Multifunctional Molecular Magnets: Magnetocaloric Effect in Octacyanometallates
Abstract
:1. Introduction
2. Deriving Magnetocaloric Effect from Calorimetric Data
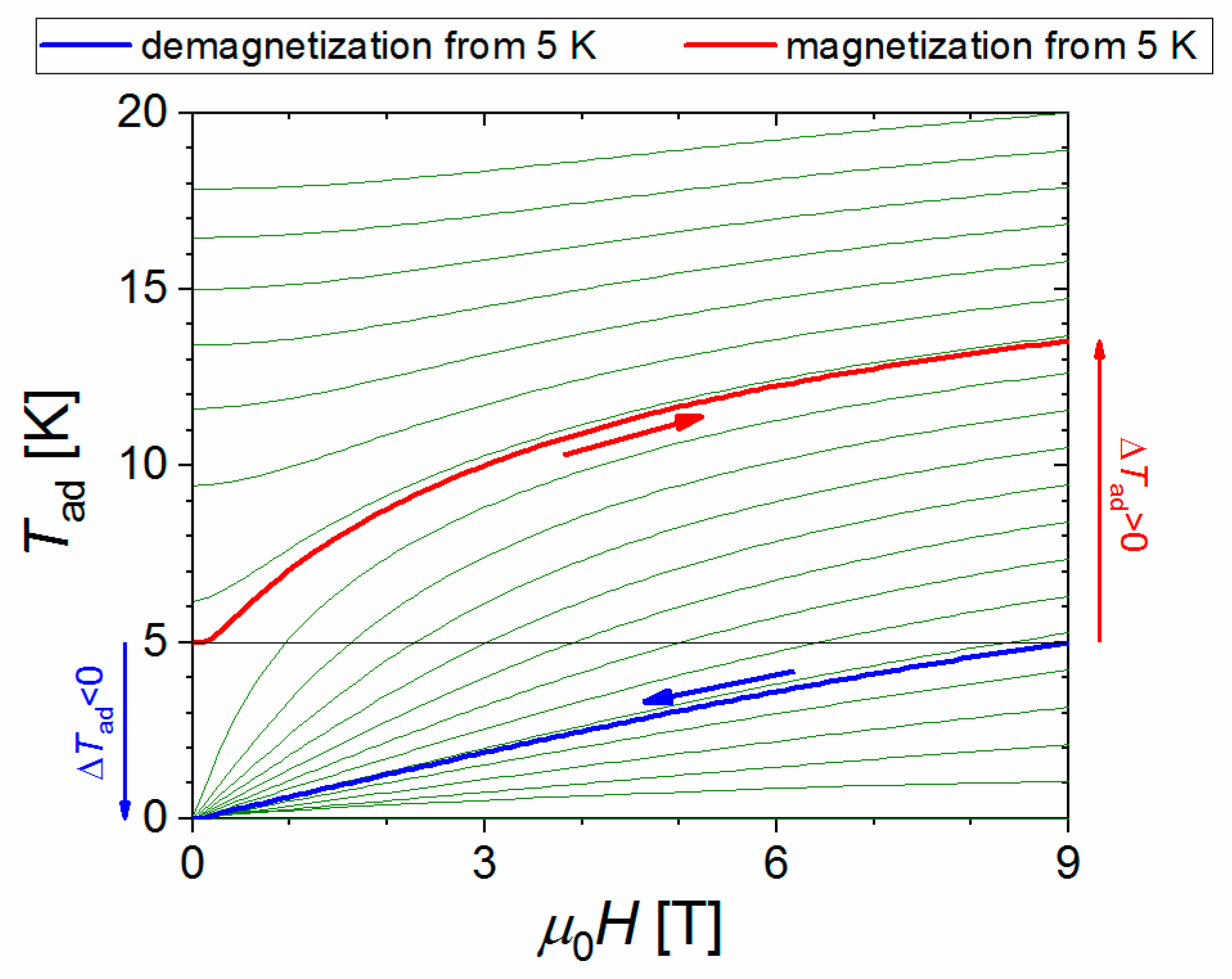
2.1. Thermodynamic Setup
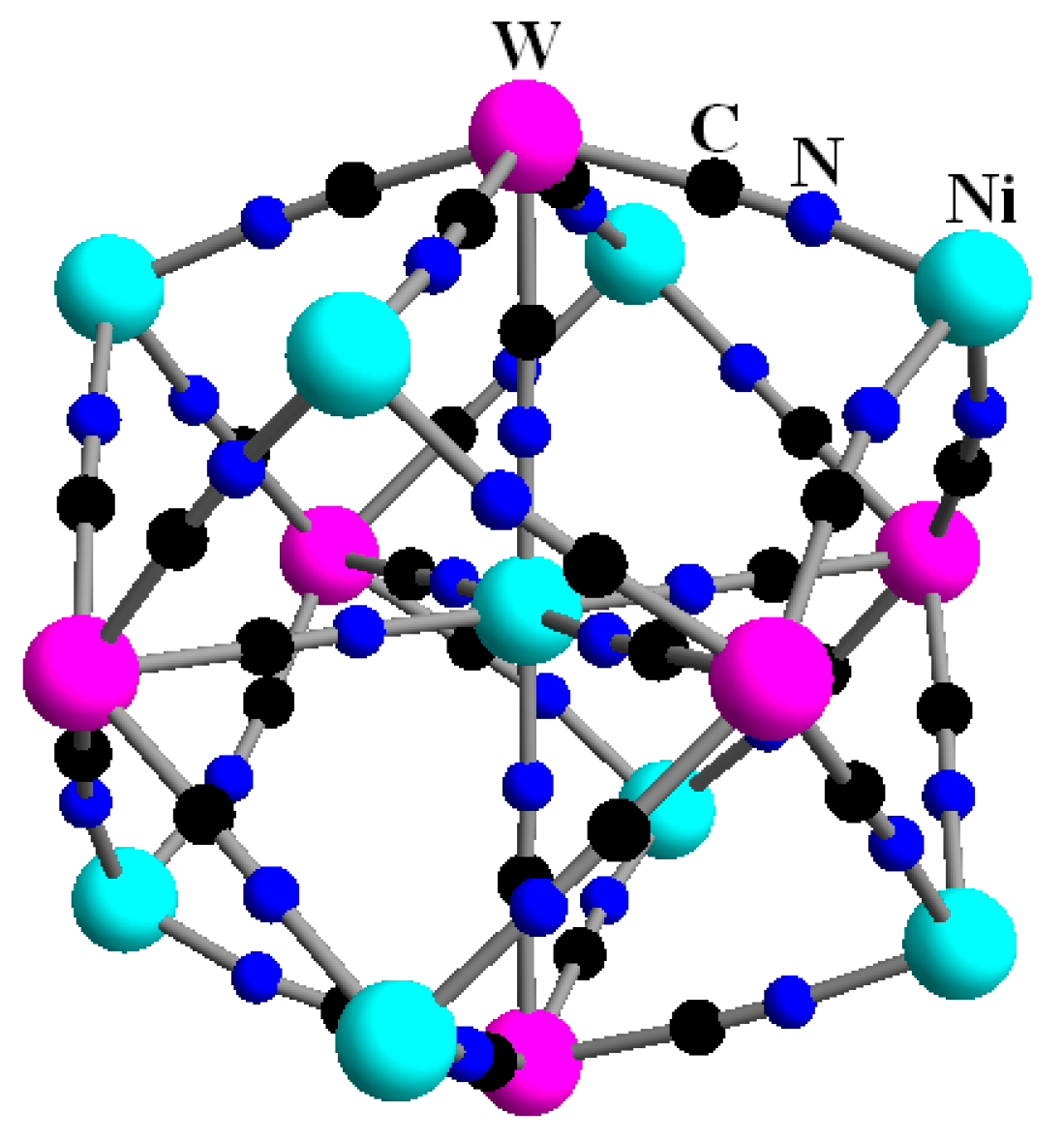
2.2. Cluster Compound Ni9[W(CN)8]6
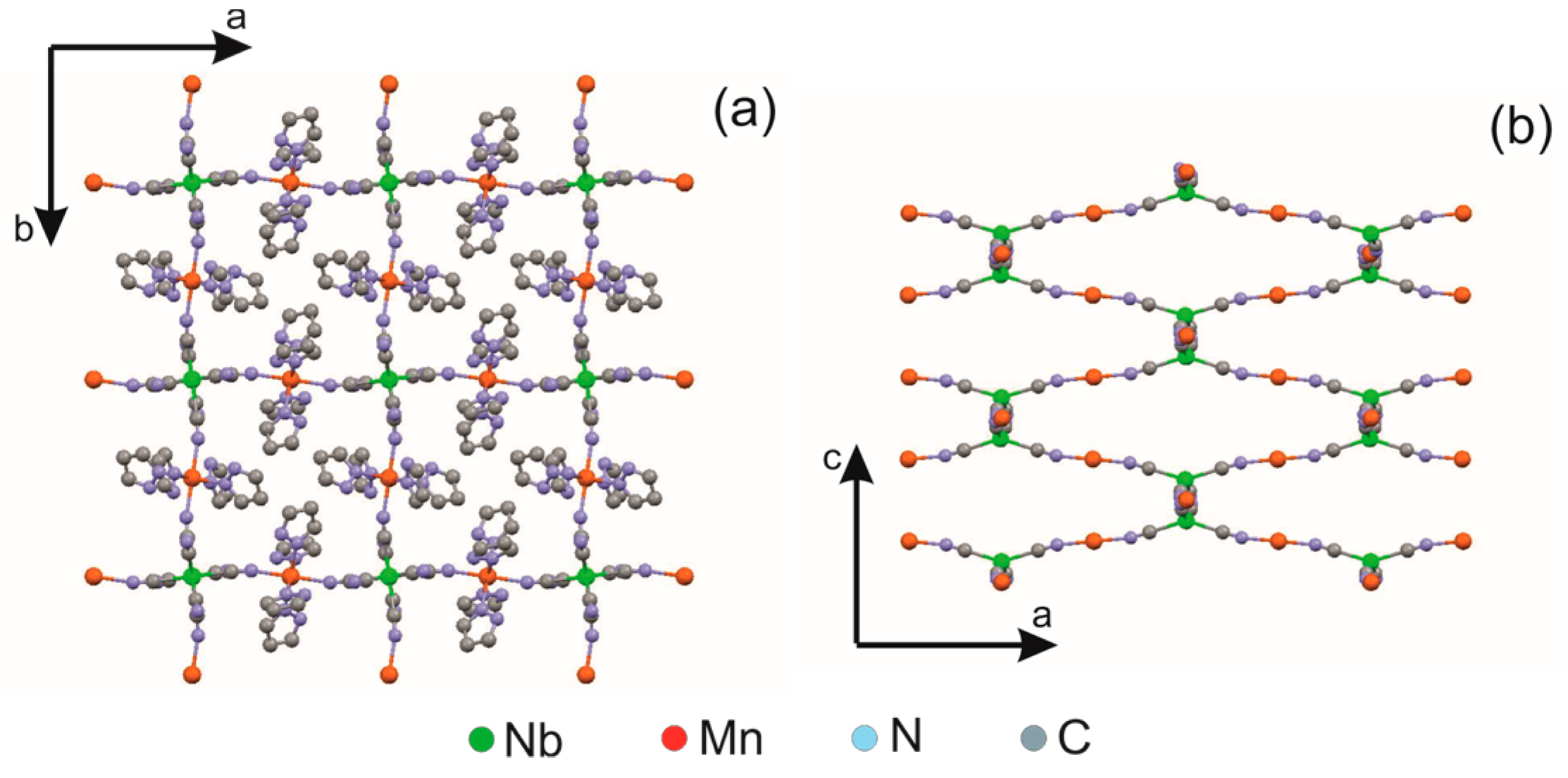
2.3. Coordination Polymer {[MnII(pyrazole)4]2[NbIV(CN)8]·4H2O}n
2.4. Coordination Polymer {[FeII(pyrazole)4]2[NbIV(CN)8]·4H2O}n
2.5. Final Remarks of Section 2
3. Magnetocaloric Properties and Critical Behaviour in Magnetically Ordered Compounds Investigated by Magnetometry
3.1. {[MII(H2O)2]2[NbIV(CN)8]·4H2O}n(M = Fe, Mn) Molecular Compounds
3.2. {[MII(pyrazole)4]2[NbIV(CN)8]3∙4H2O}n(M = Ni, Mn) Molecular Compounds
3.3. {MnII2(imH)2(H2O)4[NbIV(CN)8]∙4H2O}nMolecular Magnetic Sponge
3.4. [{[MnII(pydz)(H2O)2][MnII(H2O)2][NbIV(CN)8]}∙2H2O]nTwo-Step Molecular Magnetic Sponge
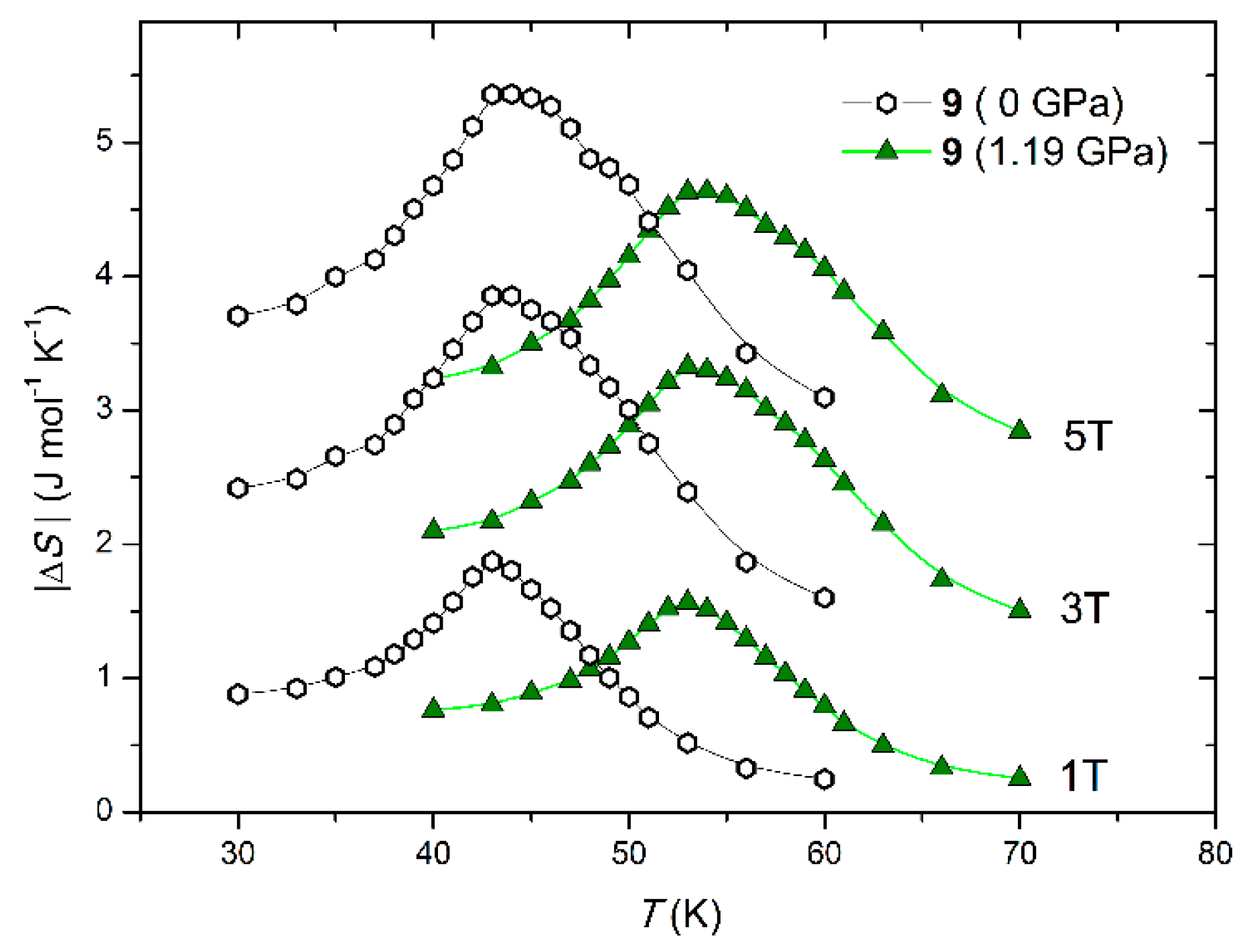
3.5. [{[MnII(pydz)(H2O)2][MnII(H2O)2][NbIV(CN)8]}∙2H2O]nMolecular Compound under Pressure
3.6. Tc−2/3 Dependence of the Maximum Entropy Change
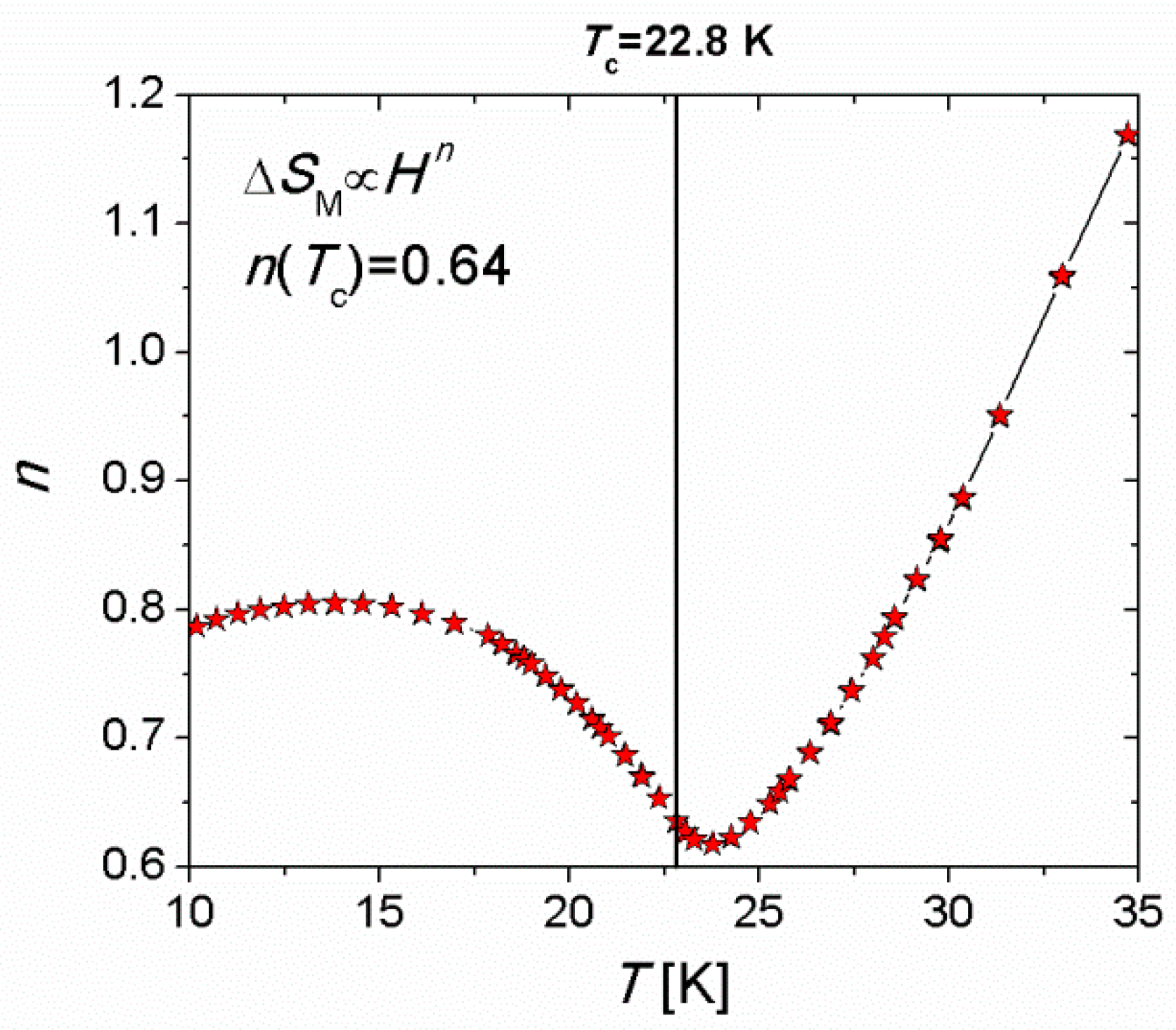
3.7. The Critical Behaviour of the 3 D Octacyanoniobate- Based Compounds
3.8. Final Remarks of Section 3
4. Rotating Magnetocaloric Effect in Anisotropic Two-Dimensional Molecular Magnets
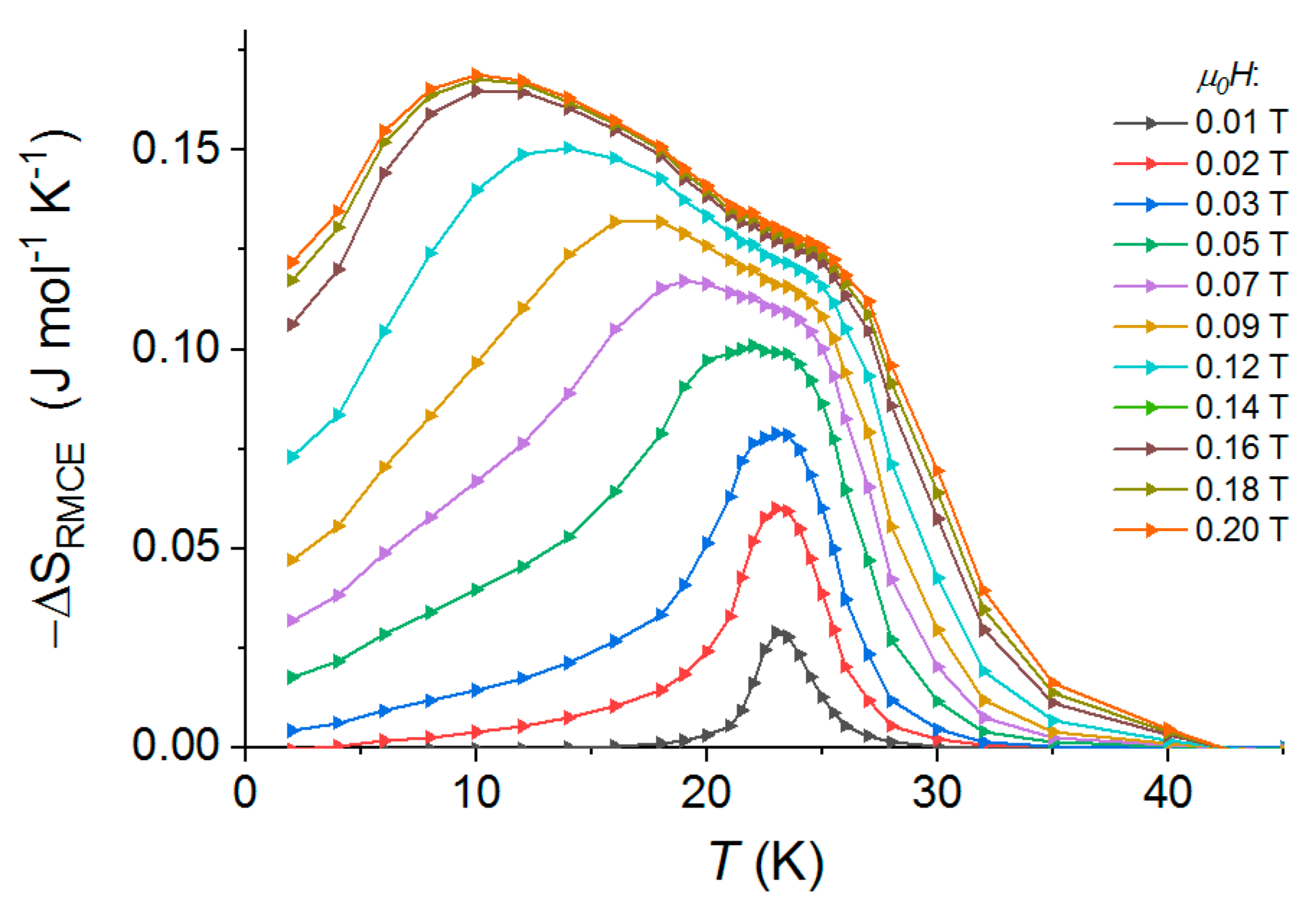
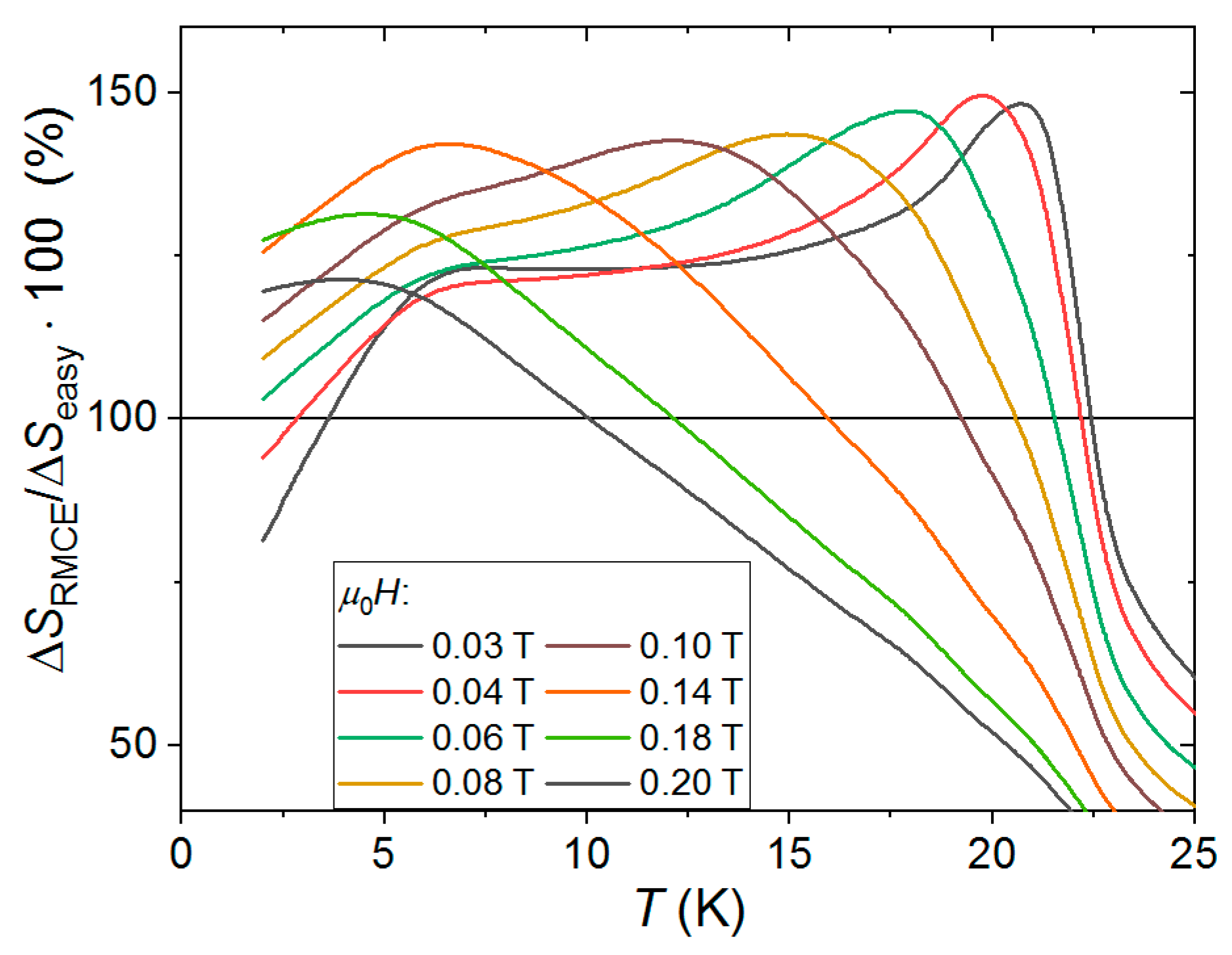
4.1. Low Anisotropy Case: {MnII(R-mpm)2]2[NbIV(CN)8]}∙4H2OCrystal
4.2. High Anisotropy Case: (tetren)Cu4[W(CN)8]4 Crystal
4.3. Final Remarks of Section 4
5. Final Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kahn, O. Molecular Magnetism; VCH Weinheim: Weinheim, Germany, 1993. [Google Scholar]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Bartolomé, J.; Luis, F.; Fernández, J.F. Molecular Magnets—Physics and Applications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Sieklucka, B.; Pinkowicz, D. Molecular Magnetic Materials—Concepts and Applications; VCH Weinheim: Weinheim, Germany, 2017. [Google Scholar]
- Zhang, Q.; Li, B.; Zhao, X.G.; Zhang, Z.D. Magnetic and reversible magnetocaloric properties of (Gd1−xDyx)4Co3 ferrimagnets. J. Appl. Phys. 2009, 105, 053902. [Google Scholar] [CrossRef]
- Samanta, T.; Das, I.; Banerjee, S. Giant magnetocaloric effect in antiferromagnetic ErRu2Si2 compound. Appl. Phys. Lett. 2007, 91, 152506. [Google Scholar] [CrossRef]
- Oesterreicher, H.; Parker, F.T. Magnetic cooling near Curie temperatures above 300 K. J. Appl. Phys. 1984, 55, 4334. [Google Scholar] [CrossRef]
- Franco, V.; Blázquez, J.S.; Conde, A. Field dependence of the magnetocaloric effect in materials with a second order phase transition: A muster curve for the magnetic entropy change. Appl. Phys. Lett. 2006, 89, 222512. [Google Scholar] [CrossRef]
- Belo, J.H.; Amaral, J.S.; Pereira, A.M.; Amaral, V.S.; Araújo, J.P. On the Curie temperature dependency of the magnetocaloric effect. Appl. Phys. Lett. 2012, 100, 242407. [Google Scholar] [CrossRef]
- Von Ranke, P.J.; Gama, S.; Coelho, A.A.; De Campos, A.; Magnus, A.; Carvalho, G.; Gandra, F.C.; de Oliveira, N.A. Theoretical description of the colossal entropic magnetocaloric effect: Application to MnAs. Phys. Rev. B 2006, 73, 014415. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic Materials and Devices for the 21st Century: Stronger, Lighter, and More Energy Efficient. Adv. Mater. 2011, 23, 821. [Google Scholar] [CrossRef]
- Sessoli, R. Chilling with Magnetic Molecules. Angew. Chem. Int. Ed. 2012, 51, 43. [Google Scholar] [CrossRef]
- Evangelisti, M.; Brechin, E.K. Recipes for enhanced molecular cooling. Dalton Trans. 2010, 39, 4672–4676. [Google Scholar] [CrossRef]
- Sibille, R.; Mazet, T.; Malaman, B.; François, M. A Metal–Organic Framework as Attractive Cryogenic Magnetorefrigerant. Chem. Eur. J. 2012, 18, 12970. [Google Scholar] [CrossRef]
- Evangelisti, M.; Roubeau, O.; Palacios, E.; Camón, A.; Hooper, T.N.; Brechin, E.K.; Alonso, J.J. Cryogenic magnetocaloric effect in a ferromagnetic molecular dimer. Angew. Chem. Int. Ed. 2011, 50, 6606–6609. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.B.; Zhang, Q.C.; Kong, X.J.; Zheng, Y.Z.; Ren, Y.P.; Long, L.S.; Huang, R.B.; Zheng, L.S.; Zheng, Z. High-Nuclearity 3d–4f Clusters as Enhanced Magnetic Coolers and Molecular Magnets. J. Am. Chem. Soc. 2012, 134, 3314–3317. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.X.; Xiong, G.; Wang, L.; Cheng, P.; Zhao, B.A. 24-Gd nanocapsule with a large magnetocaloric effect. Chem. Commun. 2013, 49, 1055. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Guo, F.S.; Liu, J.-L.; Leng, J.-D.; Vrábel, P.; Orendáč, M.; Prokleška, J.; Sechovský, V.; Tong, M.L. Switching of the Magnetocaloric Effect of MnII Glycolate by Water Molecules. Chem. Eur. J. 2014, 20, 3029. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, Y.-C.; Guo, F.-S.; Tong, M.-L. Recent advances in the design of magnetic molecules for use as cryogenic magnetic coolants. Coord. Chem. Rev. 2014, 281, 26. [Google Scholar] [CrossRef]
- Entley, W.R.; Girolami, G.S. High-Temperature Molecular Magnets Based on Cyanovanadate Building Blocks: Spontaneous Magnetization at 230 K. Science 1995, 268, 397–400. [Google Scholar] [CrossRef]
- Verdaguer, M.; Girolami, G. Magnetic Prussian Blue Analogs. In Magnetism: Molecules to Materials V; Miller, J.S., Drillon, M., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2001; pp. 283–347. [Google Scholar]
- Sieklucka, B.; Podgajny, R.; Pinkowicz, D.; Nowicka, B.; Korzeniak, T.; Bałanda, M.; Wasiutyński, T.; Pełka, R.; Makarewicz, M.; Czapla, M.; et al. Towards high Tc octacyanometalate-based networks. CrystEngComm 2009, 11, 2032. [Google Scholar] [CrossRef]
- Sieklucka, B.; Podgajny, R.; Korzeniak, T.; Nowicka, B.; Pinkowicz, D.; Kozieł, M. A decade of octacyanides in polynuclear molecular materials. Eur. J. Inorg. Chem. 2011, 3, 305. [Google Scholar] [CrossRef]
- Nowicka, B.; Korzeniak, T.; Stefańczyk, O.; Pinkowicz, D.; Chorąży, S.; Podgajny, R.; Sieklucka, B. The impact of ligands upon topology and functionality of octacyanidometallate-based assemblies. Coord.Chem.Rev. 2012, 256, 1946. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, P.; Ren, X.-M.; Shen, X.-F.; Li, Y.-Z.; You, X.-Z. Octacyanometalate-based single-molecule magnets: CoII9MV6 (M = W, Mo). J. Am. Chem. Soc. 2005, 127, 3708. [Google Scholar] [CrossRef]
- Yoo, H.S.; Ko, H.H.; Ryu, D.W.; Lee, J.W.; Yoon, J.H.; Lee, W.R.; Kim, H.C.; Koh, E.K.; Hong, C.S. Octacyanometalate-Based Ferrimagnetic MVMnIII (M = Mo, W) bimetallic chain racemates with slow magnetic relaxations. Inorg. Chem. 2009, 48, 5617. [Google Scholar] [CrossRef] [PubMed]
- Chorazy, S.; Stanek, J.; Nogas, W.; Majcher, A.M.; Rams, M.; Kozieł, M.; Juszyńska-Gałązka, E.; Nakabayashi, K.; Ohkoshi, S.; Sieklucka, B.; et al. Tuning of charge transfer assisted phase transition and slow magnetic relaxation functionalities in {Fe9−xCox[W(CN)8]6} (x = 0–9) molecular solid solution. J. Am. Chem. Soc. 2016, 138, 1635. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Rams, M.; Stadnicka, K.; Sieklucka, B. Reversible Guest-Induced Magnetic and Structural Single-Crystal-to-Single-Crystal Transformation in Microporous Coordination Network {[Ni(cyclam)]3 [W(CN)8]2}n. Inorg. Chem. 2007, 46, 8123. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Reczyński, M.; Rams, M.; Nitek, W.; Kozieł, M.; Sieklucka, B. Larger pores and higher TC: {[Ni(cyclam)]3[W(CN)8]2·solv}n—A new member of the largest family of pseudo-polymorphic isomers among octacyanometallate-based assemblies. CrystEngComm 2015, 17, 3526. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Tokoro, H.; Hozumi, T.; Zhang, Y.; Hashimoto, K.; Mathonière, C.; Bord, I.; Rombaut, G.; Verelst, M.; Moulin, C.C.D.; et al. Photoinduced Magnetization in Copper Octacyanomolybdate. J. Am. Chem. Soc. 2006, 128, 270. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, S.; Tokoro, H. Photomagnetism in Cyano-Bridged Bimetal Assemblies. Acc. Chem. Res. 2012, 45, 1749. [Google Scholar] [CrossRef] [PubMed]
- Pinkowicz, D.; Podgajny, R.; Nitek, W.; Rams, M.; Majcher, A.M.; Nuida, T.; Ohkoshi, S.; Sieklucka, B. Multifunctional Magnetic Molecular {[MnII(urea)2(H2O)]2[NbIV(CN)8]}n System: Magnetization-Induced SHG in the Chiral Polymorph. Chem. Mater. 2011, 23, 21. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Podgajny, R.; Gaweł, B.; Nitek, W.; Łasocha, W.; Oszajca, M.; Czapla, M.; Makarewicz, M.; Bałanda, M.; Sieklucka, B. Double Switching of a Magnetic Coordination Framework through Intraskeletal Molecular Rearrangement. Angew. Chem. Int. Ed. 2011, 50, 3973. [Google Scholar] [CrossRef]
- Arczyński, M.; Rams, M.; Stanek, J.; Fitta, M.; Sieklucka, B.; Dunbar, K.R.; Pinkowicz, D. A Family of Octahedral Magnetic Molecules Based on [NbIV(CN)8]4−. Inorg. Chem. 2017, 56, 4021. [Google Scholar] [CrossRef]
- Nowicka, B.; Stadnicka, K.; Nitek, W.; Rams, M.; Sieklucka, B. Geometrical isomerism in pentadecanuclear high-spin Ni9W6 clusters with symmetrical bidentate ligands detected. CrystEngComm 2012, 14, 6559. [Google Scholar] [CrossRef]
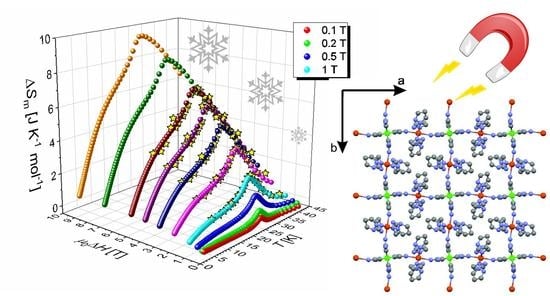
- Gajewski, M.; Pełka, R.; Fitta, M.; Miyazaki, Y.; Nakazawa, Y.; Bałanda, M.; Reczyński, M.; Nowicka, B.; Sieklucka, B. Magnetocaloric effect of high spin cluster with Ni9W6 core. J. Magn. Magn. Mater. 2016, 414, 25. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Pełka, R.; Drath, O.; Nitek, W.; Bałanda, M.; Majcher, A.M.; Poneti, G.; Sieklucka, B. Nature of magnetic interactions in 3D {[MII(pyrazole)4]2[NbIV(CN)8]·4H2O}n (M = Mn, Fe, Co, Ni) molecular magnets. Inorg. Chem. 2010, 49, 7565. [Google Scholar] [CrossRef]
- Pełka, R.; Gajewski, M.; Miyazaki, Y.; Yamashita, S.; Nakazawa, Y.; Fitta, M.; Pinkowicz, D.; Sieklucka, B. Magnetocaloric effect in Mn2-pyrazole-[Nb(CN)8] molecular magnet by relaxation calorimetry. J. Magn. Magn. Mater. 2016, 419, 435. [Google Scholar] [CrossRef]
- Pełka, R.; Konieczny, P.; Zieliński, P.M.; Wasiutyński, T.; Miyazaki, Y.; Inaba, A.; Pinkowicz, D.; Sieklcuka, B. Magnetocaloric effect in {[Fe(pyrazole)4]2[Nb(CN)8]·4H2O}n molecular magnet. J. Magn. Magn. Mater. 2014, 354, 359. [Google Scholar] [CrossRef]
- Konieczny, P.; Pełka, R.; Zieliński, P.M.; Pratt, F.L.; Pinkowicz, D.; Sieklucka, B.; Wasiutyński, T. Scaling analysis of [Fe(pyrazole)4]2[Nb(CN)8] molecular magnet. J. Magn. Magn. Mater. 2013, 344, 105. [Google Scholar] [CrossRef]
- Evangelisti, M.; Candini, A.; Affronte, M.; Pasca, E.; de Jongh, L.J.; Scott, R.T.W.; Brechin, E.K. Magnetocaloric effect in spin degenerated molecular nanomagnets. Phys. Rev. B 2009, 79, 104414. [Google Scholar] [CrossRef]
- Fitta, M.; Bałanda, M.; Mihalik, M.; Pełka, R.; Pinkowicz, D.; Sieklucka, B.; Zentková, M. Magnetocaloric effect in M-pyrazole-[Nb(CN)8] molecular compounds. J. Phys.Condens. Matter. 2012, 24, 506002. [Google Scholar] [CrossRef] [PubMed]
- Fitta, M.; Pełka, R.; Bałanda, M.; Czapla, M.; Mihalik, M.; Pinkowicz, D.; Sieklucka, B.; Wasiutyński, T.; Zentková, M. Magnetocaloric effect in Mn2-pyridazine-[Nb(CN)8] molecular magnetic sponge. Eur. J. Inorg. Chem. 2012, 2012, 3830. [Google Scholar] [CrossRef]
- Franco, V.; Conde, A.; Romero-Enrique, J.M.; Blázquez, J.S. A universal curve for the magnetocaloric effect: An analysis based on scaling relations. J. Phys. Condens. Matter 2008, 20, 285207. [Google Scholar] [CrossRef]
- Campostrini, M.; Hasenbusch, M.; Palissetto, A.; Rossi, P.; Vicari, E. Critical exponents and equation of state of the three-dimensional Heisenberg universality class. Phys. Rev. B 2002, 65, 144520. [Google Scholar] [CrossRef] [Green Version]
- Manuel, E.; Evangelisti, M.; Affronte, M.; Okubo, M.; Train, C.; Verdaguer, M. Magnetocaloric effect in hexacyanochromate Prussian blue analogs. Phys. Rev. B 2006, 73, 172406. [Google Scholar] [CrossRef]
- Herrera, J.M.; Franz, P.; Podgajny, R.; Pilkington, M.; Biner, M.; Decurtins, S.; Stoeckli-Evans, H.; Neels, A.; Garde, R.; Dromzee, Y.; et al. Three-dimensional bimetallic octacyanidometalates [MIV{(µ-CN)4MnII(H2O)2}2∙4H2O]n (M= Nb, Mo, W): Synthesis, single-crystal X-ray diffraction and magnetism. C. R. Chim. 2008, 11, 1192. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Podgajny, R.; Pelka, R.; Nitek, W.; Balanda, M.; Makarewicz, M.; Czapla, M.; Zukrowski, J.; Kapusta, C.; Zajac, D.; et al. Iron(II)-octacyanoniobate(IV) ferromagnet with TC 43 K. Dalton Trans. 2009, 7771. [Google Scholar] [CrossRef] [PubMed]
- Fitta, M.; Pełka, R.; Sas, W.; Pinkowicz, D.; Sieklucka, B. Dinuclear molecular magnets with unblocked magnetic connectivity: Magnetocaloric effect. RSC Adv. 2018, 8, 14640. [Google Scholar] [CrossRef]
- Franco, V.; Caballero-Flores, R.; Conde, A.; Knipling, K.E.; Willard, M.A. Magnetocaloric effect and critical exponents of Fe77Co5.5Ni5.5Zr7B4Cu1: A detailed study. J. Appl. Phys. 2011, 109, 07A905. [Google Scholar] [CrossRef]
- Foldeaki, M.; Schnelle, W.; Gmelin, E.; Benard, P.; Koszegi, B.; Giguere, A.; Chahine, R.; Boseet, T.K. Comparison of magnetocaloric properties from magnetic and thermal measurements. J. Appl. Phys. 1997, 82, 309. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Podgajny, R.; Bałanda, M.; Makarewicz, M.; Gaweł, B.; Łasocha, W.; Sieklucka, B. Magnetic Spongelike Behavior of 3D Ferrimagnetic {[MnII(imH)]2[NbIV(CN)8]}n with Tc = 62 K. Inorg. Chem. 2008, 47, 9745. [Google Scholar] [CrossRef] [PubMed]
- Fitta, M.; Pełka, R.; Gajewski, M.; Mihalik, M.; Zentkova, M.; Pinkowicz, D.; Sieklucka, B.; Bałanda, M. Magnetocaloric effect and critical behavior in Mn2-imidazole-[Nb(CN)8] molecular magnetic sponge. J. Magn. Magn. Mater. 2015, 396. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Kurpiewska, K.; Lewiński, K.; Bałanda, M.; Mihalik, M.; Zentkova, M.; Sieklucka, B. High-pressure single-crystal XRD and magnetic study of aoctacyanoniobate-based magnetic sponge. CrystEngComm 2012, 14, 5224. [Google Scholar] [CrossRef]
- Fitta, M.; Bałanda, M.; Pełka, R.; Konieczny, P.; Pinkowicz, D.; Sieklucka, B. Magnetocaloric effect and critical behaviour in Mn2-pyridazine-[Nb(CN)8] molecular compound under pressure. J. Phys. Condens. Matter 2013, 25, 496012. [Google Scholar] [CrossRef]
- Banerjee, S.K. On a generalised approach to first and second order magnetic transitions. Phys. Lett. 1964, 12, 16. [Google Scholar] [CrossRef]
- Kouvel, J.S.; Fisher, M.E. Detailed Magnetic Behavior of Nickel Near its Curie Point. Phys. Rev. 1964, 136, A1626. [Google Scholar] [CrossRef]
- Pełka, R.; Pinkowicz, D.; Sieklucka, B.; Fitta, M. Molecular realizations of 3D Heisenberg magnet: Critical scaling. J. Alloys Compd. 2018, 765, 520. [Google Scholar] [CrossRef]
- Nikitin, S.A.; Skokov, K.P.; Koshkid’ko, Y.S.; Pastushenkov, Y.G.; Ivanova, T.I. Giant Rotating Magnetocaloric Effect in the Region of Spin-Reorientation Transition in the NdCo5 Single Crystal. Phys. Rev. Lett. 2010, 105, 137205. [Google Scholar] [CrossRef] [PubMed]
- Orendáč, M.; Gabáni, S.; Gažo, E.; Pristáš, G.; Shitsevalova, N.; Siemensmeyer, K.; Flachbart, K. Rotating Magnetocaloric Effect and Unusual Magnetic Features in Metallic Strongly Anisotropic Geometrically Frustrated TmB4. Sci. Rep. 2018, 8, 10933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Liu, E.; Ke, Y.; Jin, J.; Long, Y.; Shen, B. Giant Rotating Magnetocaloric Effect Induced by Highly Texturing in Polycrystalline DyNiSi Compound. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Jin, J.-L.; Zhang, X.-Q.; Ge, H.; Cheng, Z.-H. Rotating Field Entropy Change in Hexagonal TmMnO3 Single Crystal with Anisotropic Paramagnetic Response. Phys. Rev. B 2012, 85, 214426. [Google Scholar] [CrossRef]
- Balli, M.; Jandl, S.; Fournier, P.; Dimitrov, D.Z. Giant Rotating Magnetocaloric Effect at Low Magnetic Fields in Multiferroic TbMn2O5 Single Crystals. Appl. Phys. Lett. 2016, 108, 102401. [Google Scholar] [CrossRef]
- Balli, M.; Jandl, S.; Fournier, P.; Gospodinov, M.M. Anisotropy-Enhanced Giant Reversible Rotating Magnetocaloric Effect in HoMn2O5 Single Crystals. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Engelbrecht, K.; Eriksen, D.; Bahl, C.R.H.; Bjørk, R.; Geyti, J.; Lozano, J.A.; Nielsen, K.K.; Saxild, F.; Smith, A.; Pryds, N. Experimental Results for a Novel Rotary Active Magnetic Regenerator. Int. J. Refrig. 2012, 35, 1498. [Google Scholar] [CrossRef]
- Tkáč, V.; Orendáčová, A.; Čižmár, E.; Orendáč, M.; Feher, A.; Anders, A.G. Giant Reversible Rotating Cryomagnetocaloric Effect in KEr(MoO4)2 Induced by a Crystal-Field Anisotropy. Phys. Rev. B 2015, 92, 24406. [Google Scholar] [CrossRef]
- Caro Patiño, J.; de Oliveira, N.A. Rotating Magnetocaloric Effect in HoAl2 Single Crystal. Intermetallics 2015, 64, 59. [Google Scholar] [CrossRef]
- Balli, M.; Mansouri, S.; Jandl, S.; Fournier, P.; Dimitrov, D.Z. Large Rotating Magnetocaloric Effect in the Orthorhombic DyMnO3 Single Crystal. Solid State Commun. 2016, 239, 9. [Google Scholar] [CrossRef]
- Lorusso, G.; Roubeau, O.; Evangelisti, M. Rotating Magnetocaloric Effect in an Anisotropic Molecular Dimer. Angew. Chem. Int. Ed. 2016, 55, 3360–3363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chorazy, S.; Podgajny, R.; Nitek, W.; Fic, T.; Görlich, E.; Rams, M.; Sieklucka, B. Natural and Magnetic Optical Activity of 2-D Chiral Cyanido-Bridged MnII–NbIV Molecular Ferrimagnets. Chem. Commun. 2013, 49, 6731. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, P.; Pełka, R.; Czernia, D.; Podgajny, R. Rotating Magnetocaloric Effect in an Anisotropic Two-Dimensional CuII[WV(CN)8]3– Molecular Magnet with Topological Phase Transition: Experiment and Theory. Inorg. Chem. 2017, 56, 11971. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, P.; Michalski, Ł.; Podgajny, R.; Chorazy, S.; Pełka, R.; Czernia, D.; Buda, S.; Mlynarski, J.; Sieklucka, B.; Wasiutyński, T. Self-Enhancement of Rotating Magnetocaloric Effect in Anisotropic Two-Dimensional (2D) Cyanido-Bridged MnII–NbIV Molecular Ferrimagnet. Inorg. Chem. 2017, 56, 2777. [Google Scholar] [CrossRef] [PubMed]
- Baranová, L.; Orendáčová, A.; Čižmár, E.; Tarasenko, R.; Tkáč, V.; Orendáč, M.; Feher, A. Fingerprints of Field-Induced Berezinskii–Kosterlitz–Thouless Transition in Quasi-Two-Dimensional S = 1/2 Heisenberg Magnets Cu(en)(H2O)2SO4 and Cu(tn)Cl2. J. Magn. Magn. Mater. 2016, 404, 53. [Google Scholar] [CrossRef]
- Bałanda, M.; Pełka, R.; Wasiutyński, T.; Rams, M.; Nakazawa, Y.; Miyazaki, Y.; Sorai, M.; Podgajny, R.; Korzeniak, T.; Sieklucka, B. Magnetic Ordering in the Double-Layered Molecular Magnet Cu(tetren)[W(CN)8]: Single-Crystal Study. Phys. Rev. B 2008, 78, 174409. [Google Scholar] [CrossRef]
- Czapla, M.; Pełka, R.; Zieliński, P.M.; Budziak, A.; Bałanda, M.; Makarewicz, M.; Pacyna, A.; Wasiutyński, T.; Miyazaki, Y.; Nakazawa, Y.; et al. Critical Behavior of Two Molecular Magnets Probed by Complementary Experiments. Phys. Rev. B 2010, 82, 94446. [Google Scholar] [CrossRef]


























| μ0ΔH (T) | Tpeak (K) | Tpeak (K) | ||
|---|---|---|---|---|
| 1 | 2.4 | 7.18 | 2.9 | 1.6 |
| 2 | 3.0 | 11.42 | 2.6 | 2.8 |
| 3 | 3.5 | 14.48 | 2.4 | 3.6 |
| 4 | 3.9 | 16.62 | 2.3 | 4.2 |
| 5 | 4.3 | 18.38 | 2.2 | 4.6 |
| 7 | 5.3 | 20.86 | 2.0 | 5.2 |
| 9 | 6.5 | 22.77 | 2.0 | 5.6 |
| μ0ΔH (T) | Tpeak (K) | Tpeak (K) | ||
|---|---|---|---|---|
| 0.1 | 23.3 | 0.29 | 23.3 | 0.06 |
| 0.2 | 23.8 | 0.68 | 23.3 | 0.14 |
| 0.5 | 23.8 | 1.50 | 23.8 | 0.30 |
| 1 | 23.8 | 2.44 | 23.8 | 0.50 |
| 2 | 24.3 | 3.85 | 23.8 | 0.80 |
| 3 | 24.3 | 4.99 | 23.8 | 1.04 |
| 4 | 24.3 | 5.97 | 23.8 | 1.24 |
| 5 | 24.3 | 6.83 | 23.8 | 1.42 |
| 7 | 24.3 | 8.30 | 23.8 | 1.73 |
| 9 | 25.5 | 9.49 | 23.8 | 1.97 |
| μ0ΔH (T) | Tpeak (K) | Tpeak (K) | ||
|---|---|---|---|---|
| 0.1 | 8.9 | 0.3 | 8.8 | 0.1 |
| 0.2 | 8.9 | 0.5 | 8.8 | 0.2 |
| 0.5 | 8.9 | 1.0 | 8.9 | 0.4 |
| 1 | 9.3 | 1.6 | 8.8 | 0.6 |
| 2 | 9.3 | 2.7 | 8.8 | 1.1 |
| 5 | 10.3 | 4.9 | 8.9 | 2.0 |
| 9 | 6.9 | 6.9 | 8.8 | 2.8 |
| Sample | Tc (K) | |ΔS|max (J mol−1 K−1) | |ΔS|max (J kg−1 K−1) | RCP (J mol−1 K−1) | RCP (J kg−1 K−1) |
|---|---|---|---|---|---|
| 4 | 50.0 | 5.07 | 9.09 | 118.40 | 212.61 |
| 5 | 43.0 | 4.82 | 8.65 | 125.43 | 225.59 |
| 6 | 23.8 | 6.70 | 6.50 | 136.9 | 132.9 |
| 7 | 13.4 | 6.10 | 5.90 | 75.6 | 73.1 |
| 8 | 25.0 | 6.70 | 8.95 | 186.2 | 248.9 |
| 8deh | 60.0 | 4.02 | 7.73 | 152.8 | 293.8 |
| 9 | 43.0 | 5.36 | 8.95 | 160.8 | 268.0 |
| 9deh | 68 | 3.33 | 5.82 | 109.9 | 192.1 |
| 9anh | 98 | 3.38 | 6.88 | 101.4 | 206.5 |
| 9HP | 52.5 | 4.63 | 7.73 | 138.9 | 231.8 |
| Sample | β | γ | δ | nMCE | ntheor |
|---|---|---|---|---|---|
| 4 | 0.41 | 1.32 | 4.39 | 0.69 | 0.66 |
| 5 | 0.37 | 1.33 | 4.37 | 0.67 | 0.63 |
| 6 | 0.64 | ||||
| 7 | 0.59 | ||||
| 8 | 0.37 | 1.35 | 4.48 | 0.65 | 0.63 |
| 8deh | 0.37 | 1.40 | 4.95 | 0.67 | 0.64 |
| 9 | 0.38 | 1.35 | 4.69 | 0.66 | 0.64 |
| 9deh | 0.43 | 1.38 | 4.23 | 0.68 | 0.69 |
| 9anh | 0.39 | 1.37 | 4.49 | 0.69 | 0.65 |
| 9HP | 0.37 | 1.40 | 4.48 | 0.67 | 0.64 |
| Mean field model | 0.500 | 1 | 3 | 0.66 | |
| Heisenberg model | 0.365 | 1.385 | 4.8 | 0.64 | |
| Ising model | 0.325 | 1.24 | 4.82 | 0.61 | |
| XY model | 0.346 | 1.316 | 4.81 | 0.57 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitta, M.; Pełka, R.; Konieczny, P.; Bałanda, M. Multifunctional Molecular Magnets: Magnetocaloric Effect in Octacyanometallates. Crystals 2019, 9, 9. https://doi.org/10.3390/cryst9010009
Fitta M, Pełka R, Konieczny P, Bałanda M. Multifunctional Molecular Magnets: Magnetocaloric Effect in Octacyanometallates. Crystals. 2019; 9(1):9. https://doi.org/10.3390/cryst9010009
Chicago/Turabian StyleFitta, Magdalena, Robert Pełka, Piotr Konieczny, and Maria Bałanda. 2019. "Multifunctional Molecular Magnets: Magnetocaloric Effect in Octacyanometallates" Crystals 9, no. 1: 9. https://doi.org/10.3390/cryst9010009