Synthesis and Crystal Structures of Cadmium(II) Cyanide with Branched-Butoxyethanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of [{Cd(CN)2(iBucel)2}{Cd(CN)2(H2O)(iBucel)}2{Cd(CN)2}6∙2(iBucel)]n, I
2.1.2. Synthesis of [{Cd(CN)2(H2O)1.06(tBucel)0.94}{Cd(CN)2(tBucel)}2{Cd(CN)2}2∙(1.06tBucel)]n, II
2.2. Single Crystal X-ray Diffraction
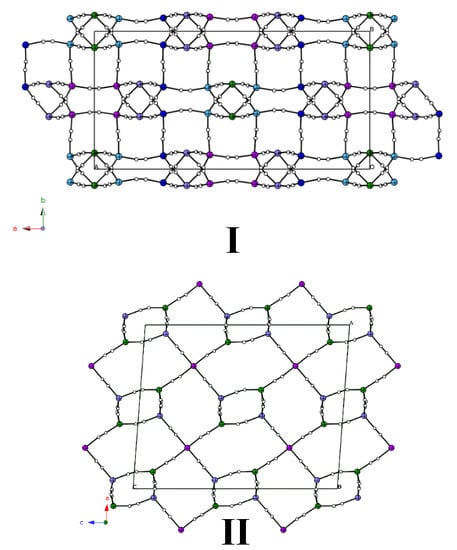
3. Results
Crystal Structure
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dyadin, Y.A.; Terekhova, I.S.; Rodionova, T.V.; Soldatov, D.V. Half century history of clathrate chemistry. J. Struct. Chem. 1999, 40, 645–653. [Google Scholar] [CrossRef]
- Zhdanov, H. The crystalline structure of Zn(CN)2. C. R. Acad. Sci. USSR 1941, 31, 352–354. [Google Scholar]
- Shugam, E.; Zhdanov, H. The crystal structure of cyanides. II. Structure of cadmium cyanide. Acta Physicochim. USSR 1945, 20, 247–252. [Google Scholar]
- Kitazawa, T.; Nishikiori, S.; Kuroda, R.; Iwamoto, T. Novel Clathrate Compound of Cadmium Cyanide Host with an Adamantane-like Caity. Cadmium Cyanide-Carbon Tetrachloride(1/1). Chem. Lett. 1988, 17, 1729–1732. [Google Scholar] [CrossRef]
- Iwamoto, T.; Nishikiori, S.; Kitazawa, T.; Yuge, H. Mineralomimetic chemistry as a modern aspect of co-ordination chemistry. J. Chem. Soc. Dalton Trans. 1997, 4127–4136. [Google Scholar] [CrossRef]
- Phillips, A.E.; Goodwin, A.L.; Halder, G.J.; Southon, P.D.; Kepert, C.J. Nanoporosity and Exceptional Negative Thermal Expansion in Single-Network Cadmium Cyanide. Angew. Chem. Int. Ed. 2008, 47, 1396–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoskins, B.F.; Robson, R. Design and Construction of New Class of Scaffolding-like Materials Comprising Infinite Polymeric Frameworks of 3D-Linked Molecular Rods. A Reappraisal of the Zn(CN)2 and Cd(CN)2 Structures and the Synthesis and Structure of the Diamond-Related Frameworks [N(CH3)4][CuIZnII(CN)4] and CuI[4,4′,4′′,4′′′-tetracyanotetraphenylmethane]BF4∙xC6H6NO2. J. Am. Chem. Soc. 1990, 112, 1546–1554. [Google Scholar]
- Abrahams, B.F.; Hardie, M.J.; Hoskins, B.F.; Robson, R.; Williams, G.A. Topological Rearrangement within a Single Crystal from a Honeycomb [Cd(CN)2]n 3D Nets to a Diamond Net. J. Am. Chem. Soc. 1992, 114, 10641–10643. [Google Scholar] [CrossRef]
- Kitazawa, T.; Nishimura, A. Mineralomimetic cadmium cyanide benzene clathrate. J. Struct. Chem. 1999, 40, 721–725. [Google Scholar] [CrossRef]
- Kitazawa, T. A new mineralomimetic Cd(CN)2 host framework which is intermediate between H- and l-cristobalite-like frameworks. Chem. Commun. 1999, 10, 891–892. [Google Scholar] [CrossRef]
- Kitazawa, T. A new type of mineralomimetic cadmium cyanide host framework containing methyl acetate. J. Mater. Chem. 1998, 8, 671–674. [Google Scholar] [CrossRef]
- Kitazawa, T.; Nishikiori, S.; Kuroda, R.; Iwamoto, T. Two novel metal-complex host structures consisting of cyanocadmate coordination polyhedra. Clay-like and zeolite-like structures. Chem. Lett. 1988, 17, 459–462. [Google Scholar] [CrossRef]
- Nishikiori, S.; Ratcliffe, C.I.; Ripmeester, J.A. Crystal Structure of Cd5(CN)10(H2O)4·4C6H11OH Studied by X-ray Diffraction and Solid-State 113Cd NMR. A New Type of Cristobalite-like Framework Host with a Site Interacting with Cyclohexanol by Hydrogen Bonding. J. Am. Chem. Soc. 1992, 114, 8590–8595. [Google Scholar] [CrossRef]
- Kim, J.; Whang, D.; Lee, J.I.; Kim, K. Guest-dependent [Cd(CN)2]n Host Structures of Cadmium Cyanide-Alcohol Clathrates: Two New [Cd(CN)2]n Frameworks formed with PrnOH and PriOH Guests. J. Chem. Soc. Chem. Commun. 1993, 18, 1400–1402. [Google Scholar] [CrossRef]
- Kim, J.; Whang, D.; Koh, Y.-S.; Kim, K. Two New [Cd(CN)2]n Frameworks with Linear Channels of Large, Elongated Hexagonal Cross-section: Structures of Cadmium Cyanide-Guest (Guest = dmf and Me2SO) Clathrates. J. Chem. Soc. Chem. Commun. 1994, 5, 637–638. [Google Scholar] [CrossRef]
- Kitazawa, T.; Kukuyama, T.; Takahashi, M.; Takeda, M. Cadmium Cyanide-Ether Clathrates: Crystal Structures of Cd8(CN)16(H2O)6·6G (G = Et2O or Pri2O) and Cd3(CN)6(H2O)2·2Prn2O. J. Chem. Soc. Dalton Trans. 1994, 20, 2933–2937. [Google Scholar] [CrossRef]
- Yuge, H.; Chong-Hyeak, K.; Iwamoto, T.; Kitazawa, T. Hofmann-H2O-type and Hofmann-H2O-Td-type host structures accommodating 1,4-dioxane: Crystal structures of trans-bis(morpholine-N)cadmium(II) tetaracyanonikelate(II), trans-diaquacadmium(II) tetracyanonickelate(II)-(1,4-dioxane)(1/2) and trans-diaquacadmium(II) tetracyanocadmate(II) (1,4-dioxane)(1/2). Inorg. Chim. Acta 1997, 257, 217–224. [Google Scholar]
- Abrahams, B.F.; Hoskins, B.F.; Liu, J.; Robson, R. The Archetype for a New Class of Simple Extended 3D Honeycomb Frameworks. The Synthesis and X-ray Crystal Structure of Cd(CN)5/3(OH)1/3∙1/3(C6H12N4), Cd(CN)2∙1/3(C6H12N4), and Cd(CN)2∙2/3H2O∙tBuOH (C6H12N4 = Hexamethylenetetramine) Revealing Tow Topologycallyt Equivalent but Geometrically Different Frameworks. J. Am. Chem. Soc. 1991, 113, 3045–3051. [Google Scholar]
- Abrahams, B.F.; Hoskins, B.F.; Lam, Y.-H.; Robson, R.; Separovic, F.; Woodberry, P. A Reexamination of the Structure of “Honeycomb Cadmium Cyanide”. J. Solid State Chem. 2001, 156, 51–56. [Google Scholar] [CrossRef]
- Ruiz, E.; Alvarez, S. Theoretical Study of Host∙Guest Interactions in Clathrates with a Cd(CN)2 Host. Inorg. Chem. 1995, 34, 5845–5851. [Google Scholar] [CrossRef]
- Nishikiori, S. Reversible reconstructive transition of the [CuZn(CN)4]− framework host induced by guest exchange. CrystEngComm 2014, 16, 10173–10176. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kitazawa, T. Three-Dimensional Cadmium(II) Cyanide Coordination Polymers with Ethoxy-, Butoxy- and Hexyloxy-ethanol. Crystals 2016, 6. [Google Scholar] [CrossRef]
- Bruker. APEX2, Version 2.0-2; Bruker AXS Inc.: Madison, WI, USA, 2006. [Google Scholar]
- Bruker. SAINT, Version 7.23A; Bruker AXS Inc.: Madison, WI, USA, 2007. [Google Scholar]
- Sheldrick, G.M. SADABS: Program for Empirical Absorption Correction; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Mighell, A.D. Monoclinic I- and C-centered cells. Acta Crystallogr. Sect. B 2003, 59, 300–302. [Google Scholar] [CrossRef]
- Feast, G.C.; Haestier, J.; Page, L.W.; Robertson, J.; Thompson, A.L.; Watkin, D.J. An unusual methylene aziridine refined in P21/c and the nonstandard setting P21/n. Acta Crystallogr. Sect. C 2009, 65, o635–o638. [Google Scholar] [CrossRef] [PubMed]
- Nishikiori, S.; Ratcliffe, C.I.; Ripmeester, J.A. 113Cd NMR studies of Hofmann-type clathrates and related compounds: Evidence form two room temperature orientational glasses. Can. J. Chem. 1990, 68, 2270–2273. [Google Scholar] [CrossRef]
- Nishikiori, S.; Ratcliffe, C.I.; Ripmeester, J.A. Framework ordering in solid cadmium cyanides from cadmium-113 NMR spectroscopy. J. Chem. Soc. Chem. Commun. 1991, 10, 735–736. [Google Scholar] [CrossRef]
- Blatov, V.A.; Delgado-Friedrichs, O.; O’Keeffe, M.; Proserpio, D.M. Three-periodic nets and tilings: Natural tilings for nets. Acta Crystallogr. Sect. A 2007, 63, 418–425. [Google Scholar] [CrossRef] [PubMed]
- O’Keefee, M.; Peskov, M.A.; Ramsden, S.J.; Yaghi, O.M. The Reticular Chemistry Structure Resource (RCSR) Database of, and Symbols for, Crystal Nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Anurova, N.A.; Blatov, V.A.; Ilyushin, G.D.; Proserpio, D.M. Natural Tilings for Zeolite-Type Frameworks. J. Phys. Chem. C 2010, 114, 10160–10170. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijin, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Siewe, A.D.; Kim, J.-Y.; Kim, S.; Park, I.-H.; Lee, S.S. Regioisomer-Dependent Endo- and Exocyclic Coordination of Bis-Dithiamacrocycles. Inorg. Chem. 2014, 53, 393–398. [Google Scholar] [CrossRef] [PubMed]



| Complex | I | II |
|---|---|---|
| Empirical formula | C54H88Cd9N18O14 | C34H58.12Cd5N10O9.06 |
| Formula weight | 2225.02 | 1314.03 |
| Temperature (K) | 90 | 90 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | I2/a |
| a (Å) | 33.8994(11) | 21.8261(7) |
| b (Å) | 16.6003(5) | 8.8155(3) |
| c (Å) | 15.8715(5) | 27.1515(12) |
| β (ᵒ) | 101.562(1) | 94.2899(4) |
| V (A3) | 8750.3(5) | 5209.5(3) |
| Z | 4 | 4 |
| dcalc (g cm−3) | 1.689 | 1.675 |
| μ (mm−1) | 2.199 | 2.059 |
| F(000) | 4328 | 2578 |
| Reflections collected | 33716 | 19,666 |
| Rint | 0.0197 | 0.0127 |
| Data/restraints/parameters | 13296/205/478 | 7912/7/319 |
| GOF | 1.072 | 1.054 |
| R1, wR2 [I > 2σ(I)] | 0.0967, 0.2291 | 0.0256, 0.0636 |
| Δρmax, Δρmin (e Å−3) | 4.314, −7.405 | 1.347, −1.357 |
| CdOC-OW/Å | |||||
| Cd1-O1 | 2.372(7) | ||||
| CdOC-OOH/Å | |||||
| Cd1-O2 | 2.343(8) | Cd2-O4 | 2.323(8) | ||
| CdOC-(CN)/Å | |||||
| Cd1-N1 | 2.282(9) | Cd1-N2 | 2.310(8) | Cd1-N3 | 2.261(7) |
| Cd1-N4 | 2.282(9) | Cd2-N5 | 2.286(8) | Cd2-N6 | 2.309(10) |
| CdT-(CN)/Å | |||||
| Cd3-C3 | 2.189(10) | Cd3-C4 | 2.214(10) | Cd3-N7 | 2.214(9) |
| Cd3-N9 | 2.224(9) | Cd4-C1 | 2.191(11) | Cd4-C2 | 2.161(9) |
| Cd4-C8 | 2.218(10) | Cd4-N10 | 2.223(14) | Cd5-C6 | 2.176(9) |
| Cd5-C5 | 2.190(9) | Cd5-C7 | 2.228(8) | Cd5-N8 | 2.205(9) |
| O-CdOC-O/ᵒ | |||||
| O1-Cd1-O2 | 86.2(3) | O4-Cd2-O4 i | 85.5(5) | ||
| trans((CN)-CdOC-O)/ᵒ | |||||
| N3-Cd1-O1 | 174.4(3) | N2-Cd1-O2 | 172.5(3) | N6-Cd2-O4 i | 172.3(4) |
| cis((CN)-CdOC-OOH)/ᵒ | |||||
| N3-Cd1-O2 | 89.1(3) | N1-Cd1-O2 | 89.1(4) | N4-Cd1-O2 | 88.7(4) |
| N6-Cd2-O4 | 87.1(4) | N5-Cd2-O4 | 87.6(3) | ||
| cis((CN)-CdOC-OW)/ᵒ | |||||
| N2-Cd1-O1 | 86.7(3) | N1-Cd1-O1 | 85.0(3) | N4-Cd1-O1 | 86.1(3) |
| trans((CN)-CdOC-(CN))/ᵒ | |||||
| N4-Cd1-N1 | 171.0(4) | N5 i-Cd2-N5 | 178.3(4) | ||
| cis((CN)-CdOC-(CN))/ᵒ | |||||
| N3-Cd1-N1 | 91.8(3) | N3-Cd1-N4 | 97.0(3) | N1-Cd1-N2 | 92.5(4) |
| N4-Cd1-N2 | 88.5(3) | N5-Cd2-N6 | 90.8(3) | ||
| N3-Cd1-N2 | 98.1(3) | N6 i-Cd2-N6 | 100.3(7) | ||
| (CN)-CdT-(CN)/ᵒ | |||||
| C3-Cd3-C4 | 115.1(4) | C3-Cd3-N7 | 109.4(3) | C4-Cd3-N7 | 105.2(3) |
| C3-Cd3-N9 | 113.0(3) | C4-Cd3-N9 | 107.0(3) | N7-Cd3-N9 | 106.6(3) |
| C2-Cd4-C1 | 118.5(3) | C2-Cd4-C8 | 115.2(4) | C1-Cd4-C8 | 106.3(4) |
| C2-Cd4-N10 | 105.3(4) | C1-Cd4-N10 | 106.4(5) | C8-Cd4-N10 | 103.8(5) |
| C6-Cd5-C5 | 117.1(4) | C6-Cd5-N8 | 115.9(4) | C5-Cd5-N8 | 107.6(3) |
| C6-Cd5-C7 | 104.8(3) | C5-Cd5-C7 | 106.0(3) | N8-Cd5-C7 | 104.1(3) |
| Hydrogen bonds/Å | |||||
| O1···O6 iv | 2.84(3) | O1···O7′ iv | 2.922(14) | O1···O7 iv | 3.323(18) |
| O1···O7′x iv | 2.922(14) | O2···O5′ vi | 2.865(18) | O4···O3 vi | 2.743(11) |
| O6···O1 iv | 2.84(3) | O6′···O1 | 3.19(3) | C11···O6 iv | 3.48(3) |
| CdT-(CN)/Å | |||||
| Cd1-C1 | 2.196(2) | Cd1-C2 | 2.219(2) | Cd1-C3 | 2.234(2) |
| Cd1-C5 | 2.192(2) | ||||
| CdSP-O/Å | |||||
| Cd2-O1 | 2.5875(17) | ||||
| CdSP-(CN)/Å | |||||
| Cd2-N1 | 2.3408(19) | Cd2-N3 | 2.215(2) | Cd2-C4 | 2.188(2) |
| Cd2-N2 | 2.215(2) | ||||
| CdOC-O/Å | |||||
| Cd3-O3 | 2.343(3) | ||||
| CdOC-(CN)/Å | |||||
| Cd3-N4 | 2.290(2) | Cd3-N5 | 2.309(2) | ||
| (CN)-CdT-(CN)/ᵒ | |||||
| C1-Cd1-C2 | 107.10(8) | C1-Cd1-C3 | 109.86(7) | C2-Cd1-C3 | 101.73(8) |
| C5-Cd1-C1 | 116.97(8) | C5-Cd1-C2 | 111.16(8) | C5-Cd1-C3 | 108.93(8) |
| (CN)-CdSP-O/ᵒ | |||||
| N1-Cd2-O1 | 167.42(6) | N3-Cd2-O1 | 85.81(6) | C4-Cd2-O1 | 81.76(7) |
| N2-Cd2-O1 | 92.99(6) | ||||
| (CN)-CdSP-(CN)/ᵒ | |||||
| C4-Cd2-N3 | 139.92(8) | C4-Cd2-N1 | 96.07(8) | N3-Cd2-N1 | 88.04(7) |
| C4-Cd2-N2 | 113.43(8) | N3-Cd2-N2 | 105.12(7) | N2-Cd2-N1 | 99.21(7) |
| cis(CN)-CdOC-O/ᵒ | |||||
| N4-Cd3-O3 | 90.07(9) | N5-Cd3-O3 | 89.45(9) | ||
| N4-Cd3-O3 i | 89.93(9) | N5-Cd3-O3 i | 90.55(9) | ||
| cis(CN)-CdOC-O/ᵒ | |||||
| N4-Cd3-N5 | 87.66(8) | N4-Cd3-N5 i | 92.34(8) | ||
| hydrogen bonds | |||||
| O1...O2 viii | 2.808(2) | O31′...O3 | 3.104(8) | ||
| C12...O31 vii | 3.178(8) | C12...O31′ vii | 3.514(8) | C21...O3 | 3.373(7) |
| C21B...O4′ | 3.21(3) | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawasaki, T.; Kitazawa, T. Synthesis and Crystal Structures of Cadmium(II) Cyanide with Branched-Butoxyethanol. Crystals 2018, 8, 221. https://doi.org/10.3390/cryst8050221
Kawasaki T, Kitazawa T. Synthesis and Crystal Structures of Cadmium(II) Cyanide with Branched-Butoxyethanol. Crystals. 2018; 8(5):221. https://doi.org/10.3390/cryst8050221
Chicago/Turabian StyleKawasaki, Takeshi, and Takafumi Kitazawa. 2018. "Synthesis and Crystal Structures of Cadmium(II) Cyanide with Branched-Butoxyethanol" Crystals 8, no. 5: 221. https://doi.org/10.3390/cryst8050221