Copper Delafossites under High Pressure—A Brief Review of XRD and Raman Spectroscopic Studies
Abstract
:1. Introduction
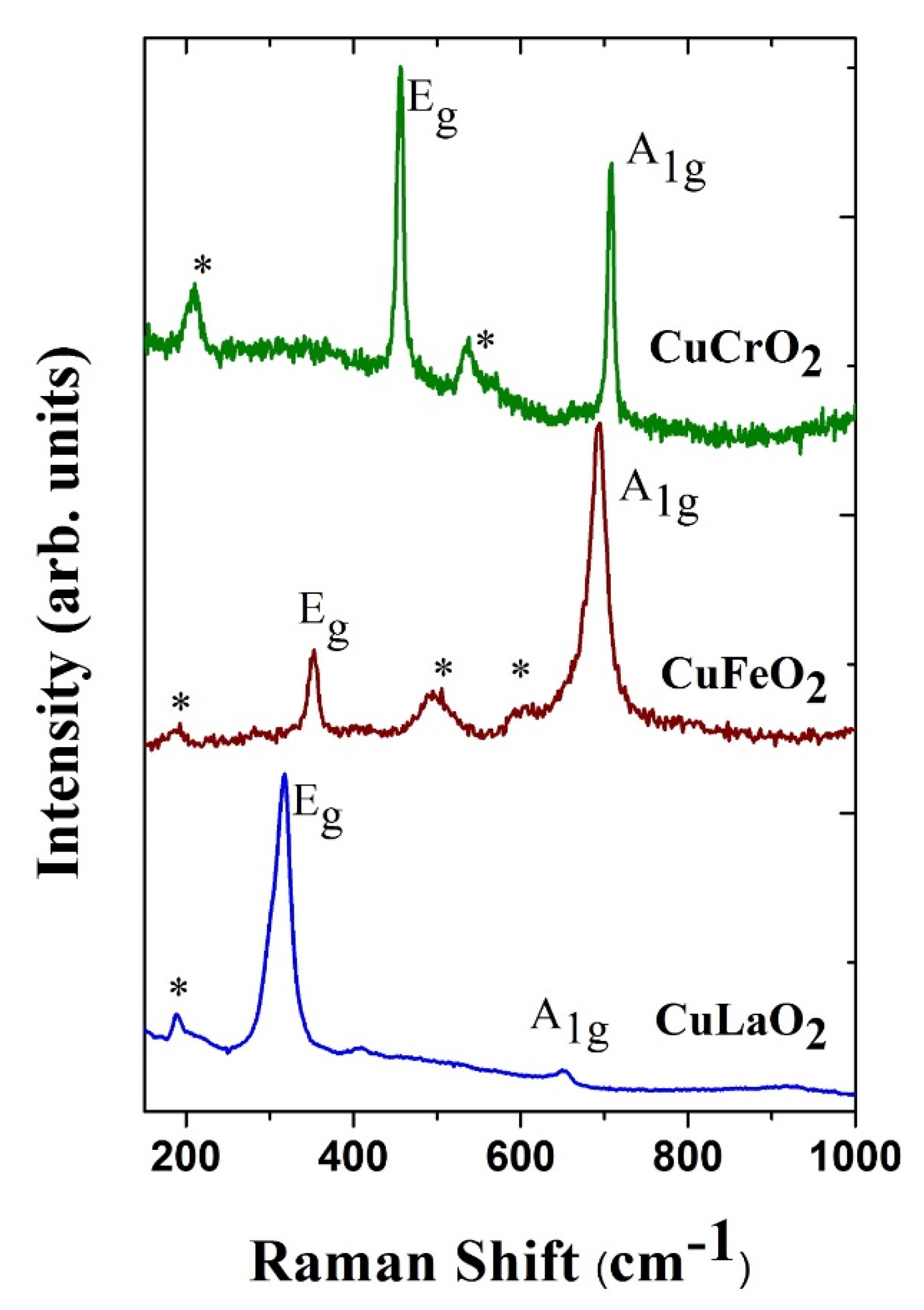
2. Ambient Crystal Structure and Vibrational Properties of ABO2
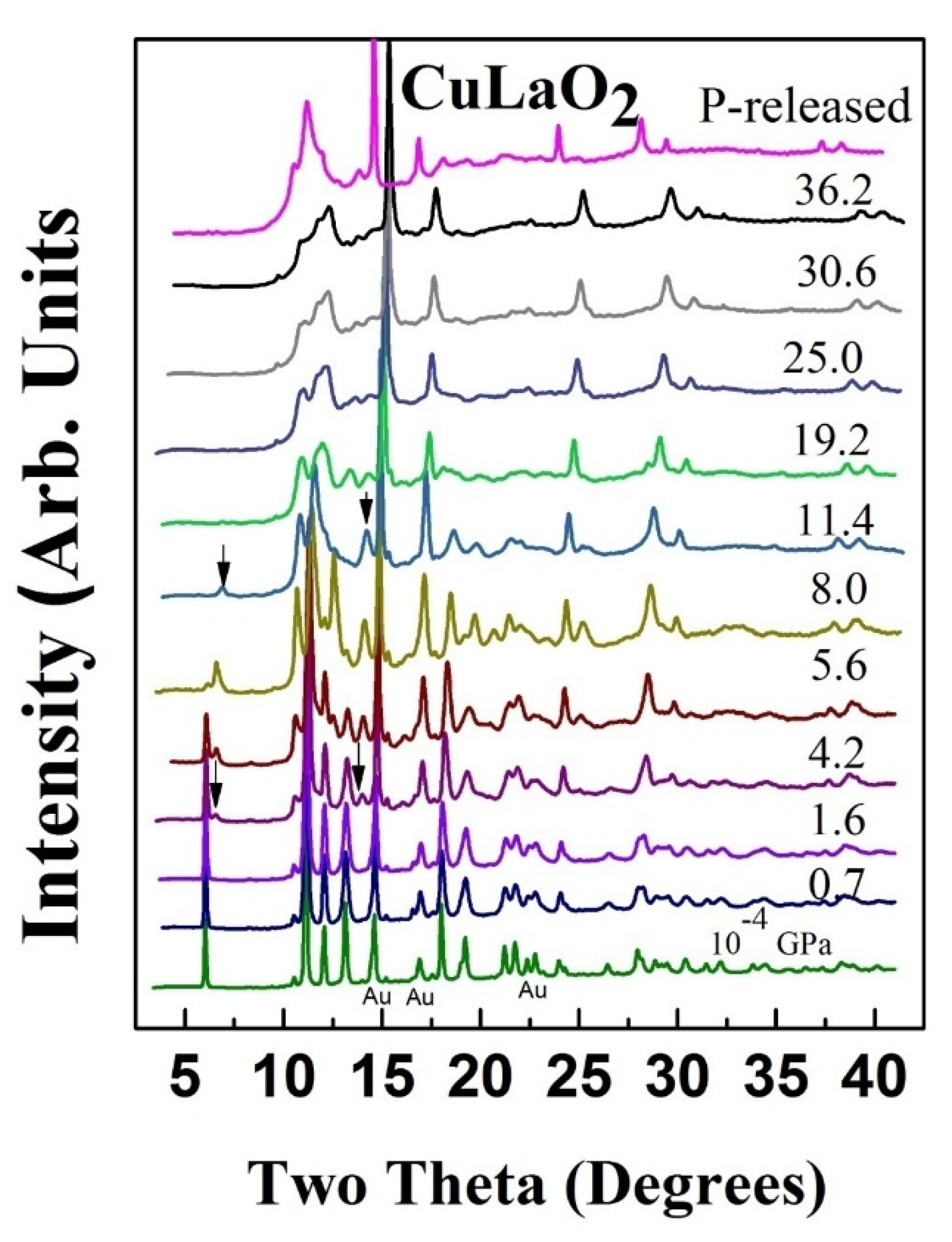
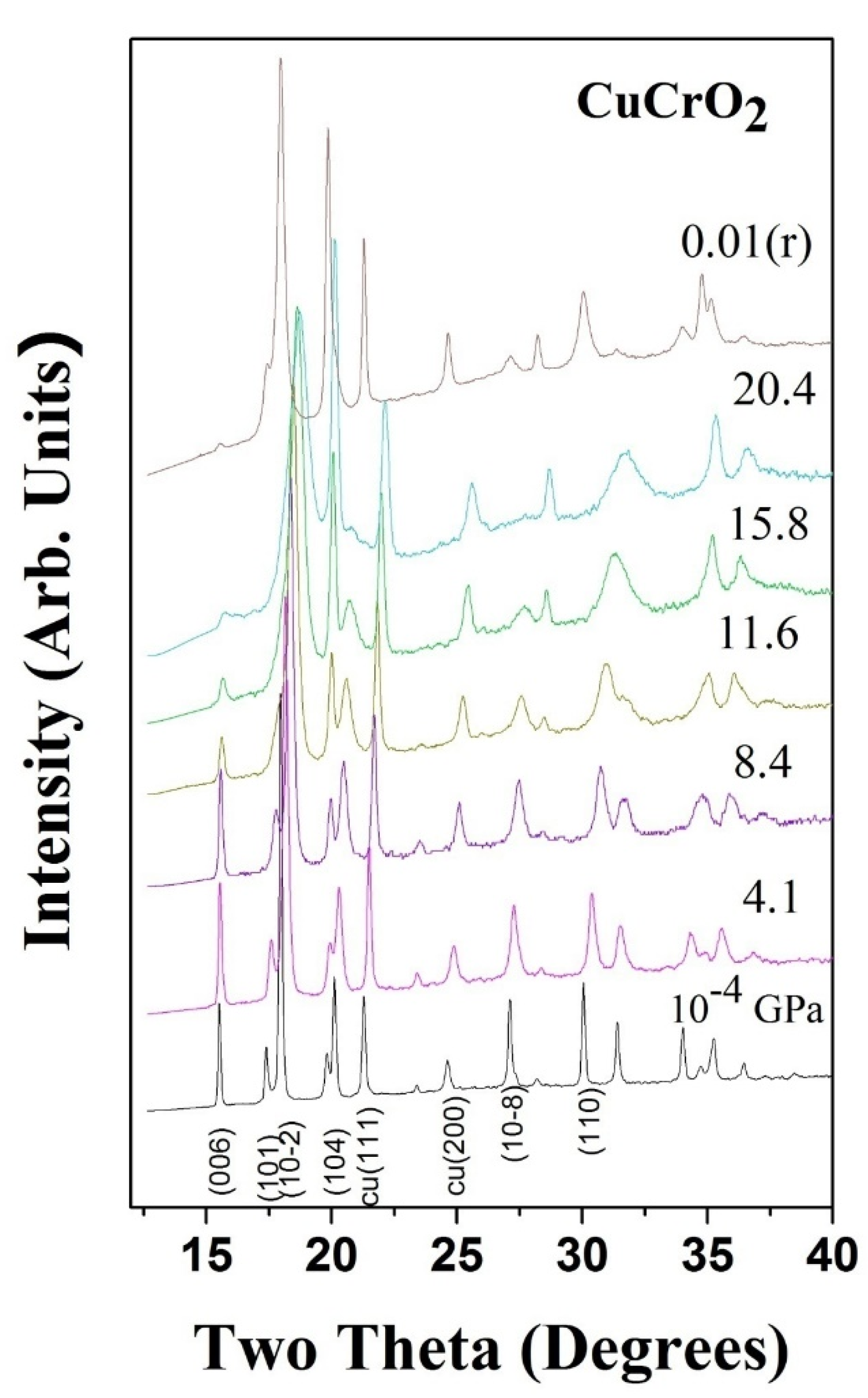
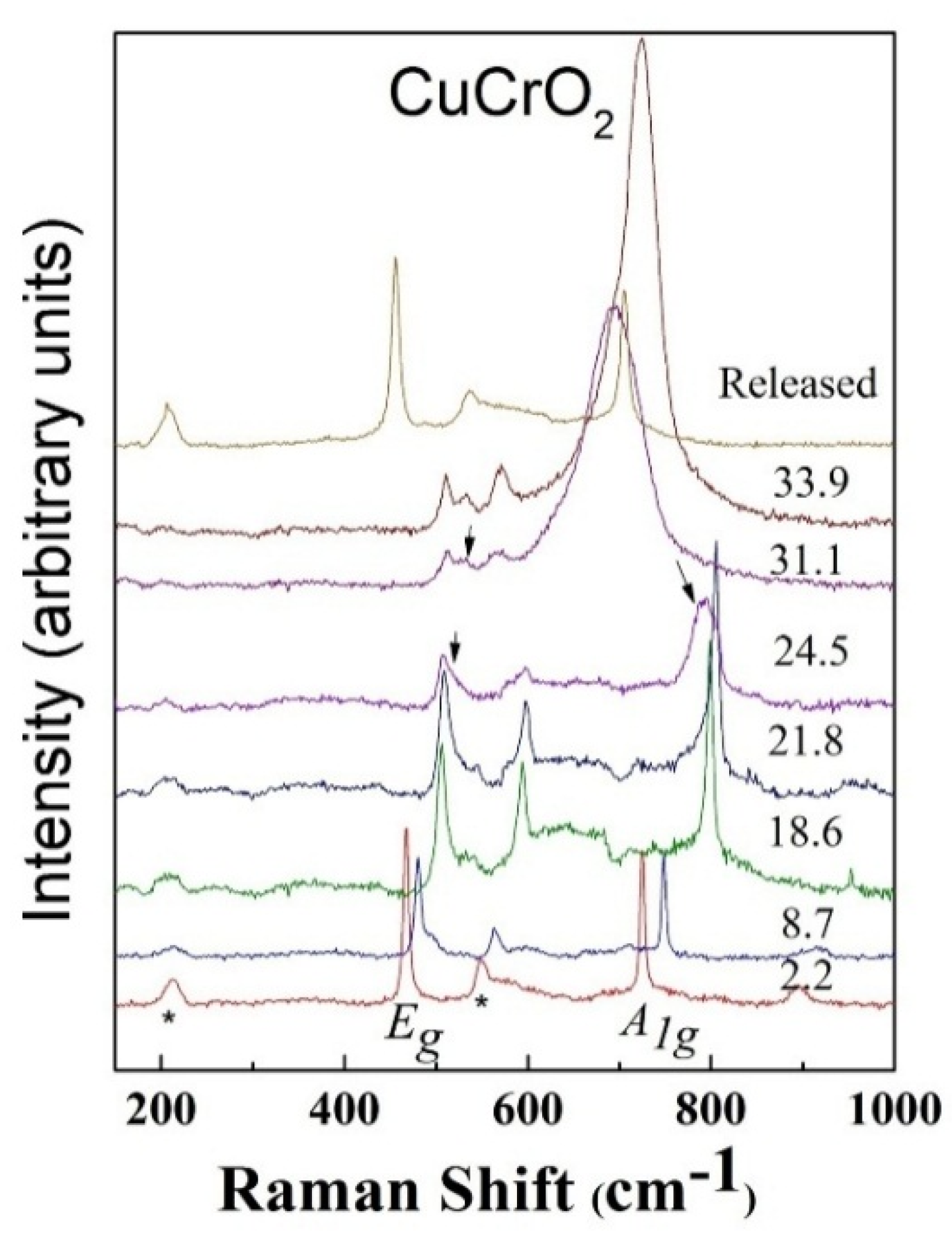
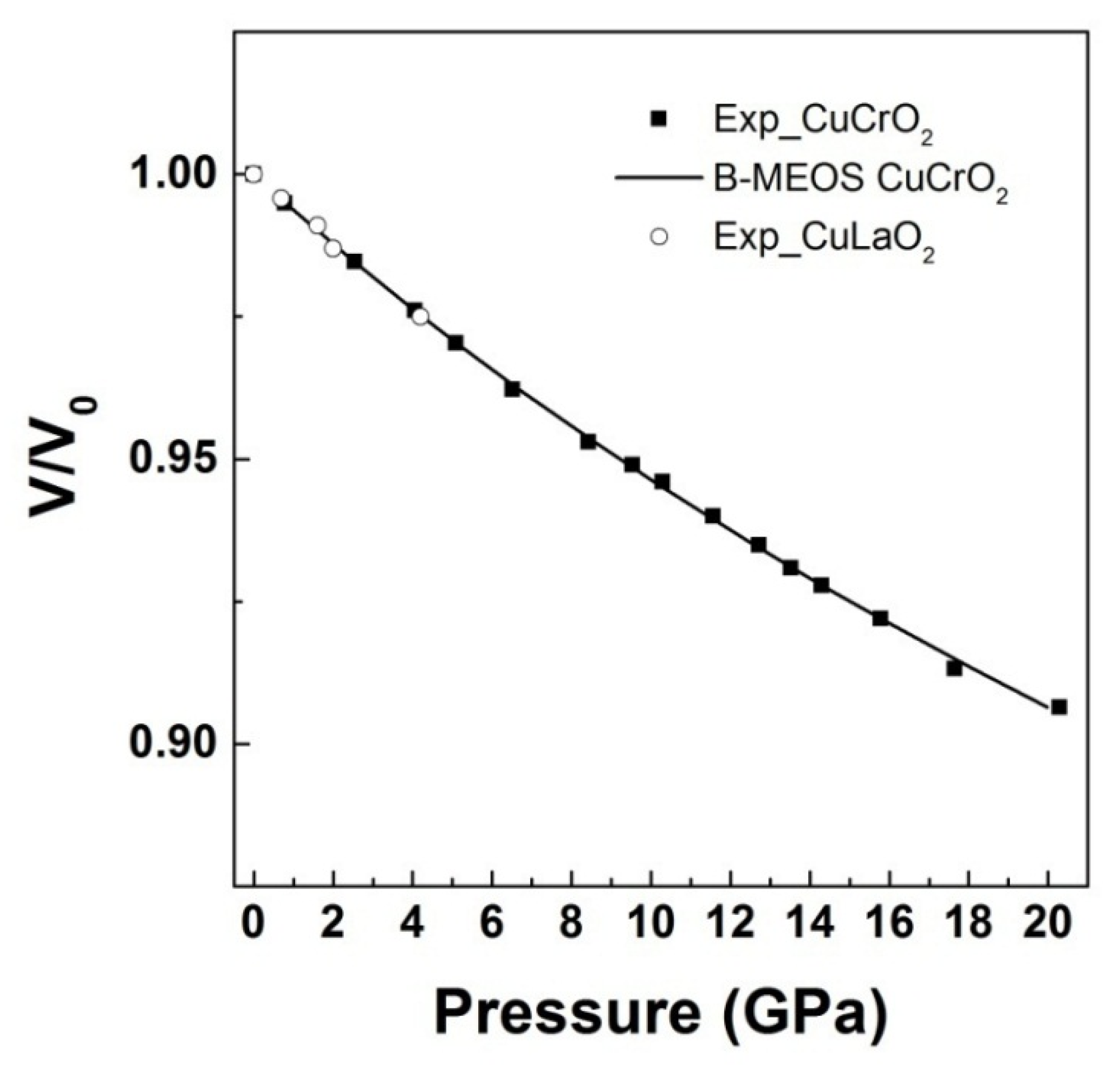
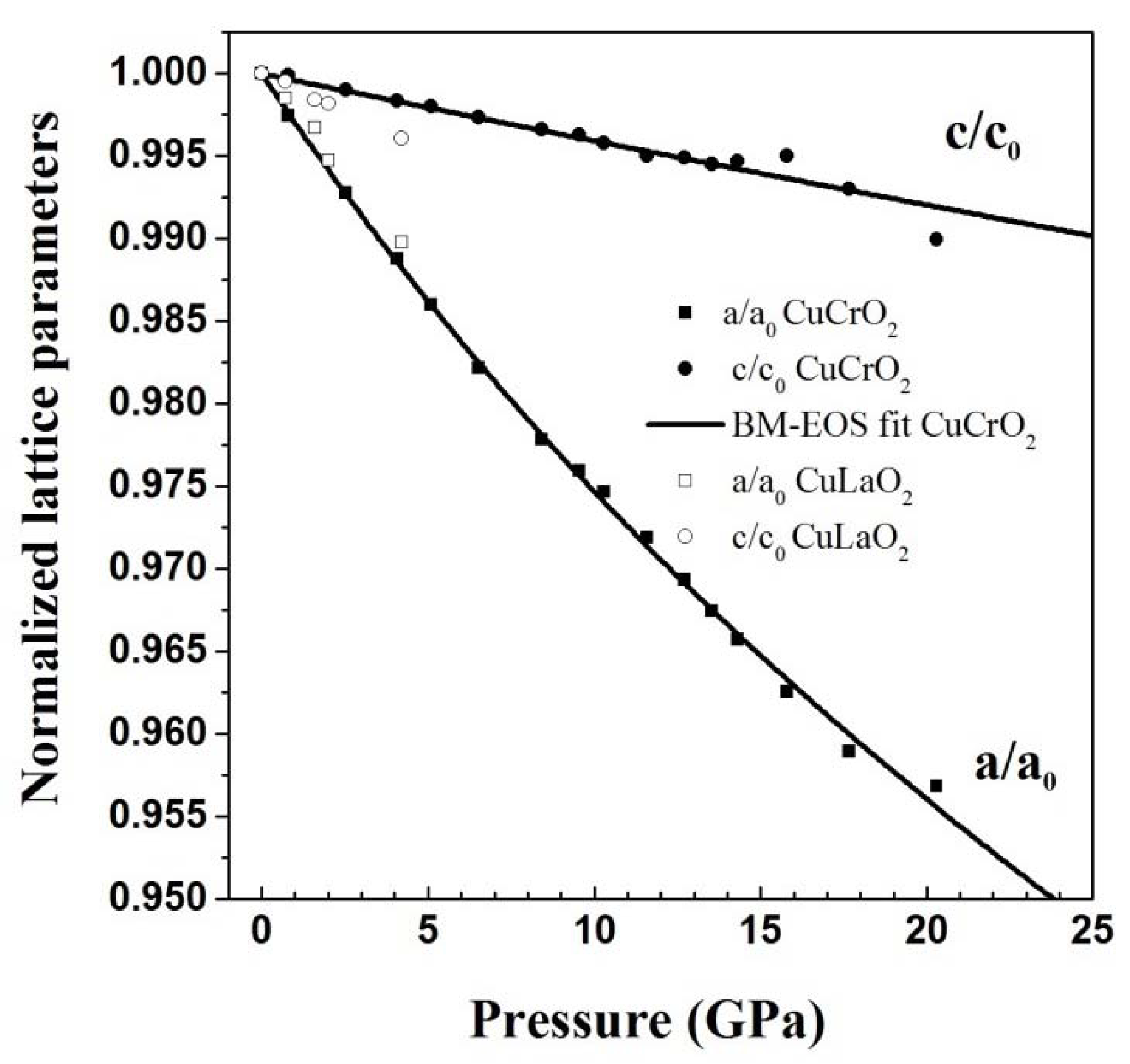
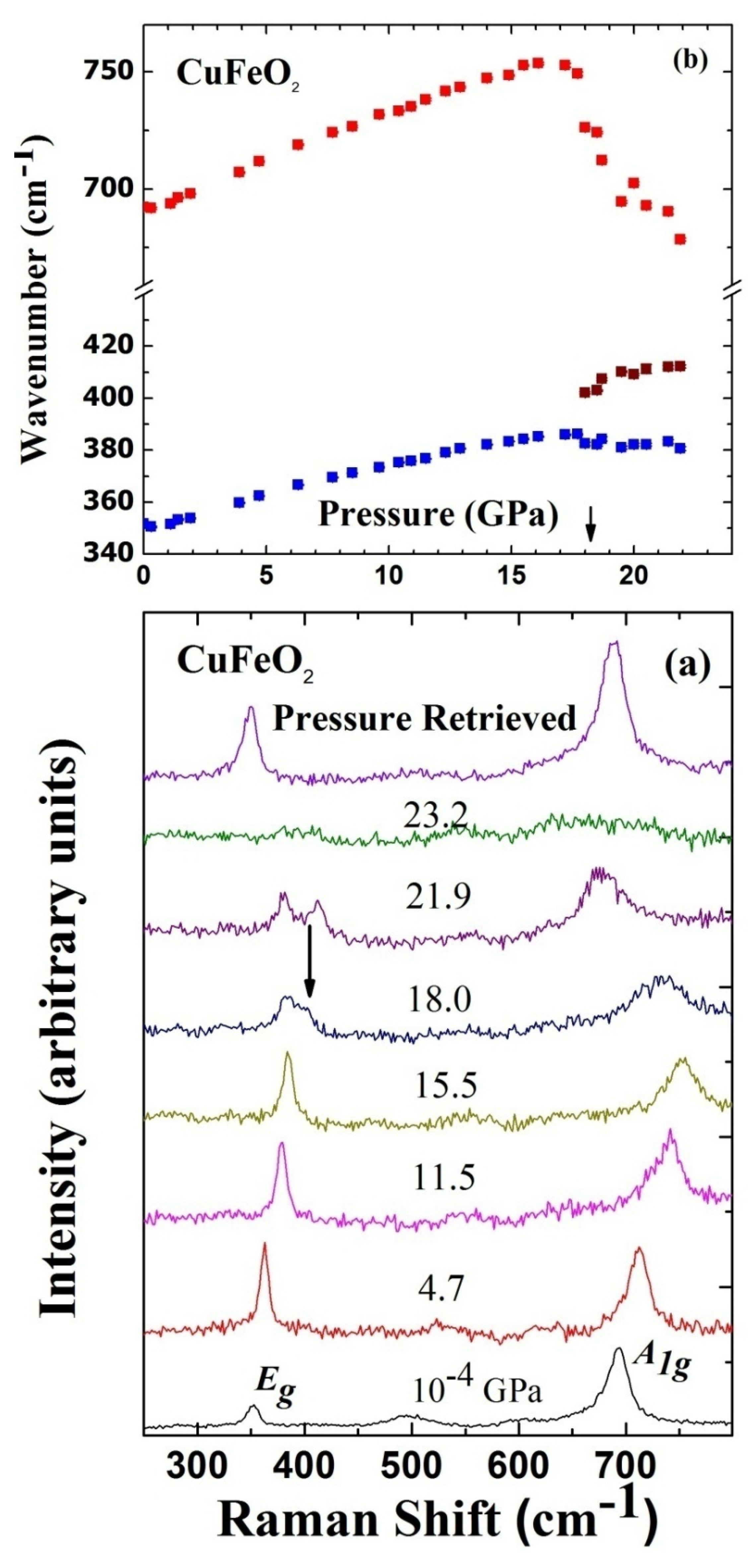
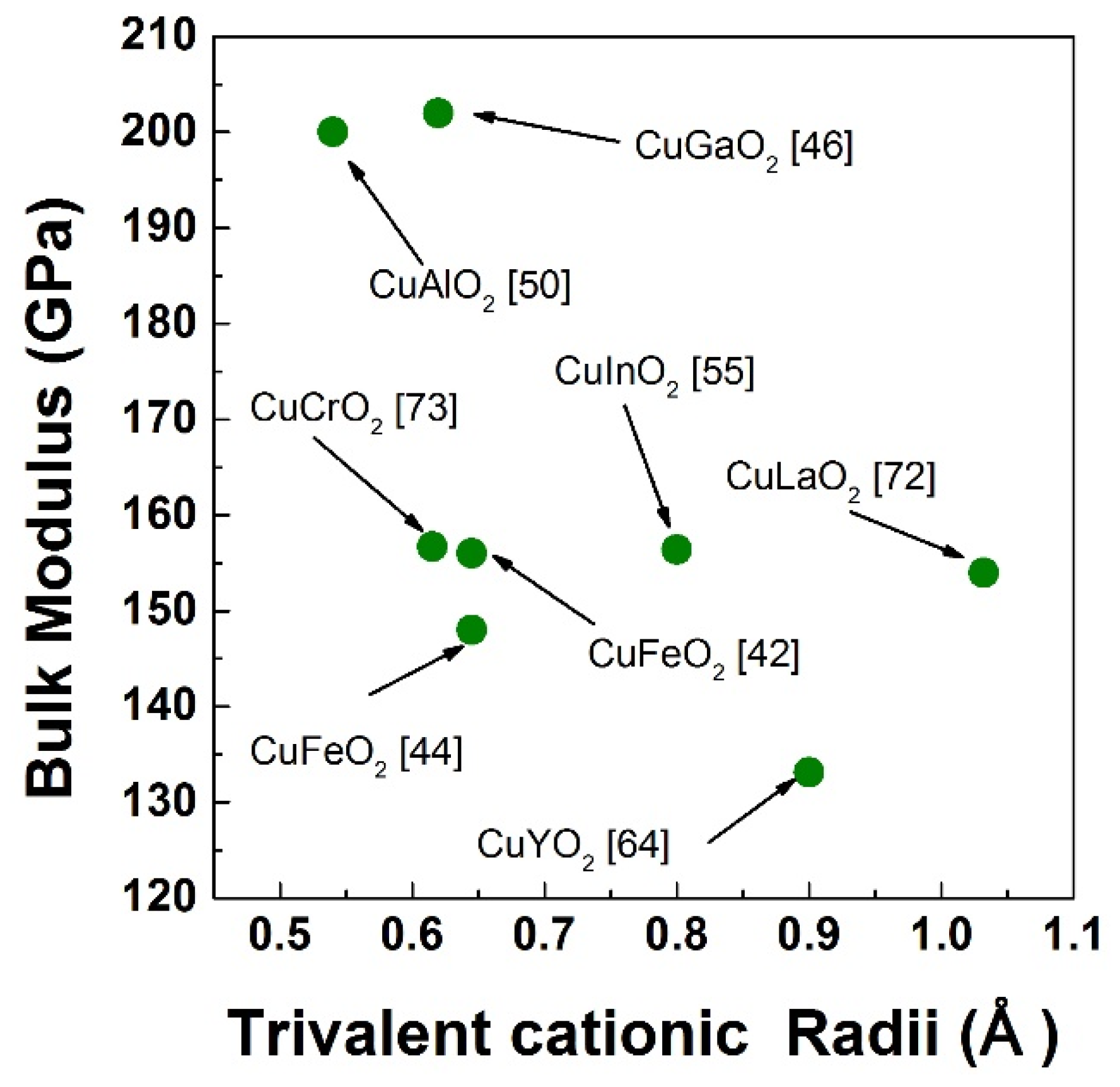
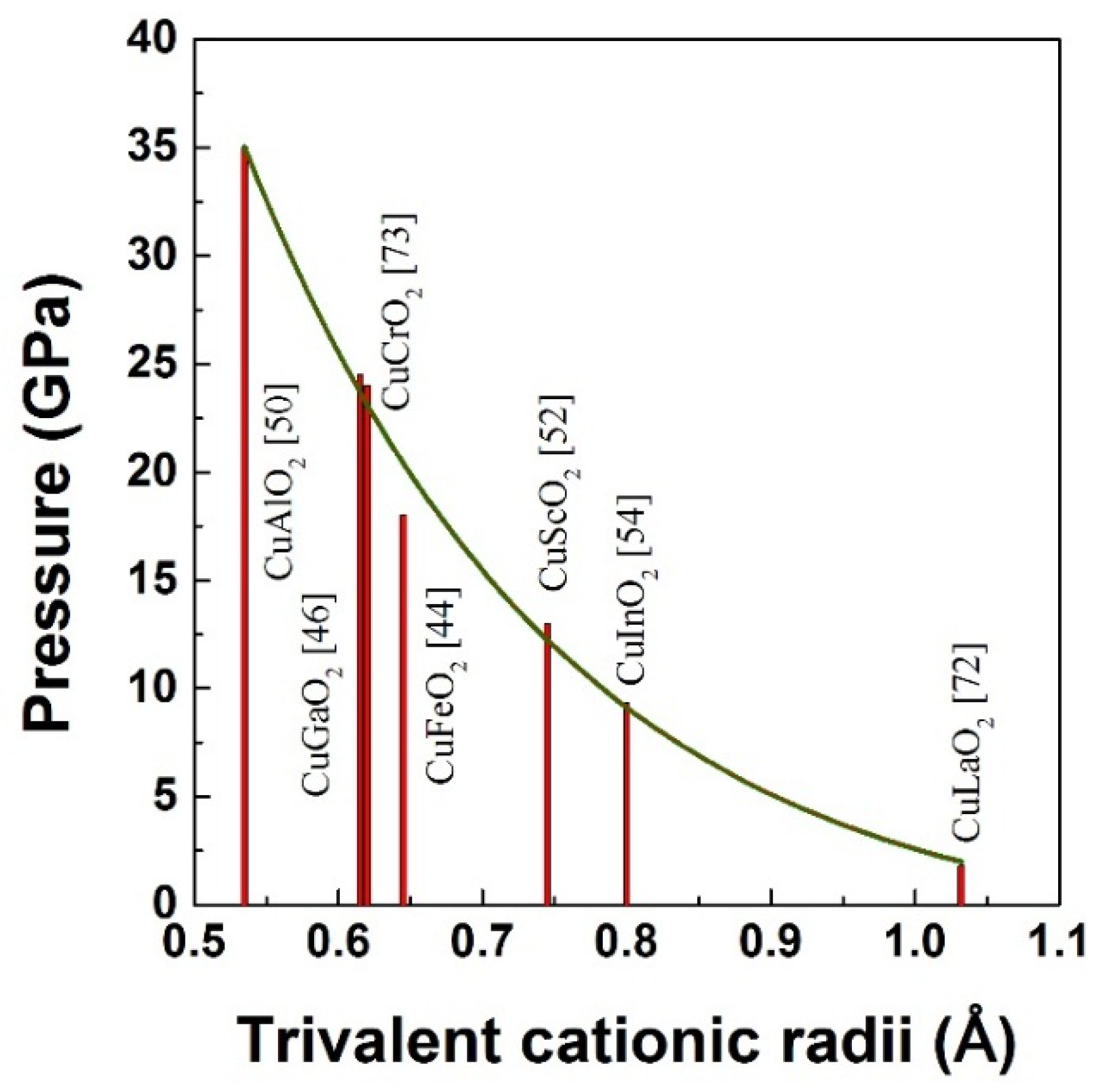
3. High Pressure Studies
4. Summary
Conflicts of Interest
References
- Shannon, R.D.; Rogers, D.B.; Prewitt, C.T. Chemistry of noble metal oxides I. Syntheses and properties of ABO2 delafossite compounds. Inorg. Chem. 1971, 10, 713–718. [Google Scholar] [CrossRef]
- Dordor, P.; Chaminade, J.P.; Wichainchai, A.; Marquestaut, E.; Doumerc, J.P.; Pouchard, M.; Hagenmuller, P. Crystal growth and electrical properties of CuFeO2 single crystals. J. Solid State Chem. 1988, 75, 105–112. [Google Scholar] [CrossRef]
- Marquardt, M.A.; Ashmore, N.A.; Cann, D.P. Crystal chemistry and electrical properties of the delafossite structure. Thin Solid Films 2006, 496, 146–156. [Google Scholar] [CrossRef]
- Kawazoe, H.; Yasukawa, M.; Hyodo, H.; Kurita, M.; Yanagi, H.; Hosono, H. P-type electrical conduction in transparent thin films of CuAlO2. Nature 1997, 389, 939–942. [Google Scholar] [CrossRef]
- Mackenzie, A.P. The Properties of ultrapure delafossite metals. Rep. Prog. Phys. 2017, 80, 032501–032519. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.N.; Chattopadhyay, K.K. Recent developments in the emerging field of crystalline p-type transparent conducting oxide thin films. Prog. Cryst. Growth Charact. Mater. 2005, 50, 52–105. [Google Scholar] [CrossRef]
- Walsh, A.; Da Silva, J.L.F.; Wei, S.-H. Multi-component transparent conducting oxides: Progress in materials modelling. J. Phys. Condens. Matter 2011, 23, 334210. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Fang, G.; Li, C.; Xu, S.; Zhao, X. P-type transparent conducting oxides. Phys. Status Solidi A 2006, 203, 1891–1900. [Google Scholar] [CrossRef]
- King, P.D.C.; Veal, T.D. Conductivity in transparent oxide semiconductors. J. Phys. Condens. Matter 2011, 23, 334214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Natu, G.; Ji, Z.; Wu, Y. P-type dye-sensitized solar cells based on delafossite CuGaO2 nanoplates with saturation photovoltages exceeding 460 mv. J. Phys. Chem. Lett. 2012, 3, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Chae, G.J. A modified transparent conducting oxide for flat panel displays only. J. Appl. Phys. 2001, 40, 1282–1286. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Azens, A.; Hjelm, A.; Kullman, L.; Niklasson, G.A.; Ronnow, D.; Mattsson, M.S.; Veszele, M.; Vaiva, G. Recent advances in electrochromics for smart windows applications. Sol. Energy 1998, 63, 199–276. [Google Scholar] [CrossRef]
- Diaz-Garcia, A.K.; Lana-Villarreal, T.; Gomez, R. Sol–gel copper chromium delafossite thin films as stable oxide photocathodes for water splitting. J. Mater. Chem. A 2015, 3, 19683–19687. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, K.; Baeg, J.O.; Lee, S.M.; Subramanian, E.; Moon, S.J.; Kong, K.J. Visible light assisted highly efficient hydrogen production from H2S decomposition by CuGaO2 and CuGa1−xInxO2 delafossite oxides bearing nanostructured co-catalysts. Catal. Commun. 2008, 9, 395–402. [Google Scholar] [CrossRef]
- Kykyneshi, R.; Nielsen, B.C.; Tate, J.; Li, J.; Sleight, A.W. Structural and transport properties of CuSc1−xMgxO2+ydelafossites. J. Appl. Phys. 2004, 96, 6188–6194. [Google Scholar] [CrossRef]
- Mazumder, N.; Sen, D.; Ghorai, U.K.; Roy, R.; Saha, S.; Das, N.S.; Chattopadhyay, K.K. Realizing direct gap, polytype, group IIIA delafossite: Ab initio forecast and experimental validation considering prototype CuAlO2. J. Phys. Chem. Lett. 2013, 4, 3539–3543. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, M.; Sunada, K.; Miyauchi, M.; Hashimoto, K. A facile one-step hydrothermal synthesis of rhombohedral CuFeO2 crystals with antivirus property. Chem. Commun. 2012, 48, 7365–7367. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cao, C.; Chui, Y.S.; Zapien, J.A. Facile hydrothermal synthesis of CuFeO2 hexagonal platelets/rings and graphene composites as anode materials for lithium ion batteries. Chem. Commun. 2014, 50, 10151–10154. [Google Scholar] [CrossRef] [PubMed]
- Patzsch, J.; Balog, I.; Krau, P.; Lehmann, C.W.; Schneider, J.J. Synthesis, characterization and p–n type gas sensing behaviour of CuFeO2delafossite type inorganic wires using Fe and Cu complexes as single source molecular precursors. RSC Adv. 2014, 4, 15348–15355. [Google Scholar] [CrossRef]
- Amrute, A.P.; Larrazabal, G.O.; Mondelli, C.; Perez-Ramirez, J. CuCrO2Delafossite: A stable copper catalyst for chlorine production. Angew. Chem. Int. Ed. 2013, 52, 9772–9775. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.I.; Dalba, G.; Fornasini, P.; Vaccari, M.; Rocca, F.; Sanson, A.; Li, J.; Sleight, A.W. Negative thermal expansion in crystals with the delafossite structure: An extended X-ray absorption fine structure study of CuScO2 and CuLaO2. Phys. Rev. B 2009, 79, 104302. [Google Scholar] [CrossRef]
- Seki, S.; Onose, Y.; Tokura, Y. Spin-driven ferroelectricity in triangular lattice antiferromagnets ACrO2 (A = Cu, Ag, Li, or Na). Phys. Rev. Lett. 2008, 101, 067204. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Cao, H.; Fang, J.; Jiang, X.; Ji, X.; Dong, Z. Spin-lattice coupling and helical-spin driven ferroelectric polarization in multiferroic CuFeO2. Appl. Phys. Lett. 2010, 97, 094103. [Google Scholar] [CrossRef]
- Omata, T.; Nagatani, H.; Suzuki, I.; Kita, M.; Yanagi, H.; Ohashi, N. A new direct and narrow band gap oxide semiconductor applicable as a solar cell absorber. J. Am. Chem. Soc. 2014, 136, 3378–3381. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, Y.; Mellott, N.P.; Wang, B.; Ye, H.; Wu, Y. Luminescence of delafossite-type CuAlO2 fibers with Eu substitution for Al cations. Sci. Technol. Adv. Mater. 2016, 17, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, F.; Wang, Y.; Wang, D.; Zhao, B.; Zhang, L.; Zhao, D.; Shen, D. Photoluminescence and photocatalytic properties of rhombohedral CuGaO2 nanoplates. Sci. Rep. 2016, 6, 21135. [Google Scholar] [CrossRef] [PubMed]
- Yassin, O.A.; Alamri, S.N.; Joraid, A.A. Effect of particle size and laser power on the Raman spectra of CuAlO2 delafossite nanoparticles. J. Phys. D Appl. Phys. 2013, 46, 235301. [Google Scholar] [CrossRef]
- Ahmed, J.; Mao, Y. Delafossite CuAlO2 nanoparticles with electrocatalytic activity toward oxygen and hydrogen evolution reactions. In Nanomaterials for Sustainable Energy; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2015; Chapter 4; Volume 1213, pp. 57–72. [Google Scholar]
- Harada, T.; Fujiwara, K.; Tsukazaki, A. Highly conductive PdCoO2 ultrathin films for transparent electrodes. APL Mater. 2018, 6, 046107. [Google Scholar] [CrossRef]
- Deng, Z.; Fang, X.; Wu, S.; Dong, W.; Shao, J.; Wang, S.; Lei, M. The morphologies and optoelectronic properties of delafossite CuFeO2 thin films prepared by PEG assisted. J. Sol-Gel Sci. Technol. 2014, 71, 297–302. [Google Scholar] [CrossRef]
- Sinnarasa, I.; Thimont, Y.; Presmanes, L.; Barnabé, A.; Tailhades, P. Thermoelectric and transport properties of delafossite CuCrO2:Mg thin films prepared by RF magnetron sputtering. Nanomaterials 2017, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Barnabe, A.; Thimont, Y.; Lalanne, M.; Presmanes, L.; Tailhades, P. P-type conducting transparent characteristics of delafossite Mg-doped CuCrO2 thin films prepared by RF-sputtering. J. Mater. Chem. C 2015, 3, 6012–6024. [Google Scholar] [CrossRef] [Green Version]
- Errandonea, D. Exploring the properties of MTO4 compounds using high-pressure powder X-ray diffraction. Cryst. Res. Technol. 2015, 50, 729–736. [Google Scholar] [CrossRef]
- Errandonea, D.; Ruiz-Fuertes, A. Brief review of the effects of pressure on wolframite-type oxides. Crystals 2018, 8, 71. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tanaka, M.; Yagi, T.; Takei, H.; Inoue, A. Compression behavior of the delafossite-type metallic oxide PdCoO2 below 10 GPa. Solid State Commun. 2003, 128, 303–307. [Google Scholar] [CrossRef]
- Sheets, W.C.; Mugnier, E.; Barnabe, A.; Marks, T.J.; Poeppelmeier, K.R. Hydrothermal synthesis of delafossite-type oxides. Chem. Mater. 2006, 18, 7–20. [Google Scholar] [CrossRef]
- Kumar, S.; Miclau, M.; Christine, M. Hydrothermal synthesis of AgCrO2 delafossite in supercritical water: A new single-step process. Chem. Mater. 2013, 25, 2083–2088. [Google Scholar] [CrossRef]
- Jin, Y.; Chuamanov, G. Solution synthesis of pure 2H CuFeO2 at low temperatures. RSC Adv. 2016, 6, 26392–26397. [Google Scholar] [CrossRef]
- Effenberger, H. Structure of Hexagonal Copper(I) Ferrite. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1991, 47, 2644–2646. [Google Scholar] [CrossRef]
- Godinho, K.G.; Morgan, B.J.; Allen, J.P.; Scanlon, D.O.; Watson, G.W. Chemical bonding in copper-based transparent conducting oxides: CuMO2 (M = In, Ga, Sc). J. Phys. Condens. Matter 2011, 23, 334201. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, D.L.; Bauman, R.P.; Porto, S.P.S. Normal mode determination in crystals. J. Raman Spectrosc. 1981, 10, 253–290. [Google Scholar] [CrossRef]
- Zhao, T.R. X-ray diffraction study of copper iron oxide [CuFeO2] under pressures up to 10 GPa. Mater. Res. Bull. 1997, 32, 151–157. [Google Scholar] [CrossRef]
- Birch, F. Finite strain isotherm and velocities for single-crystal and polycrystalline NaCl at high pressures and 300° K. J. Geophys. Res. Solid Earth 1978, 83, 1257–1268. [Google Scholar] [CrossRef]
- Xu, W.M.; Rozenberg, G.K.; Pasternak, M.P.; Kertzer, M.; Kurnosov, A.; Dubrovinsky, L.S.; Pascarelli, S.; Munoz, M.; Vaccari, M.; Hanfland, M.; et al. Pressure-induced Fe-Cu cationic valence exchange and its structural consequences: High-pressure studies of delafossite CuFeO2. Phys. Rev. B 2010, 81, 104110. [Google Scholar] [CrossRef]
- Terada, N.; Osakabe, T.; Kitazawa, H. High-pressure suppression of long range magnetic order in the triangular lattice antiferromagnet CuFeO2. Phys. Rev. B 2010, 83, 020403. [Google Scholar] [CrossRef]
- Pellicer-Porres, J.; Segura, A.; Ferrer-Roca, C.; MartıiNez-Garcıi, A.D.; Sans, J.A.; MartıiNez, E.; Itie, J.P.; Polian, A.; Baudelet, F.; Munoz, A.; et al. Structural evolution of the CuGaO2 delafossite under high pressure. Phys. Rev. B 2004, 69, 024109. [Google Scholar] [CrossRef]
- Pellicer-Porres, J.; Segura, A.; Martínez, E.; Saitta, A.M.; Polian, A.; Chervin, J.C.; Canny, B. Vibrational properties of delafossite CuGaO2 at ambient and high pressure. Phys. Rev. B 2005, 72, 064301. [Google Scholar] [CrossRef]
- Pellicer-Porres, J.; Martínez-García, D.; Segura, A.; Rodríguez-Hernández, P.; Muñoz, A.; Chervin, J.C.; Garro, N.; Kim, D. Pressure and temperature dependence of the lattice dynamics of CuAlO2 investigated by Raman scattering experiments and ab initiocalculations. Phys. Rev. B 2006, 74, 184301. [Google Scholar] [CrossRef]
- Liu, Q.J.; Liu, Z.T.; Feng, L.P.; Tian, H.; Liu, W.T.; Yan, F. Density functional theory study of 3r–and 2h–CuAlO2 under pressure. Appl. Phys. Lett. 2010, 97, 141917. [Google Scholar] [CrossRef]
- Pellicer-Porres, J.; Segura, A.; Ferrer-Roca, C.; Polian, A.; Munsch, P.; Kim, D. XRD and XAS structural study of CuAlO2 under high pressure. J. Phys. Condens. Matter 2013, 25, 115406. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Katayama-Yoshida, H. Pressure-induced structural transition and enhancement of energy gap of CuAlO2. J. Phys. Soc. Jpn. 2011, 80, 024706. [Google Scholar] [CrossRef]
- Gilliland, S.; Pellicer-Porres, J.; Segura, A.; Muñoz, A.; Rodríguez-Hernández, P.; Kim, D.; Lee, M.S.; Kim, T.Y. Electronic structure of CuAlO2 and CuScO2delafossites under pressure. Phys. Status Solidi B 2007, 244, 309–314. [Google Scholar] [CrossRef]
- Nie, X.; Su-Huai, W.; Zhang, S.B. Bipolar doping and band-gap anomalies in delafossite transparent conductive oxides. Phys. Rev. Lett. 2002, 88, 066405. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Q.; Liu, Z.-T. First principles studies of structural, mechanical, electronic, optical properties and pressure-induced phase transition of CuInO2 polymorph. Physica B 2012, 407, 4665–4670. [Google Scholar] [CrossRef]
- Jayalakshmi, V.; Murugan, R.; Palanivel, B. Electronic and structural properties of CuMO2 (M = Al, Ga, In). J. Alloy. Compd. 2005, 388, 19–22. [Google Scholar] [CrossRef]
- Aoyama, T.; Miyake, A.; Kagayama, T.; Shimizu, K.; Tsuyoshi, K. Pressure effects on the magnetoelectric properties of a multiferroic triangular-lattice antiferromagnet CuCrO2. Phys. Rev. B 2013, 87, 094401. [Google Scholar] [CrossRef]
- Piermarini, G.J.; Block, S.; Barnett, J.D. Hydrostatic limits in liquids and solids to 100 kbar. J. Appl. Phys. 1973, 44, 5377–5382. [Google Scholar] [CrossRef]
- Carter, W.T.; Marsh, S.P.; Fritz, J.N.; McQueen, R.G. Accurate Characterization of the High Pressure Environment; Lloyd, E.C., Ed.; NBS Special Pub.: Washington, DC, USA, 1971; Volume 326, p. 147. [Google Scholar]
- Larson, A.C.; Von Dreele, R.B. GSAS: General Structure Analysis System; Report LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2000. [Google Scholar]
- Petrenko, O.A.; Balakrishnan, G.; Lees, M.R.; Paul, D.M.; Hoser, A. High-magnetic-field behavior of the triangular-lattice antiferromagnet CuFeO2. Phys. Rev. B 2000, 62, 8983. [Google Scholar] [CrossRef]
- Aktas, O.; Truong, K.D.; Otani, T.; Balakrishnan, G.; Clouter, M.J.; Kimura, T.; Quirion, G. Raman scattering study of delafossite magnetoelectric multiferroic compounds: CuFeO2 and CuCrO2. J. Phys. Condens. Matter. 2012, 24, 036003. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef] [Green Version]
- Miyasaka, N.; Doi, Y.; Hinatsu, Y. Synthesis and magnetic properties of ALnO2 (A =Cu or Ag; Ln = rare earths) with the delafossite structure. J. Solid State Chem. 2009, 182, 2104–2110. [Google Scholar] [CrossRef]
- Cheng, C.; Lv, Z.L.; Cheng, Y.; Ji, G.F. Structural, elastic and electronic properties of CuYO2 from first-principles study. J. Alloy. Compd. 2014, 603, 183–189. [Google Scholar] [CrossRef]
- Shimode, M.; Sasaki, M.; Mukaida, K. Synthesis of the delafossite-type CuInO2. J. Solid State Chem. 2000, 151, 16–20. [Google Scholar] [CrossRef]
- Li, J.; Yokochi, A.F.T.; Sleight, A.W. Oxygen intercalation of two polymorphs of CuScO2. Solid State Sci. 2004, 6, 831–839. [Google Scholar] [CrossRef]
- Poienar, M.; Hardy, V.; Kundys, B.; Singh, K.; Maignan, A.; Damay, F.; Martin, C. Revisiting the properties of delafossite CuCrO2: A single crystal study. J. Solid State Chem. 2012, 185, 56–61. [Google Scholar] [CrossRef]
- Elkhouni, T.; Amami, M.; Hlil, E.K.; Salah, A.B. The structural, anisotropic magnetization, and spectroscopic study of delafossite CuCr1−xMxO2 systems. J. Supercond. Nov. Magn. 2015, 28, 1895–1903. [Google Scholar] [CrossRef]
- Elkhouni, T.; Amami, M.; Strobel, P.; Salah, A.B. Structural and magnetic properties of substituted delafossite-type oxides CuCr1−xScxO2. World J. Condens. Matter Phys. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Elkhoun, T.; Amami, M.; Hlil, E.K.; Salah, A.B. Effect of Spin dilution on the magnetic state of delafossite CuFeO2 with an S = 5/2 antiferromagnetic triangular sublattice. J. Supercond. Novel Magn. 2015, 28, 1439–1447. [Google Scholar] [CrossRef]
- Pavunny, S.P.; Kumar, A.; Katiyar, R.S. Raman spectroscopy and field emission characterization of delafossite CuFeO2. J. Appl. Phys. 2010, 107, 013522. [Google Scholar] [CrossRef]
- Salke, N.P.; Garg, A.B.; Rao, R.; Achary, S.N.; Gupta, M.K.; Mittal, R.; Tyagi, A.K. Phase transitions in delafossite CuLaO2 at high pressures. J. Appl. Phys. 2014, 115, 133507. [Google Scholar] [CrossRef]
- Garg, A.B.; Mishra, A.K.; Pandey, K.K.; Sharma, S.M. Multiferroic CuCrO2under high pressure: In situ X-ray diffraction and Raman spectroscopic studies. J. Appl. Phys. 2014, 116, 133514. [Google Scholar] [CrossRef]
- Inaba, M.; Iriyama, Y.; Ogumi, Z.; Todzuka, Y.; Tasaka, A. Raman study of layered rock-salt LiCoO2 and its electrochemical lithium deintercalation. J. Raman Spectrosc. 1997, 28, 613–617. [Google Scholar] [CrossRef]
- Gomis, O.; Lavina, B.; Rodríguez-Hernández, P.; Muñoz, A.; Errandonea, R.; Errandonea, D.; Bettinelli, M. High-pressure structural, elastic, and thermodynamic properties of zircon-type HoPO4 and TmPO4. J. Phys. Condens. Matter 2017, 29, 095401. [Google Scholar] [CrossRef] [PubMed]
- Salke, N.P.; Kamali, K.; Ravindran, T.R.; Balakrishnan, G.; Rao, R. Raman spectroscopic studies of CuFeO2 at high pressures. Vib. Spectrosc. 2015, 81, 112–118. [Google Scholar] [CrossRef]
- Mota, D.A.; Almeida, A.; Rodrigues, V.H.; Costa, M.M.R.; Tavares, P.; Bouvier, P.; Guennou, M.; Kreisel, J.; Moreira, J.A. Dynamic and structural properties of orthorhombic rare-earth manganites under high pressure. Phys. Rev. B 2014, 90, 054104. [Google Scholar] [CrossRef]
- Garg, A.B.; Errandonea, D.; Rodríguez-Hernández, P.; Muñoz, A. ScVO4 under non-hydrostatic compression: A new metastable polymorph. J. Phys. Condens. Matter 2017, 29, 055401. [Google Scholar] [CrossRef] [PubMed]





| Hexagonal P63/mmc, Z = 2 | Atomic coordinates | Vibrations at the zone centre | |||||||||
| Wyckoff position | x | y | z | A1g | E1g | E2g | B1g | A2u | E1u | E2u | B2u |
| Monovalent cation A at 2c | 1/3 | 2/3 | 1/4 | - | - | 1 | 1 | 1 | 1 | - | - |
| Trivalent cation B at 2a | 0 | 0 | 0 | - | - | - | - | 1 | 1 | 1 | 1 |
| Oxygen at 4f | 1/3 | 2/3 | 0.0892 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Rhombohedral R-3m, Z = 1 | Atomic co-ordinate | Vibrations at the zone centre | |||||||||
| Wyckoff position | x | y | z | A1g | Eg | A2u | Eu | ||||
| Monovalent cation A at 3b | 0 | 0 | 0 | - | - | 1 | 1 | ||||
| Trivalent cation B at 3a | 0 | 0 | 1/2 | - | - | 1 | 1 | ||||
| Oxygen at 6c | 0 | 0 | 0.108 | 1 | 1 | 1 | 1 | ||||
| Ordered rock salt R-3m, Z = 1 | Atomic co-ordinate | Vibrations at the zone centre | |||||||||
| Wyckoff position | x | y | z | A1g | Eg | A2u | Eu | ||||
| Monovalent cation A at 3a | 0 | 0 | 1/2 | - | - | 1 | 1 | ||||
| Trivalent cation B at 3b | 0 | 0 | 0 | - | - | 1 | 1 | ||||
| Oxygen at 6c | 0 | 0 | 0.743 | 1 | 1 | 1 | 1 | ||||
| Delafossite | Ionic Radii of Trivalent Cation (Å) [62] | Raman Mode Frequency | Lattice Parameter | Bond-Length | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Eg (cm−1) | A1g (cm−1) | a (Å) | c (Å) | Cu-O (Å) | M-O (Å) | |||
| CuLaO2 | 1.032 | 318 | 652 | 3.8326 | 17.092 | 1.760 | 2.466 | [63] |
| CuPrO2 | 0.99 | 3.7518 | 17.086 | 1.789 | 2.411 | [63] | ||
| CuNdO2 | 0.983 | 3.7119 | 17.085 | 1.836 | 2.370 | [63] | ||
| CuSmO2 | 0.958 | 3.6628 | 17.078 | 1.880 | 2.325 | [63] | ||
| CuEuO2 | 0.947 | 3.6316 | 17.074 | 1.895 | 2.302 | [63] | ||
| CuYO2 | 0.90 | 3.5330 | 17.136 | 1.827 | 2.285 | [64] | ||
| CuInO2 | 0.8 | 378 | 678 | 3.2922 | 17.388 | 1.845 | 2.172 | [65] |
| CuScO2 | 0.745 | 3.2204 | 17.099 | 1.831 | 2.121 | [66] | ||
| CuFeO2 | 0.645 | 352 | 692 | 3.0351 | 17.166 | 1.835 | 2.033 | [61] |
| CuGaO2 | 0.62 | 368 | 729 | 2.9770 | 17.171 | 1.848 | 1.996 | [46] |
| CuCrO2 | 0.615 | 454 | 703 | 2.9767 | 17.111 | 1.8455 | 1.989 | [67] |
| CuAlO2 | 0.535 | 418 | 767 | 2.8584 | 16.958 | 1.8617 | 1.912 | [50] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, A.B.; Rao, R. Copper Delafossites under High Pressure—A Brief Review of XRD and Raman Spectroscopic Studies. Crystals 2018, 8, 255. https://doi.org/10.3390/cryst8060255
Garg AB, Rao R. Copper Delafossites under High Pressure—A Brief Review of XRD and Raman Spectroscopic Studies. Crystals. 2018; 8(6):255. https://doi.org/10.3390/cryst8060255
Chicago/Turabian StyleGarg, Alka B., and Rekha Rao. 2018. "Copper Delafossites under High Pressure—A Brief Review of XRD and Raman Spectroscopic Studies" Crystals 8, no. 6: 255. https://doi.org/10.3390/cryst8060255




