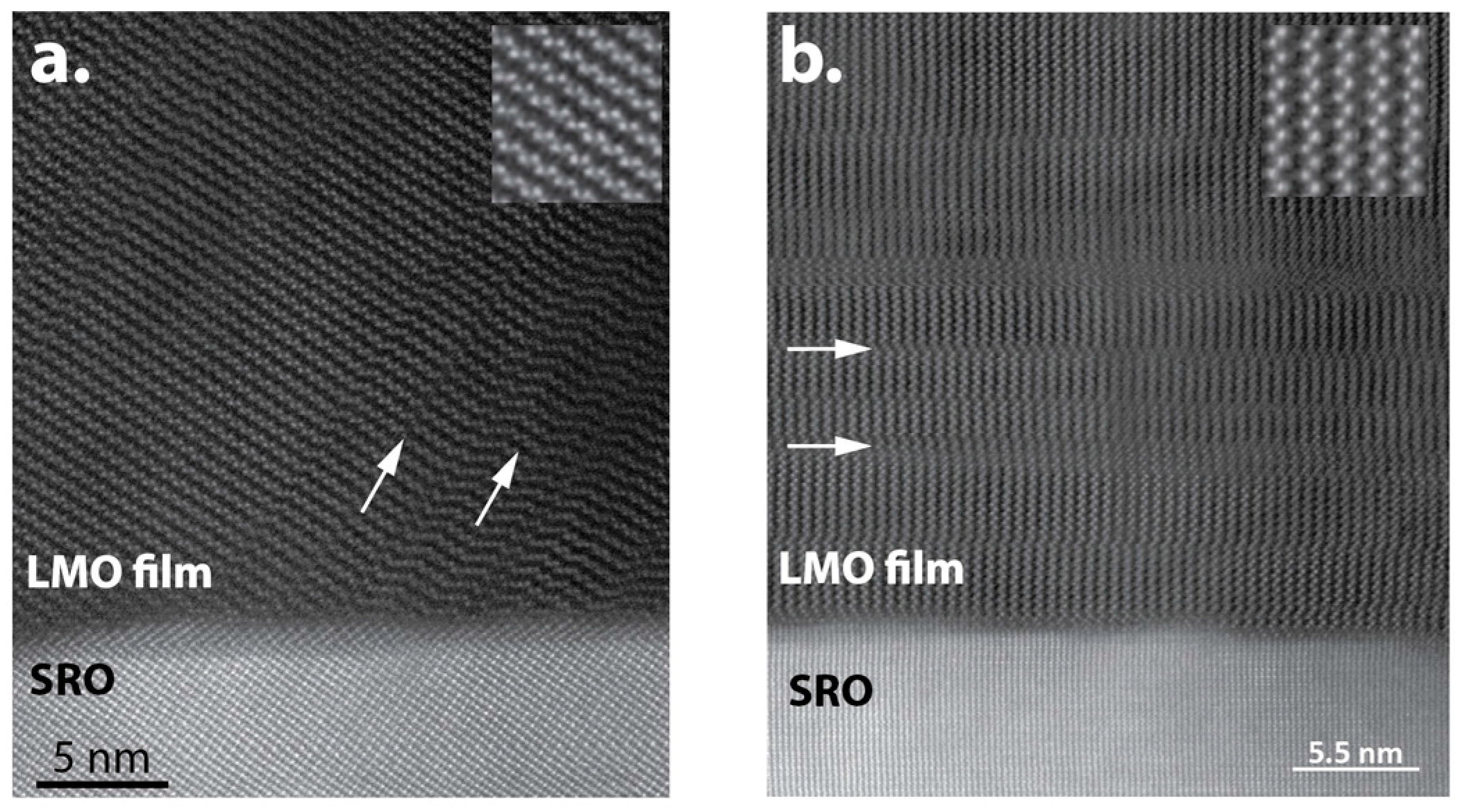

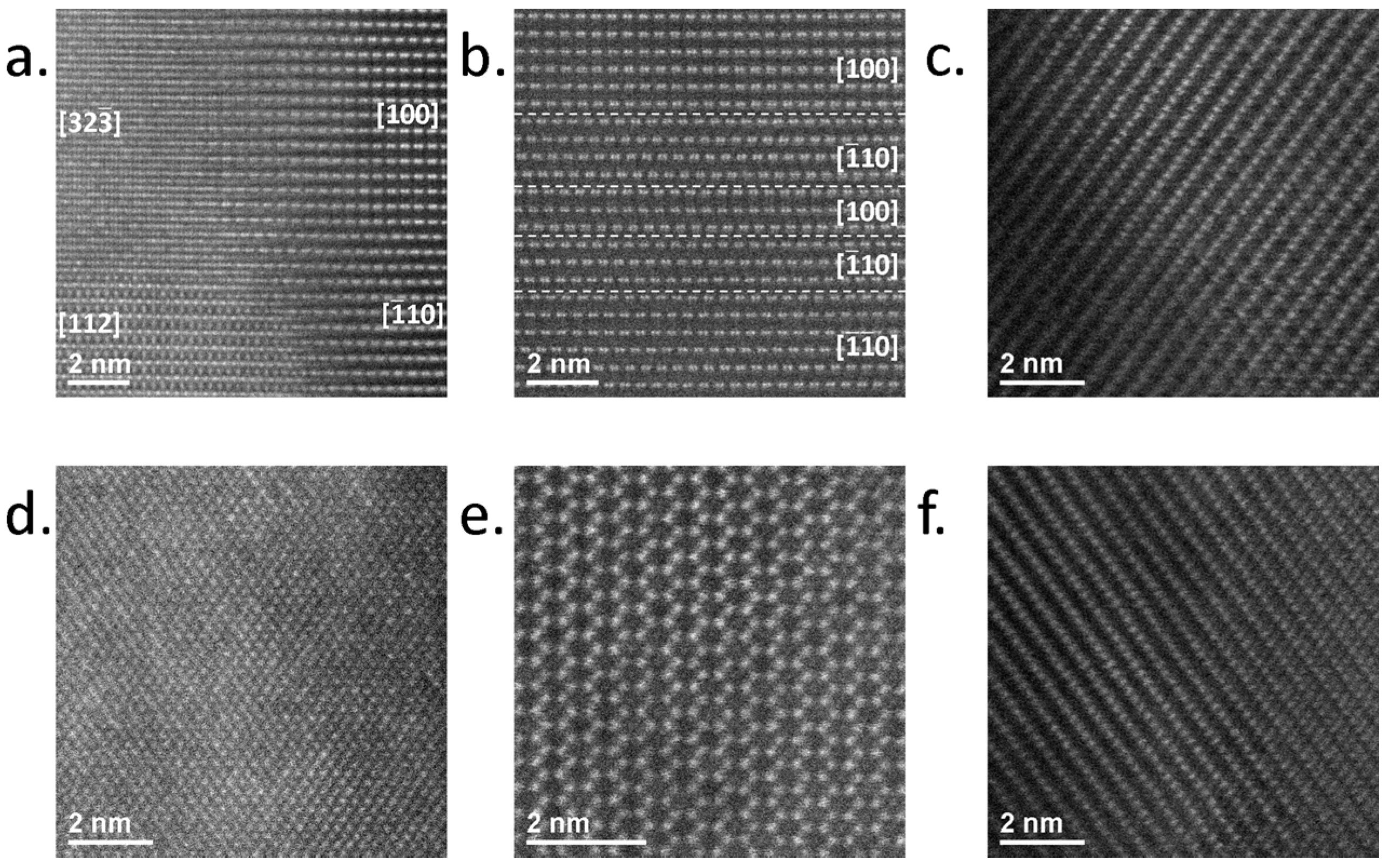
| Phases | Space Group Structure Type | Lattice Parameters | Specific Capacity (mAhg−1): Theoretical/Practical | Ref. |
|---|---|---|---|---|
| CoO, (Co,Li)O Co2+O2− | Fmm NaCl-type | a = 0.425 nm | [25,26,27] | |
| HT-LiCoO2 (Li1+)(Co3+)(O2−)2 | Rm NaCoO2-type | a = 0.2814 nm; c = 1.405 nm | 272/140 | [28] |
| Li(Co1/3Ni1/3Mn1/3)O2 | Rm NaCoO2-type | a = 0.2867 nm; c = 1.425 nm | 280/160 | [29,30] |
| LT-LiCoO2 (Li1+)(Co3+)(O2−)2 | Fdm pseudo-spinel | a = 0.802 nm | 172/84 | [37,38,39] |
| LiMn2O4 | Fdm Spinel | a = 0.824 nm | 148/120 | [45] |
| LiMnO2 | Pmnm Orthorh. | a = 0.457 nm; b = 0.575 nm; c = 0.28 nm | 285/140 | [51] |
| Li2MnO3 | C2/m layered | a = 0.494 nm; b = 0.853 nm; c = 0.503 nm β = 109.4 | 458/180 | [55] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendersky, L.A.; Tan, H.; Bharathi Karuppanan, K.; Li, Z.-P.; Johnston-Peck, A.C. Crystallography and Growth of Epitaxial Oxide Films for Fundamental Studies of Cathode Materials Used in Advanced Li-Ion Batteries. Crystals 2017, 7, 127. https://doi.org/10.3390/cryst7050127
Bendersky LA, Tan H, Bharathi Karuppanan K, Li Z-P, Johnston-Peck AC. Crystallography and Growth of Epitaxial Oxide Films for Fundamental Studies of Cathode Materials Used in Advanced Li-Ion Batteries. Crystals. 2017; 7(5):127. https://doi.org/10.3390/cryst7050127
Chicago/Turabian StyleBendersky, Leonid A., Haiyan Tan, Kamala Bharathi Karuppanan, Zhi-Peng Li, and Aaron C. Johnston-Peck. 2017. "Crystallography and Growth of Epitaxial Oxide Films for Fundamental Studies of Cathode Materials Used in Advanced Li-Ion Batteries" Crystals 7, no. 5: 127. https://doi.org/10.3390/cryst7050127
APA StyleBendersky, L. A., Tan, H., Bharathi Karuppanan, K., Li, Z.-P., & Johnston-Peck, A. C. (2017). Crystallography and Growth of Epitaxial Oxide Films for Fundamental Studies of Cathode Materials Used in Advanced Li-Ion Batteries. Crystals, 7(5), 127. https://doi.org/10.3390/cryst7050127





