Thermal Conductivity of an AZ31 Sheet after Accumulative Roll Bonding
Abstract
1. Introduction
2. Materials and Methods
3. Results
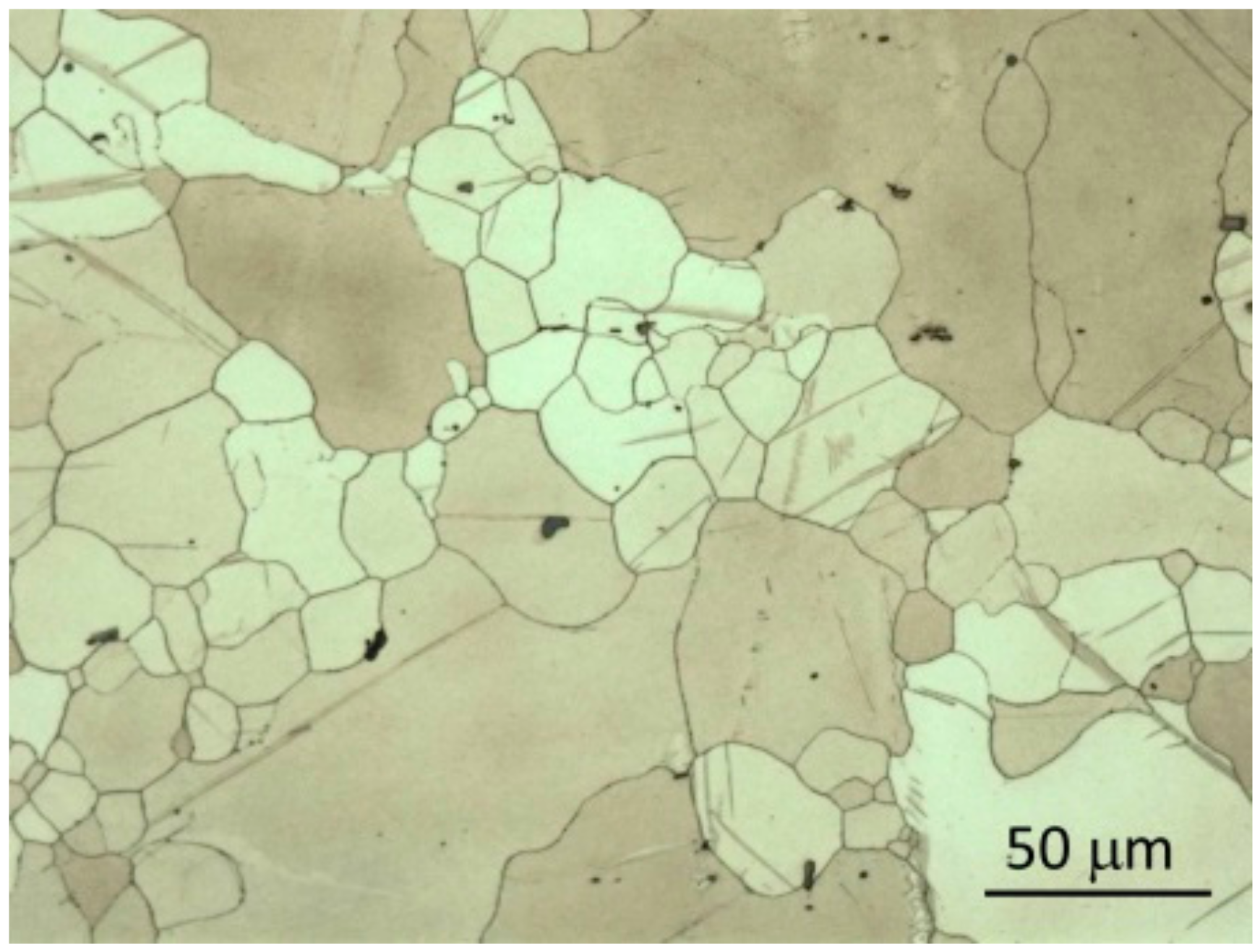
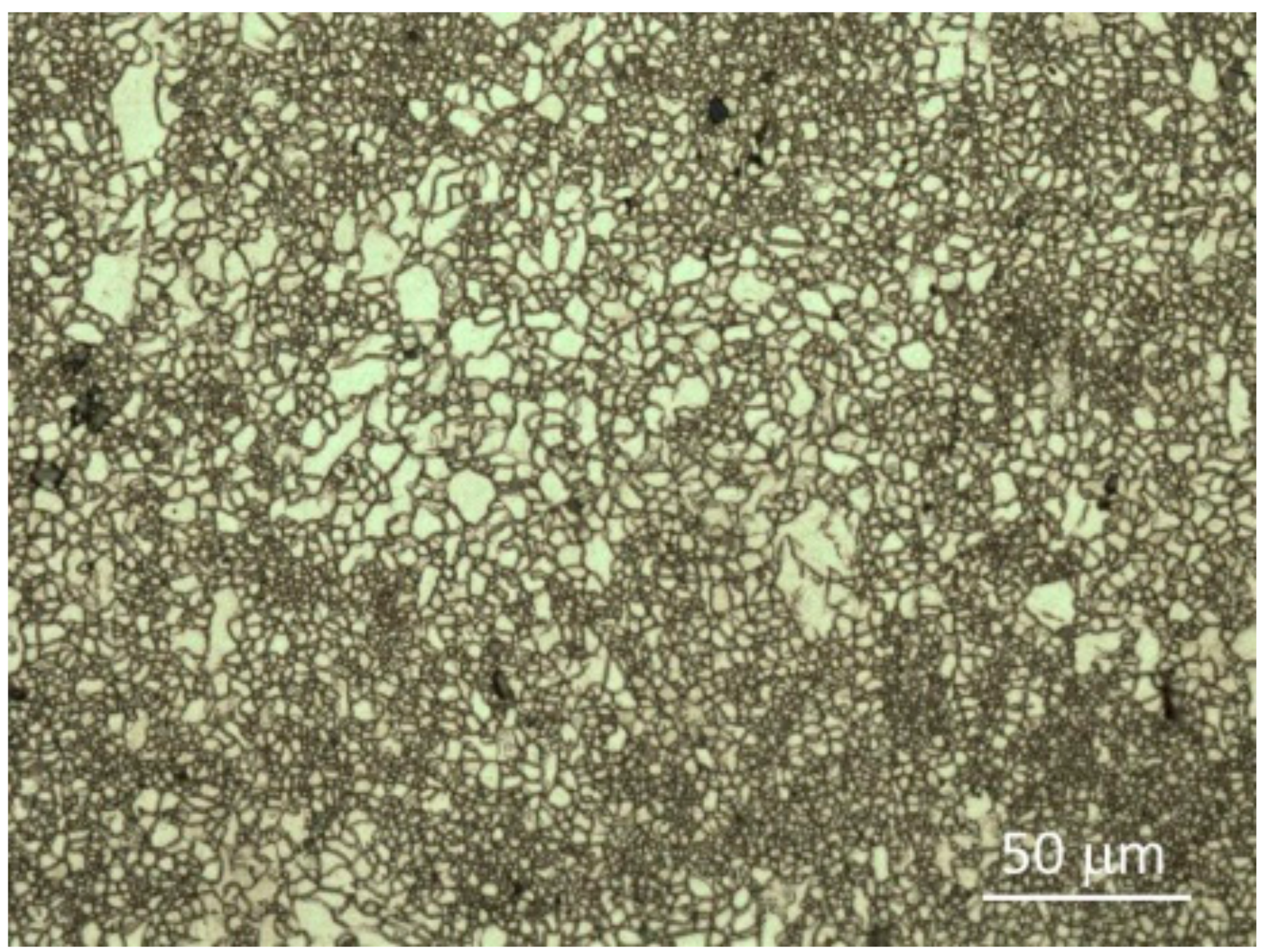
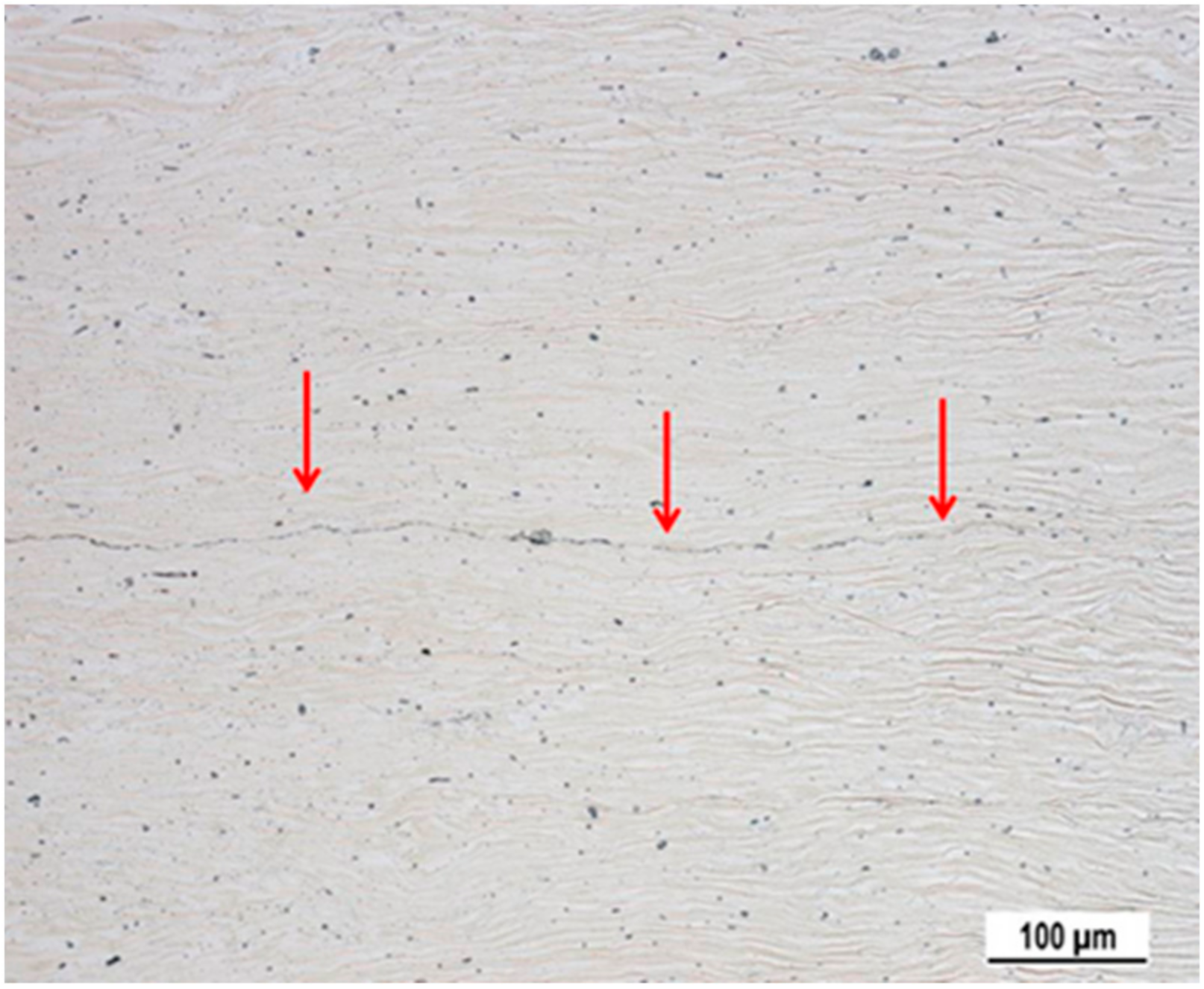
3.1. Microstructure of Samples
3.2. Thermal Measurements
4. Discussion
5. Conclusions
- ARB process refined the sheets microstructure.
- Rolled sheets exhibit developed texture where basal planes (0001) are preferentially parallel to the sheet surface.
- Thermal conductivity increases with temperature and increasing number of rolling passes.
- The observed increase of thermal conductivity with the increasing number of rolling passes can be explained with the texture improvement and anisotropy of thermal properties of magnesium.
- This anisotropy can be of advantage in cases where the heat dissipation occurs in one direction.
Author Contributions
Funding
Conflicts of Interest
References
- Watanabe, H.; Mukai, T.; Ishikawa, K. Differential speed rolling of an AZ31 magnesium alloy and resulting mechanical properties. J. Mater. Sci. 2004, 36, 1477–1480. [Google Scholar] [CrossRef]
- Huang, X.; Suzuki, K.; Saito, N. Textures and stretched formability of Mg-6Al-1Zn magnesium alloy sheets rolled at high temperatures up to 793 K. Scripta Mater. 2009, 60, 651–654. [Google Scholar] [CrossRef]
- Chino, Y.; Mabuchi, M.; Kishihara, R.; Hosokawa, H.; Amada, Y.; Wen, C.; Shimojima, K.; Iwasaki, H. Mechanical properties and press formability at room temperature of AZ31 Mg alloy processed by single roller drive rolling. Mater. Trans. 2002, 43, 2554–2560. [Google Scholar] [CrossRef]
- Schwarz, F.; Eilers, C.; Krüger, L. Mechanical properties of an AM20 magnesium alloy processed by accumulative roll bonding. Mater. Charact. 2015, 105, 144–153. [Google Scholar] [CrossRef]
- Del Valle, J.A.; Pérez-Prado, M.T.; Ruano, O.A. Accumulative roll bonding of a Mg based AZ61 alloy. Mater. Sci. Eng. A 2005, 410–411, 353–357. [Google Scholar] [CrossRef]
- Pérez-Prado, M.T.; Del Valle, J.A.; Ruano, O.A. Grain refinement of Mg-Al-Zn alloys via accumulative roll bonding. Scripta Mater. 2004, 51, 1093–1097. [Google Scholar] [CrossRef]
- Agnew, S.R. Plastic anisotropy of magnesium alloy AZ31B sheet. In Magnesium Technology; Kaplan, H., Ed.; TMS: Warendale, PA, USA, 2002; pp. 169–174. [Google Scholar]
- Bohlen, J.; Chmelík, F.; Dobroň, P.; Letzig, D.; Kaiser, F.; Lukáč, P.; Kainer, K.U. Orientation effects on acoustic emission during tensile deformation of hot rolled magnesium alloy AZ31. J. Alloys Compd. 2004, 378, 207. [Google Scholar] [CrossRef]
- Trojanová, Z.; Džugan, J.; Halmešová, K.; Németh, G.; Minárik, P.; Lukáč, P.; Bohlen, J. Influence of accumulative roll bonding on the texture and tensile properties of an AZ31 Magnesium alloy sheets. Materials 2018, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.; Zheng, M.Y.; Li, Z.T.; Qiao, X.G. Thermal conductivity of as-cast and as-extruded binary Mg-Al alloys. J. Alloys Compd. 2014, 608, 19–24. [Google Scholar] [CrossRef]
- Ying, T.; Zheng, M.Y.; Li, Z.T.; Qiao, X.G.; Xu, S.W. Thermal conductivity of as-cast and as-extruded Binary Mg-Zn alloys. J. Alloys Compd. 2015, 621, 250–255. [Google Scholar] [CrossRef]
- Pan, H.; Pan, F.; Peng, J.; Gou, J.; Tang, A.; Wu, L.; Dong, H. High conductivity Binary Mg-Zn sheet processed by cold rolling. J. Alloys Compd. 2013, 578, 493–500. [Google Scholar] [CrossRef]
- Zhong, L.; Peng, J.; Sung, Y.; Wang, Y.; Lu, Y.; Pan, F. Microstructure and thermal conductivity of cast and as-extruded Mg-Mn alloys. Mater. Sci. Technol. 2017, 33, 92–97. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, Q.; Le, Q.; Zhang, Z.; Cui, J. Analysis of temperature distribution in the hot plate rolling of Mg alloy by experiment and finite element method. J. Mater. Process. Technol. 2015, 225, 286–294. [Google Scholar] [CrossRef]
- Trojanová, Z.; Podrábsky, T.; Lukáč, P.; Armstrong, R.W.; Pesička, J.; Forejt, M. Influence of the strain rate on deformation mechanisms of an AZ31 magnesium alloy. Int. J. Mater. Res. 2013, 104, 762–768. [Google Scholar] [CrossRef]
- Ohno, M.; Mirkovic, D.; Schmid-Fetzer, R. Liquidus and solidus temperatures of rich Mg-Al-Zn alloys. Acta Mater. 2006, 54, 3883–3891. [Google Scholar] [CrossRef]
- Braszczyńska-Malik, K.N. Discontinuous and continuous precipitation in magnesium-aluminium type alloys. J. Alloys Compd. 2009, 477, 870–876. [Google Scholar] [CrossRef]
- Rudajevová, A.; Staněk, M.; Lukáč, P. Determination of thermal diffusivity and thermal conductivity of Mg-Al alloys. Mater. Sci. Eng. A 2003, 341, 152–157. [Google Scholar] [CrossRef]
- Su, C.; Li, D.; Ying, T.; Zhou, L.; Li, L.; Zeng, X. Effect of Nd content and heat treatment on the thermal conductivity of Mg-Nd alloys. J. Alloys Compd. 2015, 685, 114–121. [Google Scholar] [CrossRef]
- Yamasaki, M.; Kawamura, Y. Thermal diffusivity and thermal conductivity of Mg-Zn-rare earth element alloys with long period stacking ordered phase. Scripta Mater. 2009, 60, 264–267. [Google Scholar] [CrossRef]
- Rudajevová, A.; Lukáč, P. Comparison of the thermal properties of AM20 and AS21 magnesium alloys. Mater. Sci. Eng. A 2005, 397, 16–21. [Google Scholar] [CrossRef]
- Lee, S.; Ham, H.J.; Kwon, S.Y.; Kim, S.W.; Suh, C.M. Thermal conductivity of magnesium alloys in the temperature range from −125 °C to 400 °C. Int. J. Thermophys. 2013, 34, 2343–2350. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Z.; Liu, H.; Chen, Y.; Ding, W.; Xiao, S. Electrical and thermal conductivity in Mg-5Sn alloy at different aging status. Mater. Design 2015, 74, 48–52. [Google Scholar] [CrossRef]
- Ying, T.; Chi, H.; Zheng, M.; Li, Z.; Uher, C. Low temperature electrical resistivity and thermal conductivity of Binary magnesium alloys. Acta Mater. 2014, 80, 288–295. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, Y.; Gong, M.; Zheng, X.; Peng, J. Effects of precipitates and its interface on thermal conductivity of Mg–12Gd alloy during aging treatment. Mater. Charact. 2018, 138, 284–288. [Google Scholar] [CrossRef]
- Zhong, L.; Peng, J.; Sun, S.; Wang, Y.; Lu, Y.; Pan, F. Microstructure and thermal conductivity of as cast and as-solutionized Mg-rare earth binary alloys. J. Mater. Sci. Technol. 2017, 33, 1240–1248. [Google Scholar] [CrossRef]
- Zhong, L.; Peng, J.; Li, M.; Wang, Y.; Lu, Y.; Pan, F. Effect of Ce addition on the microstructure, thermal conductivity and mechanical properties of Mg-0.5Mn alloys. J. Alloys Compd. 2016, 661, 402–410. [Google Scholar] [CrossRef]
- Li, B.; Hou, L.; Wu, R.; Zhang, J.; Li, X.; Zhang, M. Microstructure and thermal conductivity of Mg-2Zn-Zr alloy. J. Alloys Compd. 2017, 722, 772–777. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, K.; Li, T.; Li, X.; Li, Y.; Ma, M.; Luo, P. Anisotropy of thermal conductivity and mechanical properties in Mg-5Zn-1Mn alloy. Mater. Design 2012, 40, 257–261. [Google Scholar] [CrossRef]
- Del Valle, J.A.; Pérez-Prado, M.T.; Ruano, O.A. Texture evolution during large-strain hot rolling of the Mg AZ61 alloy. Mater. Sci. Eng. A 2005, 355, 68–78. [Google Scholar] [CrossRef]
- Ion, S.E.; Humphreys, F.J.; White, H.S. Dynamic recrystallization and the development of microstructure during the high temperature deformation of magnesium. Acta Metall. 1982, 30, 1909–1919. [Google Scholar] [CrossRef]
- Grimvall, G. Thermophysical Properties of Materials, 1st ed.; Elsevier, North Holland: Amsterdam, The Netherlands, 1999; p. 262. ISBN 9780444827944. [Google Scholar]
- Berman, B. Thermal Conduction in Solids; Claredon Press: Oxford, UK, 1976. [Google Scholar]
- Uher, C. Thermal conductivity of metals. In Thermal Conductivity: Theory, Properties and Application; Tritt, T.M., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2004; ISBN 0-306-48327-0. Available online: ftp://nozdr.ru/biblio/kolxo3/P/PS/PSa/Tritt%20T.M.%20(ed.)%20Thermal%20conductivity%20(Kluwer,%202004)(ISBN%200306483270)(O)(306s)_PSa_.pdf (accessed on 11 Apil 2018).
- Bass, J.; Fisher, K.H. Landolt-Börnstein Database; New Series III/15a; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar] [CrossRef]





| Al | Zn | Mn | Si | Ce | Fe | Mg |
|---|---|---|---|---|---|---|
| 3.16 | 1.29 | 0.41 | 0.015 | 0.055 | 0.02 | Bal. |
| Material | ARB_0 | ARB_1 | ARB_2 | |||
|---|---|---|---|---|---|---|
| T (°C) | cp (Jkg−1K−1) | α × 10−6 (K−1) | cp (Jkg−1K−1) | α × 10−6 (K−1) | cp (Jkg−1K−1) | α × 10−6 (K−1) |
| 20 | 1010 | 26.53 | 1002 | 26.27 | 1036 | 25.73 |
| 50 | 1012 | 26.72 | 1037 | 26.47 | 1087 | 25.94 |
| 100 | 1060 | 27.04 | 1070 | 26.80 | 1101 | 26.31 |
| 150 | 1090 | 27.36 | 1107 | 27.12 | 1133 | 26.67 |
| 200 | 1112 | 27.68 | 1120 | 27.45 | 1159 | 27.04 |
| 250 | 1130 | 27.99 | 1113 | 27.77 | 1186 | 27.40 |
| 300 | 1161 | 28.31 | 1146 | 28.10 | 1186 | 27.77 |
| 350 | 1184 | 28.63 | 1166 | 28.43 | 1203 | 28.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trojanová, Z.; Halmešová, K.; Drozd, Z.; Šíma, V.; Lukáč, P.; Džugan, J.; Minárik, P. Thermal Conductivity of an AZ31 Sheet after Accumulative Roll Bonding. Crystals 2018, 8, 278. https://doi.org/10.3390/cryst8070278
Trojanová Z, Halmešová K, Drozd Z, Šíma V, Lukáč P, Džugan J, Minárik P. Thermal Conductivity of an AZ31 Sheet after Accumulative Roll Bonding. Crystals. 2018; 8(7):278. https://doi.org/10.3390/cryst8070278
Chicago/Turabian StyleTrojanová, Zuzanka, Kristýna Halmešová, Zdeněk Drozd, Vladimír Šíma, Pavel Lukáč, Ján Džugan, and Peter Minárik. 2018. "Thermal Conductivity of an AZ31 Sheet after Accumulative Roll Bonding" Crystals 8, no. 7: 278. https://doi.org/10.3390/cryst8070278
APA StyleTrojanová, Z., Halmešová, K., Drozd, Z., Šíma, V., Lukáč, P., Džugan, J., & Minárik, P. (2018). Thermal Conductivity of an AZ31 Sheet after Accumulative Roll Bonding. Crystals, 8(7), 278. https://doi.org/10.3390/cryst8070278







