Selenium-Doped Hydroxyapatite Nanocrystals–Synthesis, Physicochemical Properties and Biological Significance
Abstract
:1. Introduction
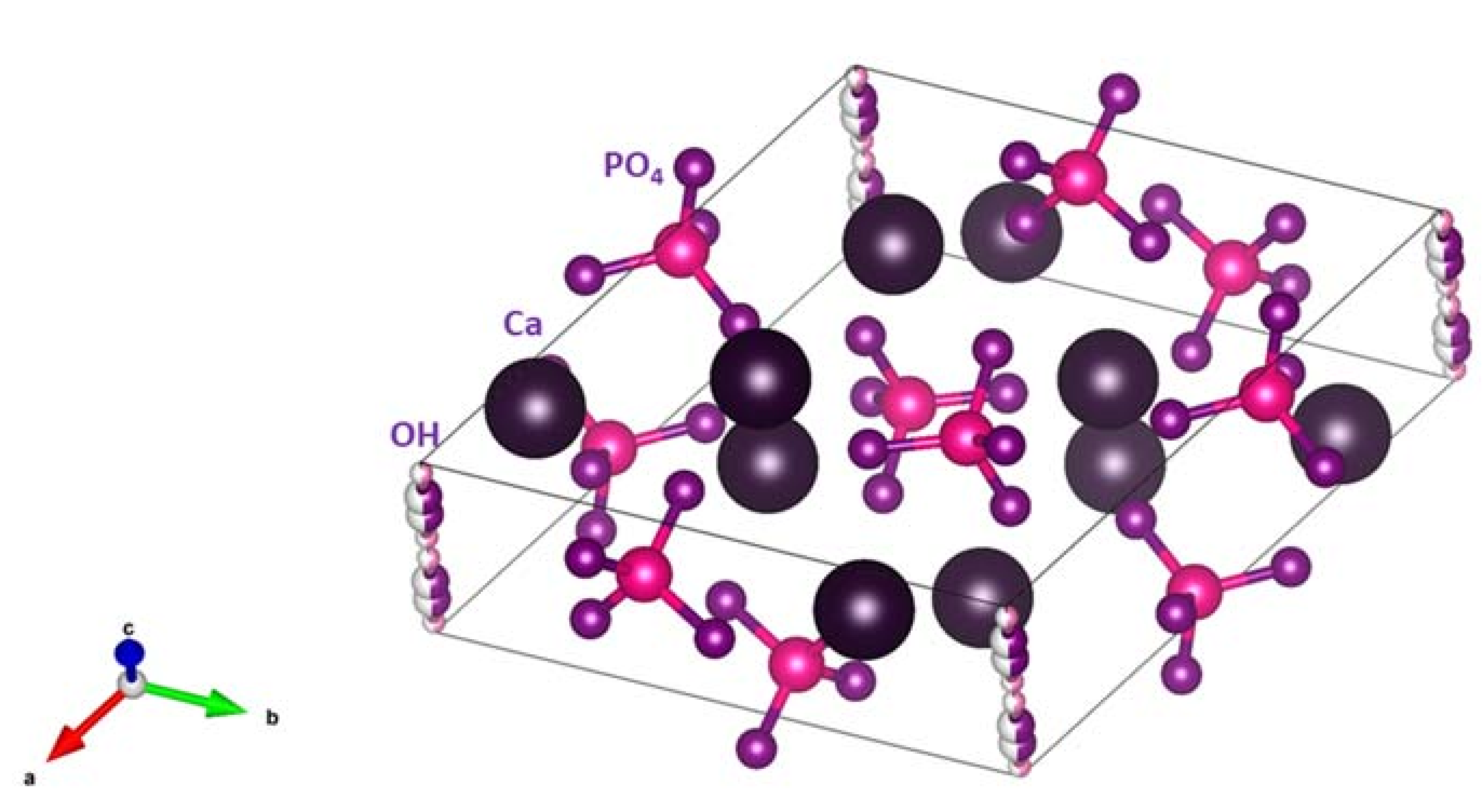
1.1. Hydroxyapatite–Structure and Function
1.2. The Role of Selenium in Human Organisms
2. Synthesis of Hydroxyapatites Doped with Selenite and Selenate Ions
3. Physicochemical Examination of Hydroxyapatites Doped with Selenate and Selenite Ions
3.1. Powder Diffractometry (PXRD)
3.2. Examinations Using Electron Microscopy Methods (SEM, TEM)
3.3. Examinations Using Mid-Infrared Spectroscopy (FT-IR)
3.4. Examinations Using the Raman Spectroscopy Method
3.5. Examinations Using Nuclear Magnetic Resonance Spectroscopy (NMR)
4. Biological Examinations of Hydroxyapatite Materials Doped with Selenium
4.1. Antibacterial Activity
4.2. Anticancer, Cytotoxic, and Osteoinductive Effects
4.2.1. In Vitro Studies
4.2.2. In Vivo Studies
5. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. Engl. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kawashita, M. Electrochemical deposition of hydroxyapatite and its biomedical applications. In Hydroxyapatite Coatings for Biomedical Applications; Zhang, S., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 31–54. [Google Scholar]
- Palazzo, B.; Sidoti, M.C.; Roveri, N.; Tampieri, A.; Sandri, M.; Bertolazzi, L.; Galbusera, F.; Dubini, G.; Vena, P.; Contro, R. Controlled drug delivery from porous hydroxyapatite grafts: An experimental and theoretical approach. Mater. Sci. Eng. C 2005, 25, 207–213. [Google Scholar] [CrossRef]
- Sakae, T.; Nakada, H.; John, P.L. Historical review of biological apatite crystallography. J. Hard Tissue Biol. 2015, 24, 111–122. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.A.; Posner, A.S. Crystal structure of hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Szurkowska, K.; Kolmas, J. Hydroxyapatites enriched in silicon—Bioceramic materials for biomedical and pharmaceutical applications. Prog. Nat. Sci. Mater. Int. 2017, 27, 401–409. [Google Scholar] [CrossRef]
- Kolmas, J.; Piotrowska, U.; Kuras, M.; Kurek, E. Effect of carbonate substitution on physicochemical and biological properties of silver containing hydroxyapatites. Mater. Sci. Eng. C 2017, 74, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S. Selenium: Significance and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef] [PubMed]
- Lenz, M.; Lens, P.N.L. The essential toxin: The changing perception of selenium in environmental sciences. Sci. Total Environ. 2009, 407, 3620–3633. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.M.; Beckham, C.; Yosioka, A.; Darban, H.; Watson, R.R. β-carotene and selenium supplementation enhances immune response in aged humans. Integr. Med. 2000, 2, 85–92. [Google Scholar] [CrossRef]
- Broome, C.S.; McArdle, F.; Kyle, J.A.M.; Andrews, F.; Lowe, N.M.; Hart, C.A.; Arthur, J.R.; Jackson, M.J. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J. Clin. Nutr. 2004, 80, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.W.; Hashimoto, A.C.; Shafer, L.A.; Dow, S.; Berry, M.J.; Hoffmann, P.R. Dietary selenium modulates activation and differentiation of CD4(+) T cells in mice through a mechanism involving cellular free thiols. J. Nutr. 2010, 140, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, M.R.; Shabanian, R.; Abbaskhanian, A.; Nasirian, A.; Ghofrani, M.; Mohammadi, M.; Zamani, G.R.; Kayhanidoost, Z.; Ebrahimi, S.; Pourpak, Z. Selenium and intractable epilepsy: Is there any correlation? Pediatr. Neurol. 2007, 36, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Selenoprotein p—Expression, functions, and roles in mammals. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2009, 1790, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, W.C.; Turek, P.J. Effects of dietary selenium on sperm motility in healthy men. J. Androl. 2001, 22, 764–772. [Google Scholar] [PubMed]
- Ursini, F.; Heim, S.; Kiess, M.; Maiorino, M.; Roveri, A.; Wissing, J.; Flohé, L. Dual function of the selenoprotein PHGPx during sperm maturation. Science 1999, 285, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Contempré, B.; de Escobar, G.M.; Denef, J.-F.; Dumont, J.E.; Many, M.-C. Thiocyanate induces cell necrosis and fibrosis in selenium- and iodine-deficient rat thyroids: A potential experimental model for myxedematous endemic cretinism in Central Africa. Endocrinology 2004, 145, 994–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmersson, J.; Ärnlöv, J.; Vessby, B.; Larsson, A.; Alfthan, G.; Basu, S. Serum selenium predicts levels of F2-isoprostanes and prostaglandin F2α in a 27 year follow-up study of Swedish men. Free Radic. Res. 2005, 39, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Holben, D.H.; Smith, A.M. The diverse role of selenium within selenoproteins. J. Am. Diet. Assoc. 1999, 99, 836–843. [Google Scholar] [CrossRef]
- Ju, W.; Li, X.; Li, Z.; Wu, G.R.; Fu, X.F.; Yang, X.M.; Zhang, X.Q.; Gao, X.B. The effect of selenium supplementation on coronary heart disease: A systematic review and meta-analysis of randomized controlled trials. J. Trace Elem. Med. Biol. 2017, 44, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.P.; Geiger, P.G.; Girotti, A. Lethal damage to endothelial cells by oxidized low density lipoprotein: Role of selenoperoxidases in cytoprotection against lipid hydroperoxide- and iron-mediated reactions. J. Lipid Res. 1993, 34, 479–490. [Google Scholar] [PubMed]
- Czeczot, H.; Scibior, D.; Skrzycki, M.; Podsiad, M. Glutathione and GSH-dependent enzymes in patients with liver cirrhosis and hepatocellular carcinoma. Acta Biochim. Pol. 2006, 53, 237–242. [Google Scholar] [PubMed]
- Schomburg, L. Dietary selenium and human health. Nutrients 2017, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Reyes, R.; Egrise, D.; Nève, J.; Pasteels, J.L.; Schoutens, A. Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J. Bone Miner. Res. 2001, 16, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Wang, X.Y.; Xiao, D.M.; Hu, L.F.; Lu, M.; Wu, Z.Y.; Bian, J.S. Hydrogen sulfide protects MC3T3-E1 osteoblastic cells against H2O2-induced oxidative damage—Implications for the treatment of osteoporosis. Free Radic. Biol. Med. 2011, 50, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Cao, J.J.; Combs, G.F. Selenium in bone health: Roles in antioxidant protection and cell proliferation. Nutrients 2013, 5, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Hoeg, A.; Gogakos, A.; Murphy, E.; Mueller, S.; Köhrle, J.; Reid, D.M.; Glüer, C.C.; Felsenberg, D.; Roux, C.; Eastell, R.; et al. Bone turnover and bone mineral density are independently related to selenium status in healthy euthyroid postmenopausal women. J. Clin. Endocrinol. Metab. 2012, 97, 4061–4070. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Pang, D.; He, L.; Deng, C. Crystal structure analysis of selenium-doped hydroxyapatite samples and their thermal stability. Ceram. Int. 2017, 43, 16141–16148. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, H.; Zhang, S. Biomimetic coprecipitation of silk fibrin and calcium phosphate: Influence of selenite ions. Biol. Trace Elem. Res. 2017, 178, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zheng, X.; Li, H.; Fan, D.; Song, Z.; Ma, H.; Hua, X.; Hui, J. Monodisperse selenium-substituted hydroxyapatite: Controllable synthesis and biocompatibility. Mater. Sci. Eng. C 2017, 73, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Kolmas, J.; Pajor, K.; Pajchel, L.; Przekora, A.; Ginalska, G.; Oledzka, E.; Sobczak, M. Fabrication and physicochemical characterization of porous composite microgranules with selenium oxyanions and risedronate sodium for potential applications in bone tumors. Int. J. Nanomed. 2017, 12, 5633–5642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Hao, H.; Cai, M.; Wang, S.; Ma, J.; Li, Y.; Mao, C.; Zhang, S. In vitro and in vivo mechanism of bone tumor inhibition by selenium-doped bone mineral nanoparticles. ACS Nano 2016, 10, 9927–9937. [Google Scholar] [CrossRef] [PubMed]
- Yanhua, W.; Hao, H.; Li, Y.; Zhang, S. Selenium-substituted hydroxyapatite nanoparticles and their in vivo antitumor effect on hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2016, 140, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hao, H.; Zhang, S. Lysozyme loading and release from Se doped hydroxyapatite nanoparticles. Mater. Sci. Eng. C 2016, 61, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, J.; Zhang, S. Synthesis and thermal stability of selenium-doped hydroxyapatite with different substitutions. Front. Mater. Sci. 2015, 9, 392–396. [Google Scholar] [CrossRef]
- Kolmas, J.; Kuras, M.; Oledzka, E.; Sobczak, M. A solid-state NMR study of selenium substitution into nanocrystalline hydroxyapatite. Int. J. Mol. Sci. 2015, 16, 11452–11464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chai, Y.; Cao, N.; Wang, Y. Synthesis and characterization of selenium substituted hydroxyapatite via a hydrothermal procedure. Mater. Lett. 2014, 134, 123–125. [Google Scholar] [CrossRef]
- Kolmas, J.; Oledzka, E.; Sobczak, M.; Nałęcz-Jawecki, G. Nanocrystalline hydroxyapatite doped with selenium oxyanions: A new material for potential biomedical applications. Mater. Sci. Eng. C 2014, 39, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, P.; Ma, Z.; Zhang, J. Enhanced healing of rat calvarial critical size defect with selenium-doped lamellar biocomposites. Biol. Trace Elem. Res. 2013, 155, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Y.; Zhou, L.; Zhang, S. Preparation and characterization of selenite substituted hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, J.; Zhou, L.; Chen, J.; Liu, Y.; Qiu, Z. Dual functional selenium-substituted hydroxyapatite. Interface Focus 2012, 2, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Uskokovic, V.; Iyer, M.A.; Wu, V.M. One ion to rule them all: The combined antibacterial, osteoinductive and anticancer properties of selenite-incorporated hydroxyapatite. J. Mater. Chem. B 2017, 5, 1430–1445. [Google Scholar] [CrossRef] [PubMed]
- Renard, F.; Montes-Hernandez, G.; Ruiz-Agudo, E.; Putnis, C.V. Selenium incorporation into calcite and its effect on crystal growth: An atomic force microscopy study. Chem. Geol. 2013, 340, 151–161. [Google Scholar] [CrossRef]
- Aurelio, G.; Fernández-Martínez, A.; Cuello, G.J.; Román-Ross, G.; Alliot, I.; Charlet, L. Structural study of selenium(IV) substitutions in calcite. Chem. Geol. 2010, 270, 249–256. [Google Scholar] [CrossRef]
- Duc, M.; Lefevre, G.; Fedoroff, M.; Jeanjean, J.; Rouchaud, J.C.; Monteil-Rivera, F.; Dumonceau, J.; Milonjic, S. Sorption of selenium anionic species on apatites and iron oxides from aqueous solutions. J. Environ. Radioact. 2003, 70, 61–72. [Google Scholar] [CrossRef]
- Monteil-Rivera, F.; Masset, S.; Dumonceau, J.; Fedoroff, M.; Jeanjean, J. Sorption of selenite ions on hydroxyapatite. J. Mater. Sci. Lett. 1999, 18, 1143–1145. [Google Scholar] [CrossRef]
- Monteil-Rivera, F.; Fedoroff, M.; Jeanjean, J.; Minel, L.; Barthes, M.-G.; Dumonceau, J. Sorption of selenite (SeO32−) on hydroxyapatite: An exchange process. J. Colloid Interface Sci. 2000, 221, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Valencia, C.; Freixeiro, P.; Serra, J.; Ferreirós, C.M.; González, P.; López-Álvarez, M. In vitro evaluation of the antibacterial and osteogenic activity promoted by selenium-doped calcium phosphate coatings. Biomed. Mater. 2017, 12, 015028. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Valencia, C.; Lopez-Alvarez, M.; Cochon-Cores, B.; Pereiro, I.; Serra, J.; Gonzalez, P. Novel selenium-doped hydroxyapatite coatings for biomedical applications. J. Biomed. Mater. Res. A 2013, 101, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Evis, Z.; Tezcaner, A.; Banerjee, S. Surface characterization and biocompatibility of selenium-doped hydroxyapatite coating on titanium alloy. Int. J. Appl. Ceram. Technol. 2016, 13, 1059–1068. [Google Scholar] [CrossRef]
- Aksakal, B.; Say, Y.; Buyukpinar, Ç.; Bakirdere, S. Biodegradation of hydroxyapatite coated Rex-734 alloy with silver and selenium/chitosan substitutions: In vitro analysis. Ceram. Int. 2017, 43, 12609–12615. [Google Scholar] [CrossRef]
- Pajchel, L.; Kolodziejski, W. Solid-state MAS NMR, TEM, and TGA studies of structural hydroxyl groups and water in nanocrystalline apatites prepared by dry milling. J. Nanopart. Res. 2013, 15, 1868. [Google Scholar] [CrossRef] [PubMed]
- Kolmas, J.; Groszyk, E.; Piotrowska, U. Nanocrystalline hydroxyapatite enriched in selenite and manganese ions: Physicochemical and antibacterial properties. Nanoscale Res. Lett. 2015, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Murugan, N.; Kavitha, L.; Shinyjoy, E.; Rajeswari, D.; Vimala, K.; Kannan, S.; Gopi, D. Smart rose flower like bioceramic/metal oxide dual layer coating with enhanced anti-bacterial, anti-cancer, anti-corrosive and biocompatible properties for improved orthopedic applications. RSC Adv. 2015, 5, 85831–85844. [Google Scholar] [CrossRef]
- Kramer, G.F.; Ames, B.N. Mechanims of mutagenicity and toxicity of sodium selenite (Na2SeO3) in Salmonella typhimurium. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1988, 201, 169–180. [Google Scholar] [CrossRef]
- Seko, Y.; Imura, N. Active oxygen generation as a possible mechanism of selenium toxicity. Biomed. Environ. Sci. 1997, 10, 333–339. [Google Scholar] [PubMed]
- Vekariya, K.K.; Kaur, J.; Tikoo, K. Alleviating anastrozole induced bone toxicity by selenium nanoparticles in SD rats. Toxicol. Appl. Pharmacol. 2013, 268, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Taskin, E.; Dursun, N. The protection of selenium on adriamycin-induced mitochondrial damage in rat. Biol. Trace Elem. Res. 2012, 147, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.P.; Wei, S.Q.; Gao, X.C.; Yu, N.N.; Hu, W.Z.; Bi, S.; Cui, H. Ursodeoxycholic acid prevents selenite-induced oxidative stress and alleviates cataract formation: In vitro and in vivo studies. Mol. Vis. 2012, 18, 151–160. [Google Scholar] [PubMed]
- Rooban, B.N.; Sasikala, V.; Gayathri Devi, V.; Sahasranamam, V.; Abraham, A. Prevention of selenite induced oxidative stress and cataractogenesis by luteolin isolated from Vitex negundo. Chem. Biol. Interact. 2012, 196, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Rajamannan, N.M. Oxidative-mechanical stress signals stem cell niche mediated LRP5 osteogenesis in eNOS(−/−) null mice. J. Cell. Biochem. 2012, 113, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Wang, S.; Zhao, J.; Xu, L.; Zhu, C.; Zeng, D.; Chen, J.; Zhang, Z.; Kaplan, D.L.; et al. The use of injectable sonication-induced silk hydrogel for VEGF165 and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials 2011, 32, 9415–9424. [Google Scholar] [CrossRef] [PubMed]
- Andreas, S.S.; Parag, K.J.; Wasim, S.K. Clinical applications of mesenchymal stem cells in the treatment of fracture non-union and bone defects. Curr. Stem Cell Res. Ther. 2012, 7, 127–133. [Google Scholar]
- Bauer, T.W.; Togawa, D. Bone graft substitutes: Towards a more perfect union. Orthopedics 2003, 26, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, X.; Chai, Y.; Wang, Y. Synthesis and characterization of Zn2+ and SeO32− co-substituted nano-hydroxyapatite. Adv. Powder Technol. 2016, 27, 1857–1861. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Engin Pazarceviren, A.; Tezcaner, A.; Evis, Z. Fe3+/SeO42− dual doped nano hydroxyapatite: A novel material for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 340–352. [Google Scholar] [CrossRef] [PubMed]






| Bands (Wavenumber cm−1) | Assignment |
|---|---|
| 3700–2500 | ν3 and ν1 stretching modes of hydrogen-bonded H2O molecules |
| 3570 | stretching modes of structural hydroxyl groups |
| 1630–1640 | Bending modes of hydrogen-bonded H2O molecules |
| 1200–900 | ν3 and ν1 of PO43− |
| 605–500 | ν4 PO43− |
| 475–470 | ν2 PO43− |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajor, K.; Pajchel, L.; Kolodziejska, B.; Kolmas, J. Selenium-Doped Hydroxyapatite Nanocrystals–Synthesis, Physicochemical Properties and Biological Significance. Crystals 2018, 8, 188. https://doi.org/10.3390/cryst8050188
Pajor K, Pajchel L, Kolodziejska B, Kolmas J. Selenium-Doped Hydroxyapatite Nanocrystals–Synthesis, Physicochemical Properties and Biological Significance. Crystals. 2018; 8(5):188. https://doi.org/10.3390/cryst8050188
Chicago/Turabian StylePajor, Kamil, Lukasz Pajchel, Barbara Kolodziejska, and Joanna Kolmas. 2018. "Selenium-Doped Hydroxyapatite Nanocrystals–Synthesis, Physicochemical Properties and Biological Significance" Crystals 8, no. 5: 188. https://doi.org/10.3390/cryst8050188




