The Mechanical Properties and Corrosion Resistance of Magnesium Alloys with Different Alloying Elements for Bone Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Specimen Preparation
2.2. Microstructure
2.3. Mechanical Property Tests
2.4. Electrochemical Noise Tests
2.5. Immersion Tests
3. Results
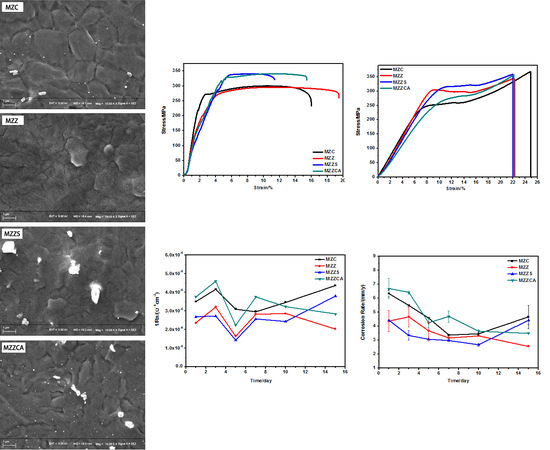
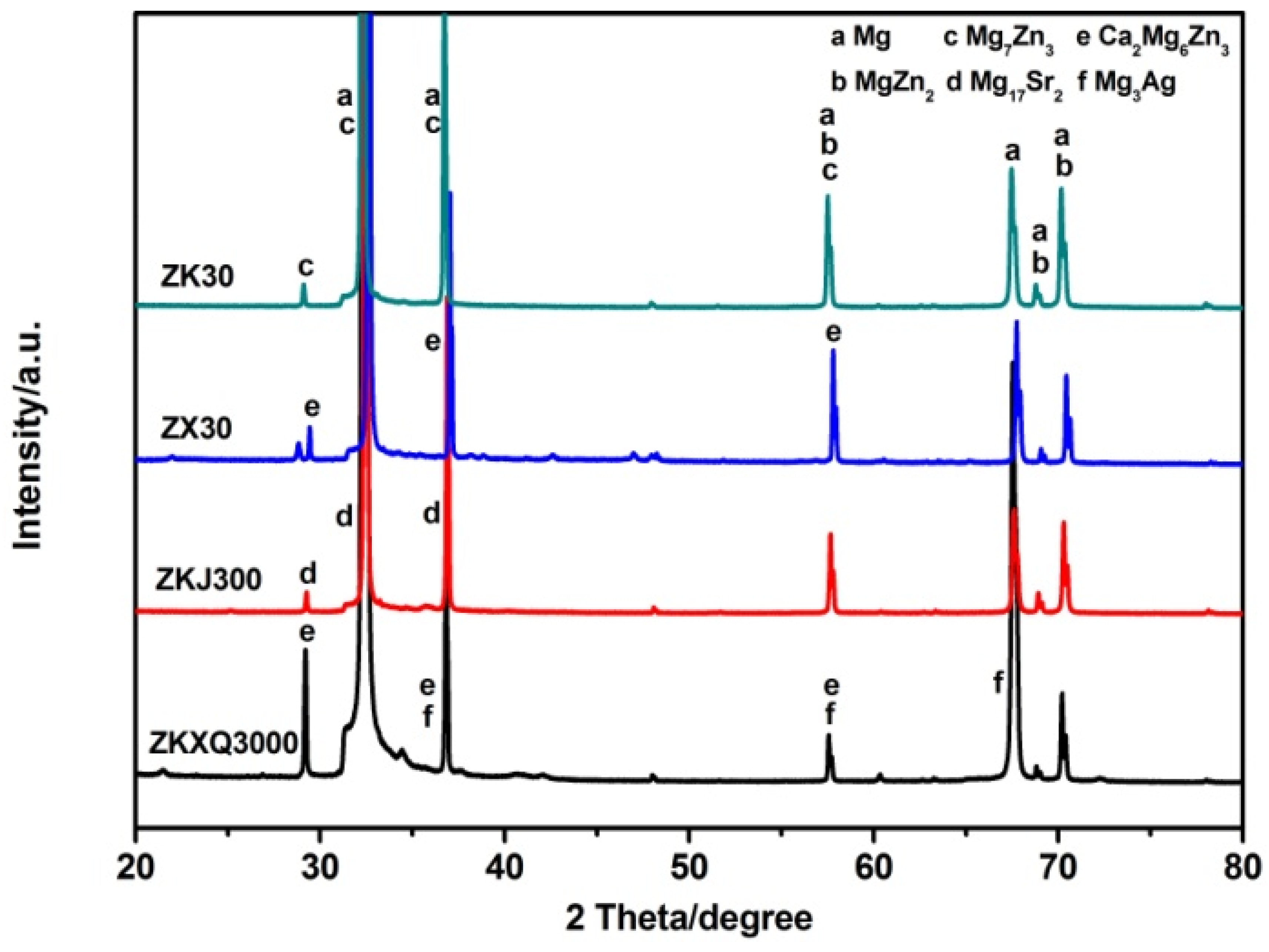
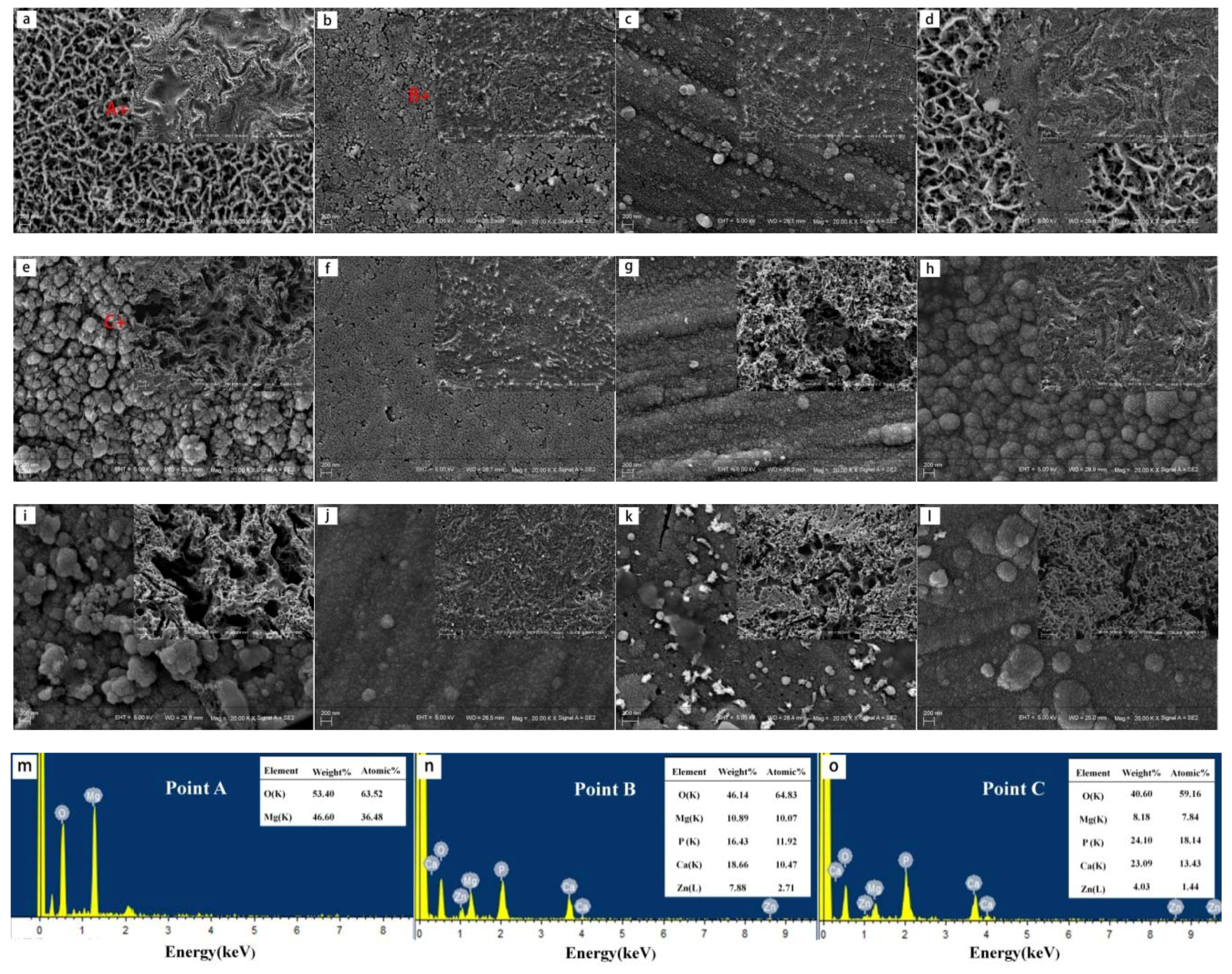
3.1. Microstructural Characterization
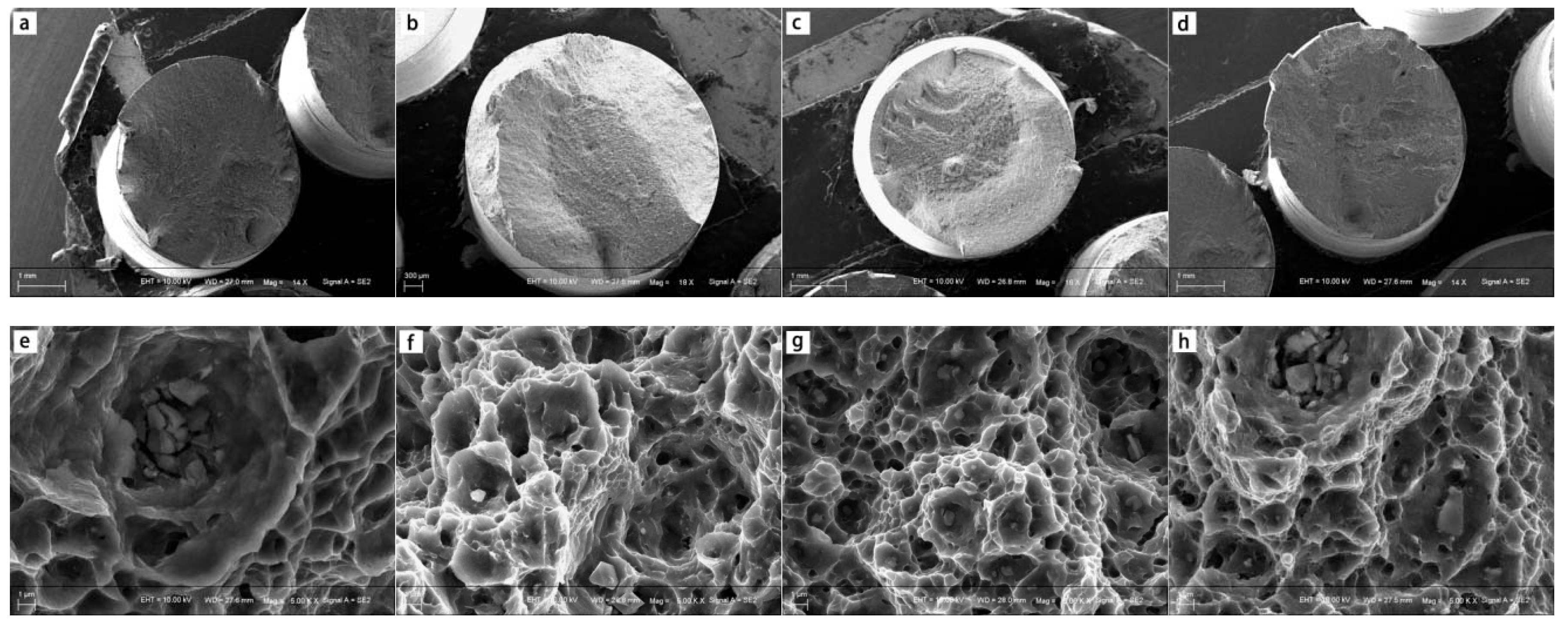
3.2. Mechanical Property Analysis
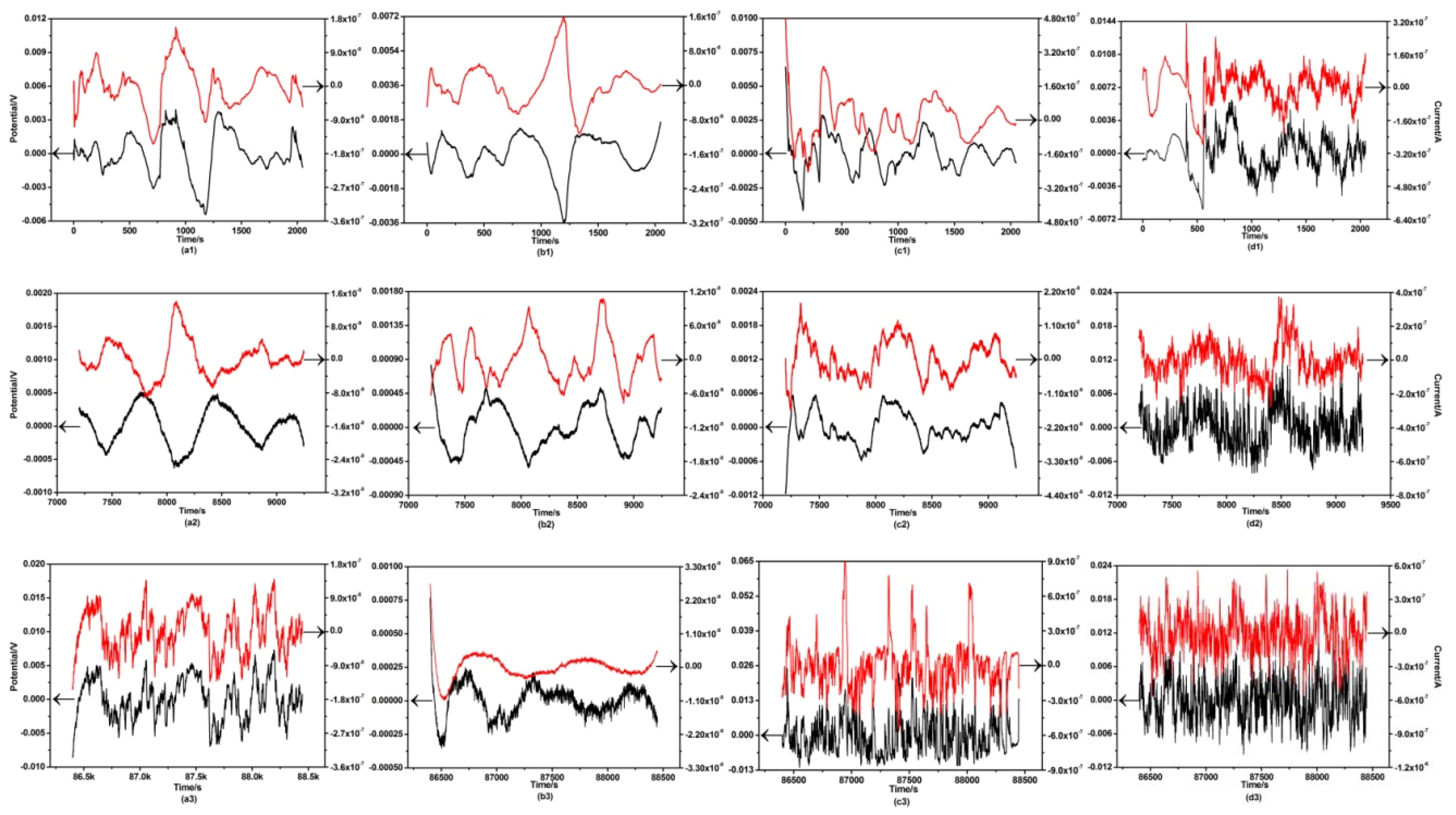
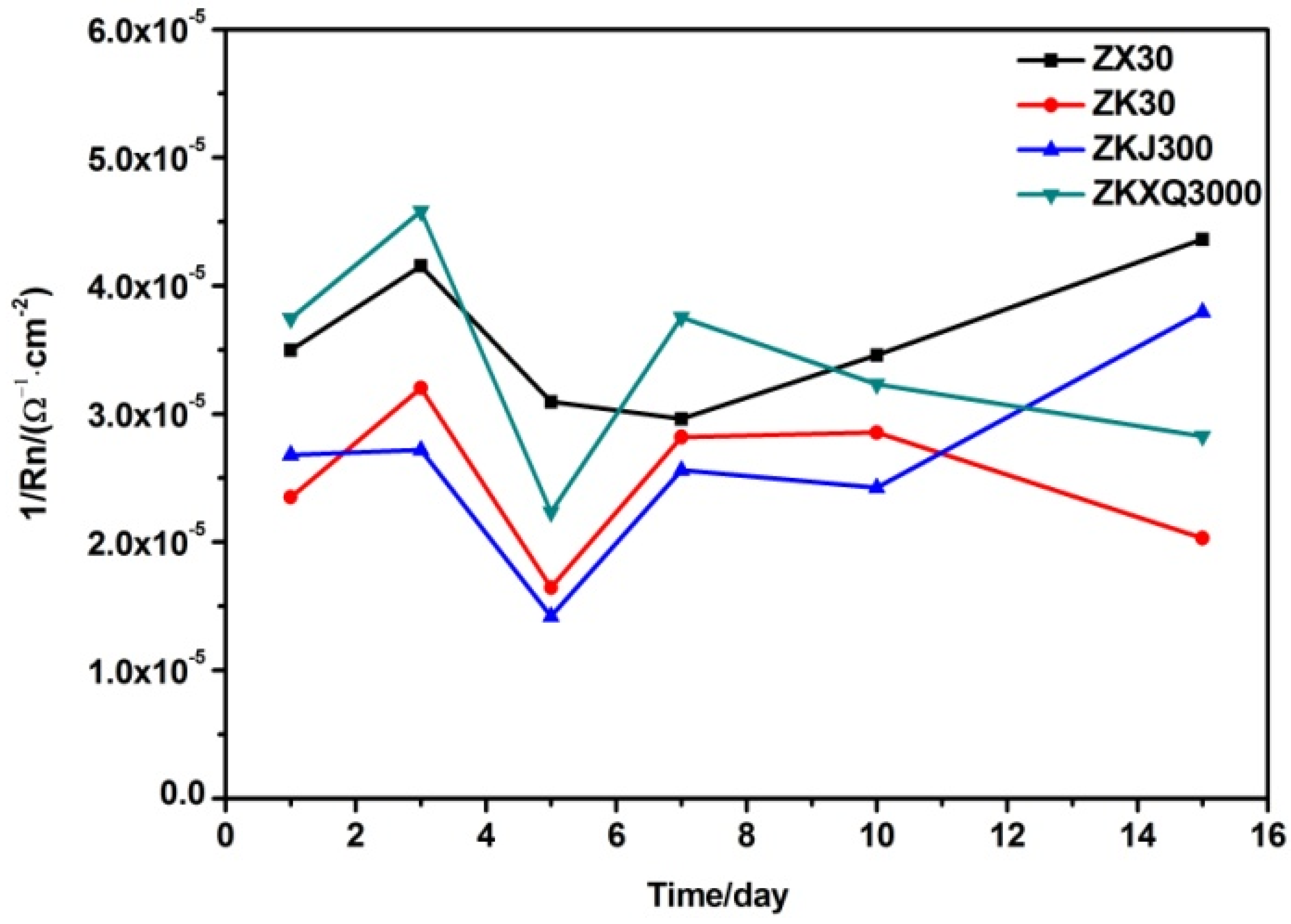
3.3. Electrochemical Noise Analysis
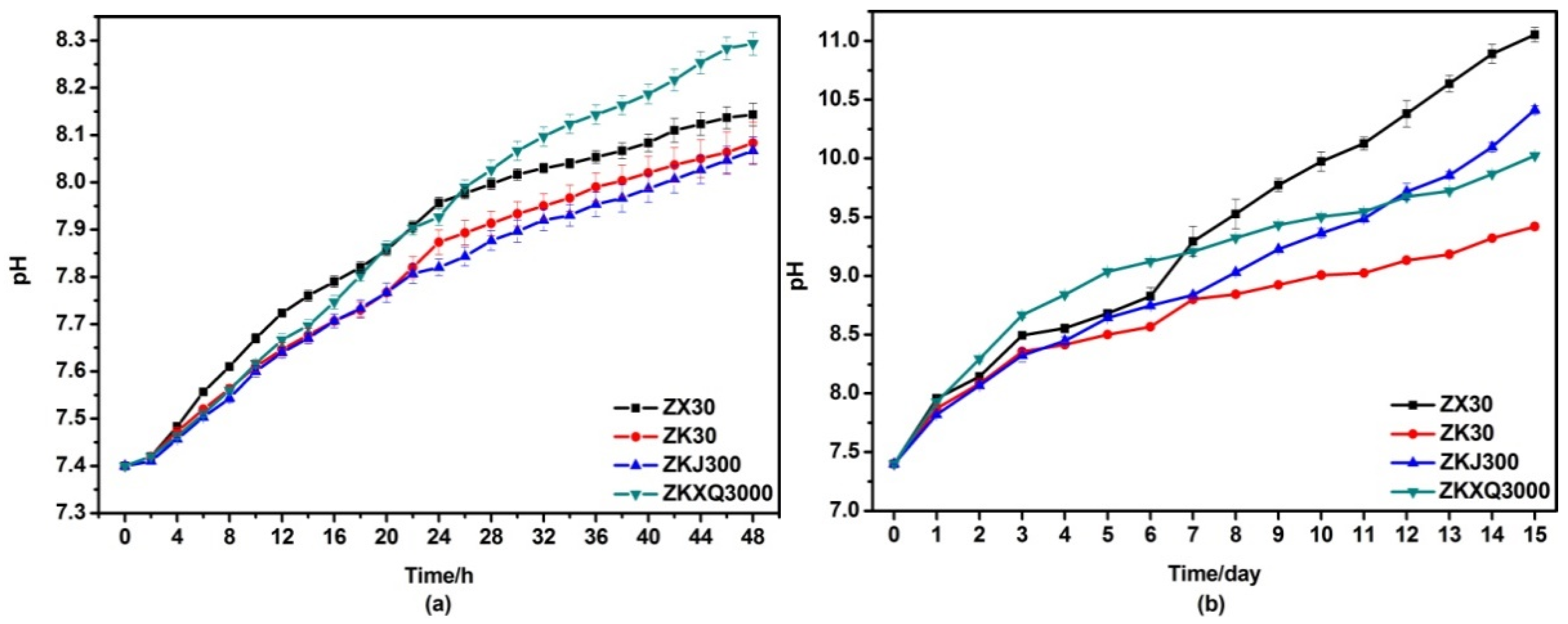
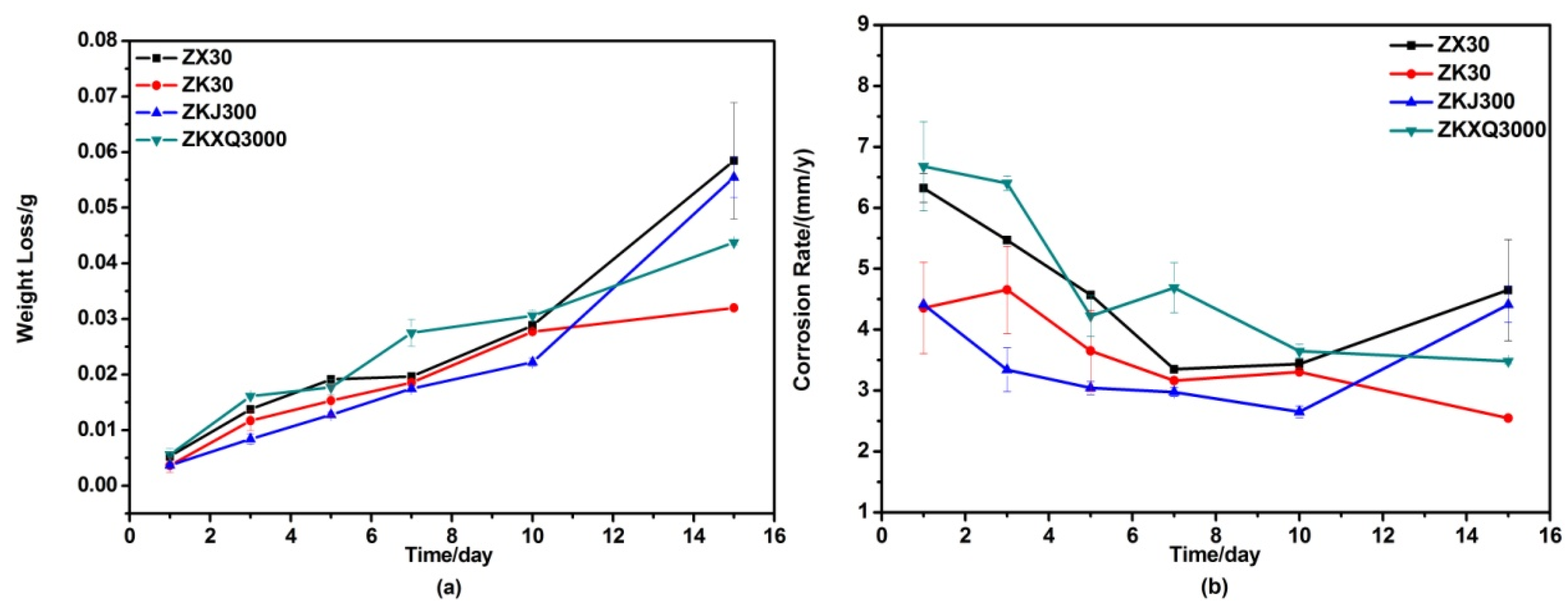
3.4. Immersion Testing In Vitro
4. Discussion
4.1. Effects of Alloying Elements on Microstructure of ZX30, ZK30, ZKJ300 and ZKXQ3000 Alloys
4.2. Effects of Alloying Elements on Mechanical Property of ZX30, ZK30, ZKJ300 and ZKXQ3000 Alloys
4.3. Effects of Alloying Elements on the Corrosion Behaviors of ZX30, ZK30, ZKJ300 and ZKXQ3000 Alloys
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Singh Raman, R.K.; Jafari, S.; Harandi, S.E. Corrosion fatigue fracture of magnesium alloys in bioimplant applications: A review. Eng. Fract. Mech. 2015, 137, 97–108. [Google Scholar] [CrossRef]
- Aghion, E.; Levy, G.; Ovadia, S. In vivo behavior of biodegradable Mg-Nd-Y-Zr-Ca alloy. J. Mater. Sci. Mater. Med. 2012, 23, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Pichler, K.; Fischerauer, S.; Ferlic, P.; Martinelli, E.; Brezinsek, H.P.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Immunological Response to Biodegradable Magnesium Implants. JOM 2014, 66, 573–579. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Razavi, S.M.; Heidari, F.; Manshaei, M.; Vashaee, D.; Tayebi, L. In vivo study of nanostructured diopside (CaMgSi2O6) coating on magnesium alloy as biodegradable orthopedic implants. Appl. Surf. Sci. 2014, 313, 60–66. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.; Qin, L. Progress of biodegradable metals. Prog. Nat. Sci. Mater. Int. 2014, 24, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Li, J. Microstructure, mechanical properties, corrosion behavior and film formation mechanism of Mg-Zn-Mn-xNd in Kokubo’s solution. J. Alloys Compd. 2018, 730, 458–470. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Bian, D.; Gao, S.; Leeflang, S.; Guo, H.; Zheng, Y.; Zhou, J. Study on the Mg-Li-Zn ternary alloy system with improved mechanical properties, good degradation performance and different responses to cells. Acta Biomater. 2017, 62, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, J.; Yang, X.; Li, M.; Wan, P.; Tan, L.; Zhang, Y.; Yang, K. Novel biocompatible magnesium alloys design with nutrient alloying elements Si, Ca and Sr: Structure and properties characterization. Mater. Sci. Eng. B 2016, 214, 26–36. [Google Scholar] [CrossRef]
- Mendis, C.L.; Oh-ishi, K.; Hono, K. Enhanced age hardening in a Mg–2.4 at.% Zn alloy by trace additions of Ag and Ca. Scr. Mater. 2007, 57, 485–488. [Google Scholar] [CrossRef]
- Aral, H.; Vecchio-Sadus, A. Toxicity of lithium to humans and the environment-A literature review. Ecotoxicol. Environ. Saf. 2008, 70, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Roth, J.; Salvi, R. Manganese is toxic to spiral ganglion neurons and hair cells in vitro. Neurotoxicology 2011, 32, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Hou, Y.; Wang, X.; Wang, Y.; Geng, L. Mechanical properties, degradation performance and cytotoxicity of Mg–Zn–Ca biomedical alloys with different compositions. Mater. Sci. Eng. C 2011, 31, 1667–1673. [Google Scholar] [CrossRef]
- Li, Y.; Hodgson, P.D.; Wen, C.E. The effects of calcium and yttrium additions on the microstructure, mechanical properties and biocompatibility of biodegradable magnesium alloys. J. Mater. Sci. 2011, 46, 365–371. [Google Scholar] [CrossRef]
- StJohn, D.H.; Qian, M.; Easton, M.A.; Cao, P.; Hildebrand, Z. Grain refinement of magnesium alloys. Metall. Mater. Trans. A 2005, 36, 1669–1679. [Google Scholar] [CrossRef]
- Cheng, M.; Chen, J.; Yan, H.; Su, B.; Yu, Z.; Xia, W.; Gong, X. Effects of minor Sr addition on microstructure, mechanical and bio-corrosion properties of the Mg-5Zn based alloy system. J. Alloys Compd. 2017, 691, 95–102. [Google Scholar] [CrossRef]
- Li, R.G.; Xin, R.L.; Liu, Q.; Liu, J.A.; Fu, G.Y.; Zong, L.; Yu, Y.M.; Guo, S.G. Effect of Ag addition on microstructure and mechanical properties of Mg–14Gd–0.5Zr alloy. Mater. Charact. 2015, 109, 43–49. [Google Scholar] [CrossRef]
- Du, Y.Z.; Qiao, X.G.; Zheng, M.Y.; Wang, D.B.; Wu, K.; Golovin, I.S. Effect of microalloying with Ca on the microstructure and mechanical properties of Mg-6 mass%Zn alloys. Mater. Des. 2016, 98, 285–293. [Google Scholar] [CrossRef]
- Huang, X.; Du, Y.; Li, W.; Chai, Y.; Huang, W. Effects of Ag content on the solid-solution and age-hardening behavior of a Mg-5Sn alloy. J. Alloys Compd. 2017, 696, 850–855. [Google Scholar] [CrossRef]
- Huang, X.; Wu, A.; Li, Q.; Huang, W. Effects of extrusion and Ag, Zn addition on the age-hardening response and microstructure of a Mg-7Sn alloy. Mater. Sci. Eng. A 2016, 661, 233–239. [Google Scholar] [CrossRef]
- L, W.; Huang, X.; Huang, W. Effects of Ca, Ag addition on the microstructure and age-hardening behavior of a Mg-7Sn (wt%) alloy. Mater. Sci. Eng. A 2017, 692, 75–80. [Google Scholar] [CrossRef]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloys 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W.; et al. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials 2016, 81, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Han, H.S.; Han, K.J.; Park, J.; Jeon, H.; Seok, H.K.; Ahn, J.P.; Lee, K.E.; Lee, D.H.; Yang, S.J.; et al. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, M.; Li, W.; Zheng, H.; You, C.; Liu, D.; Jin, F. The synergistic effect of trace Sr and Zr on the microstructure and properties of a biodegradable Mg-Zn-Zr-Sr alloy. J. Alloys Compd. 2017, 702, 290–302. [Google Scholar] [CrossRef]
- Li, Z.; Sun, S.; Chen, M.; Fahlman, B.D.; Liu, D.; Bi, H. In vitro and in vivo corrosion, mechanical properties and biocompatibility evaluation of MgF2-coated Mg-Zn-Zr alloy as cancellous screws. Mater. Sci. Eng. C 2017, 75, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, H.; Bi, Y.; Sun, J.; Chen, M.; Liu, D. Effects of gas produced by degradation of Mg-Zn-Zr Alloy on cancellous bone tissue. Mater. Sci. Eng. C 2015, 55, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Dahle, A.K.; Stjohn, D.H. The role of solute in grain refinement of magnesium. Metall. Mater. Trans. A 2000, 31, 2895–2906. [Google Scholar] [CrossRef]
- Wang, D.; Gao, J.; Zhang, J.; Li, Y.; Li, Z. Manipulation of squeezed state in electromagnetically induced transparency system via dynamic Stark effect. J. Opt. Soc. Am. B 2012, 29, 3177–3182. [Google Scholar] [CrossRef]
- Casajús, P.; Winzer, N. Electrochemical noise analysis of the corrosion of high-purity Mg–Al alloys. Corros. Sci. 2015, 94, 316–326. [Google Scholar] [CrossRef]
- Jamali, S.S.; Mills, D.J. A critical review of electrochemical noise measurement as a tool for evaluation of organic coatings. Prog. Org. Coat. 2016, 95, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Curioni, M.; Cottis, R.A.; Di Natale, M.; Thompson, G.E. Corrosion of dissimilar alloys: Electrochemical noise. Electrochim. Acta 2011, 56, 6318–6329. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, Y.; Meng, G.; Wang, F. Electrochemical noise analysis of the corrosion of AZ91D magnesium alloy in alkaline chloride solution. Electrochim. Acta 2007, 53, 561–568. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Song, Y.; Zhao, C.; Zhang, X.; Xie, C.; Zhang, Y.; Tao, H.; He, Y.; Jiang, Y.; et al. In Vitro degradation, hemolysis and MC3T3-E1 cell adhesion of biodegradable Mg–Zn alloy. Mater. Sci. Eng. C 2009, 29, 1907–1912. [Google Scholar] [CrossRef]






| YTS (MPa) | UTS (MPa) | EL (%) | YCS (MPa) | UCS (MPa) | |
|---|---|---|---|---|---|
| ZX30 | 262.67 ± 4.10 | 298.17 ± 0.93 | 18.33 ± 0.67 | 256.70 ± 0.94 | 361.32 ± 9.52 |
| ZK30 | 246.00 ± 6.81 | 293.26 ± 2.71 | 20.00 ± 2.31 | 296.49 ± 2.76 | 342.62 ± 6.29 |
| ZKJ300 | 301.33 ± 2.40 | 334.61 ± 2.92 | 13.33 ± 1.33 | 315.69 ± 0.78 | 359.78 ± 2.93 |
| ZKXQ3000 | 315.33 ± 5.67 | 337.56 ± 2.19 | 14.67 ± 1.76 | 279.16 ± 3.06 | 354.45 ± 11.27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, G.; Chen, M.; Zhao, Y.; Sasikumar, Y.; Tie, D. The Mechanical Properties and Corrosion Resistance of Magnesium Alloys with Different Alloying Elements for Bone Repair. Crystals 2018, 8, 271. https://doi.org/10.3390/cryst8070271
Lin G, Chen M, Zhao Y, Sasikumar Y, Tie D. The Mechanical Properties and Corrosion Resistance of Magnesium Alloys with Different Alloying Elements for Bone Repair. Crystals. 2018; 8(7):271. https://doi.org/10.3390/cryst8070271
Chicago/Turabian StyleLin, Guangyi, Minfang Chen, Yun Zhao, Yesudass Sasikumar, and Di Tie. 2018. "The Mechanical Properties and Corrosion Resistance of Magnesium Alloys with Different Alloying Elements for Bone Repair" Crystals 8, no. 7: 271. https://doi.org/10.3390/cryst8070271
APA StyleLin, G., Chen, M., Zhao, Y., Sasikumar, Y., & Tie, D. (2018). The Mechanical Properties and Corrosion Resistance of Magnesium Alloys with Different Alloying Elements for Bone Repair. Crystals, 8(7), 271. https://doi.org/10.3390/cryst8070271