Thermotropic Liquid-Crystalline Properties of Viologens Containing 4-n-alkylbenzenesulfonates †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. General Procedure for the Synthesis of 4-n-alkylbenzenesulfonic Acids (n = 12, 14, 16, 18)
2.3. General Procedure for the Synthesis of Sodium 4-n-alkylbenzenesulfonates (n = 6, 7, 8, 9, 10, 12)
2.4. Synthesis of 1,1′-di-n-butyl-4,4′-bipridinium Dibromide
2.5. General Procedure for the Synthesis1,1′-di-n-butyl-4,4′-bipyridinium di(4-n-alkylbenzenesulfonates) by Metathesis Reaction (V12, V14, V16, V18) Using 4-n-alkylbenzenesulfonic Acid [35]
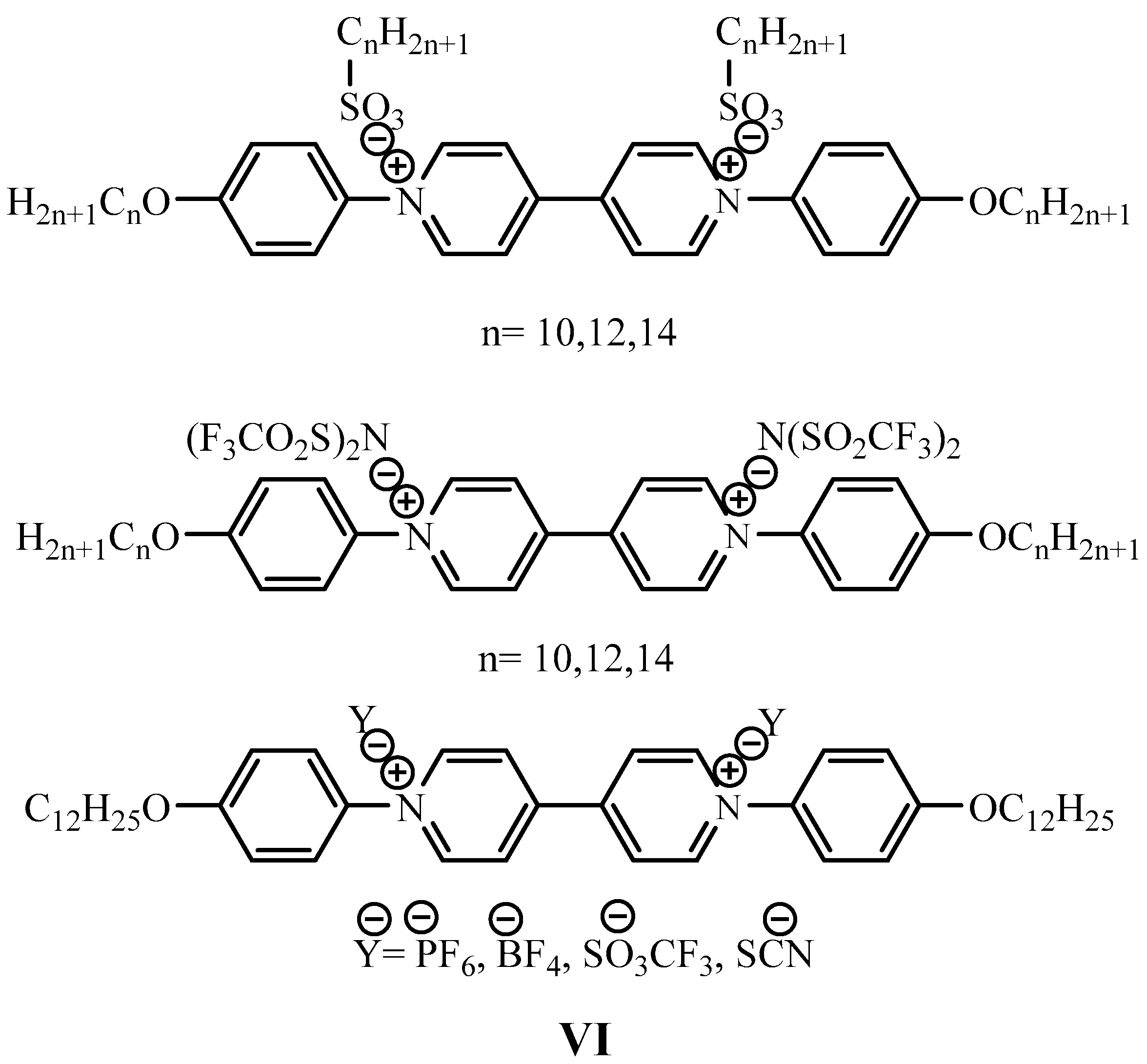
2.6. General Procedure for the Synthesis 1,1′-di-n-butyl-4,4′-bipyridinium di(4-n-alkylbenzenesulfonates) by Metathesis Reaction (V6, V7, V8, V9, V10, V12,) Using Sodium 4-n-alkylbenzenesulfonate
3. Results and Discussion
3.1. Synthesis of Viologen Salts (V6–V18)
3.2. Thermotropic LC Properties of 4-n-alkylbenzenesulfonic Acids (n = 12, 14, 16, 18) by DSC and POM [36,37,38,39,40]
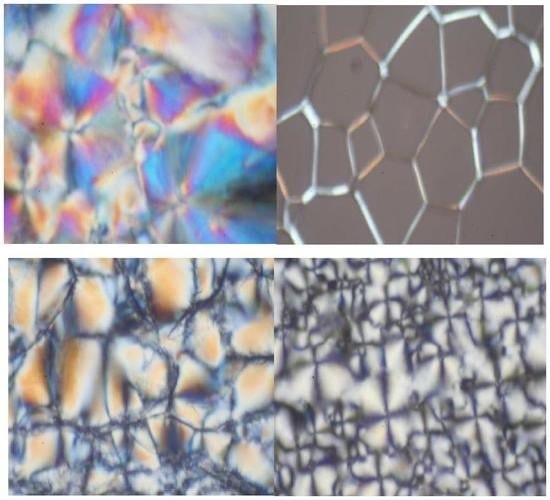
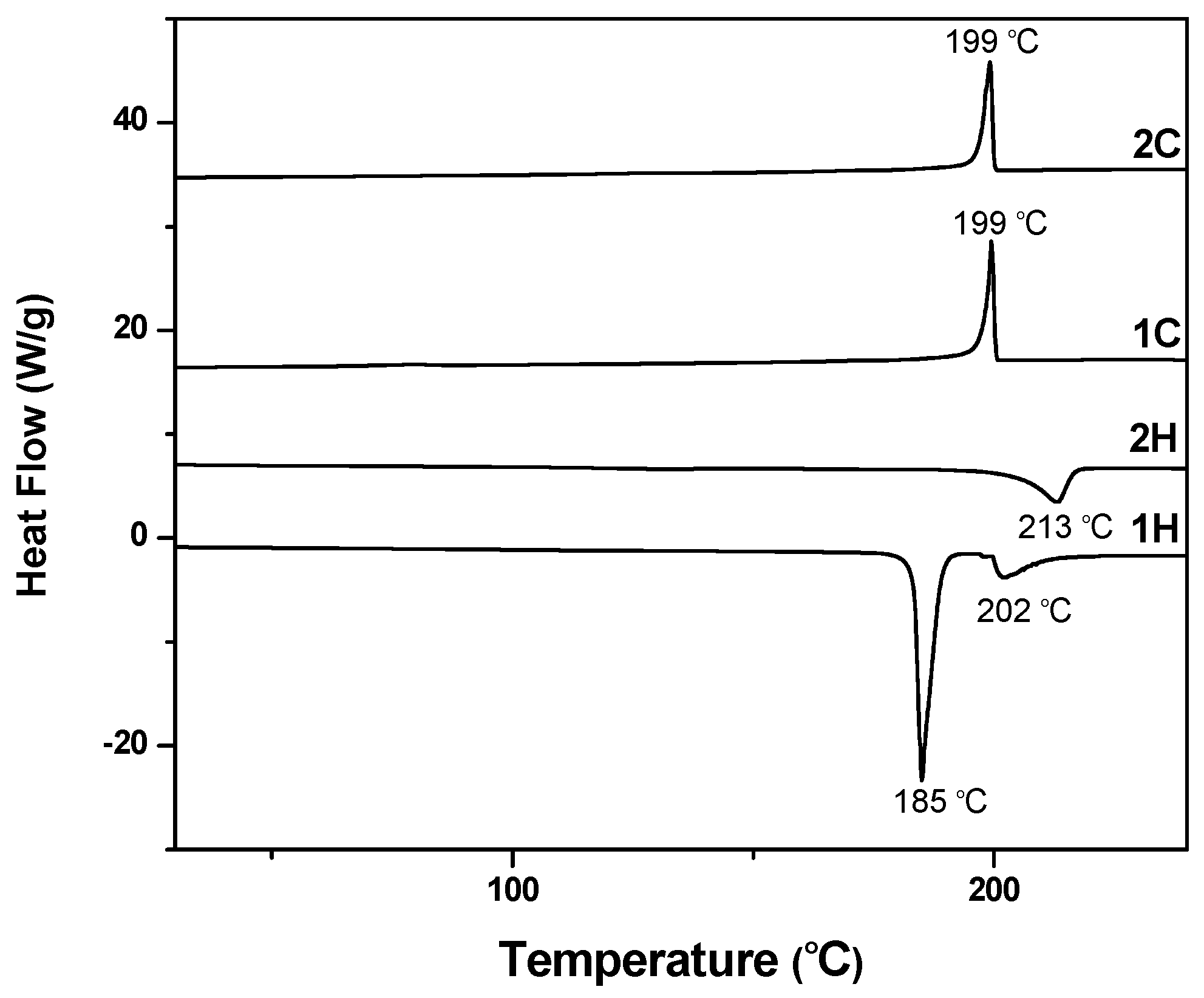
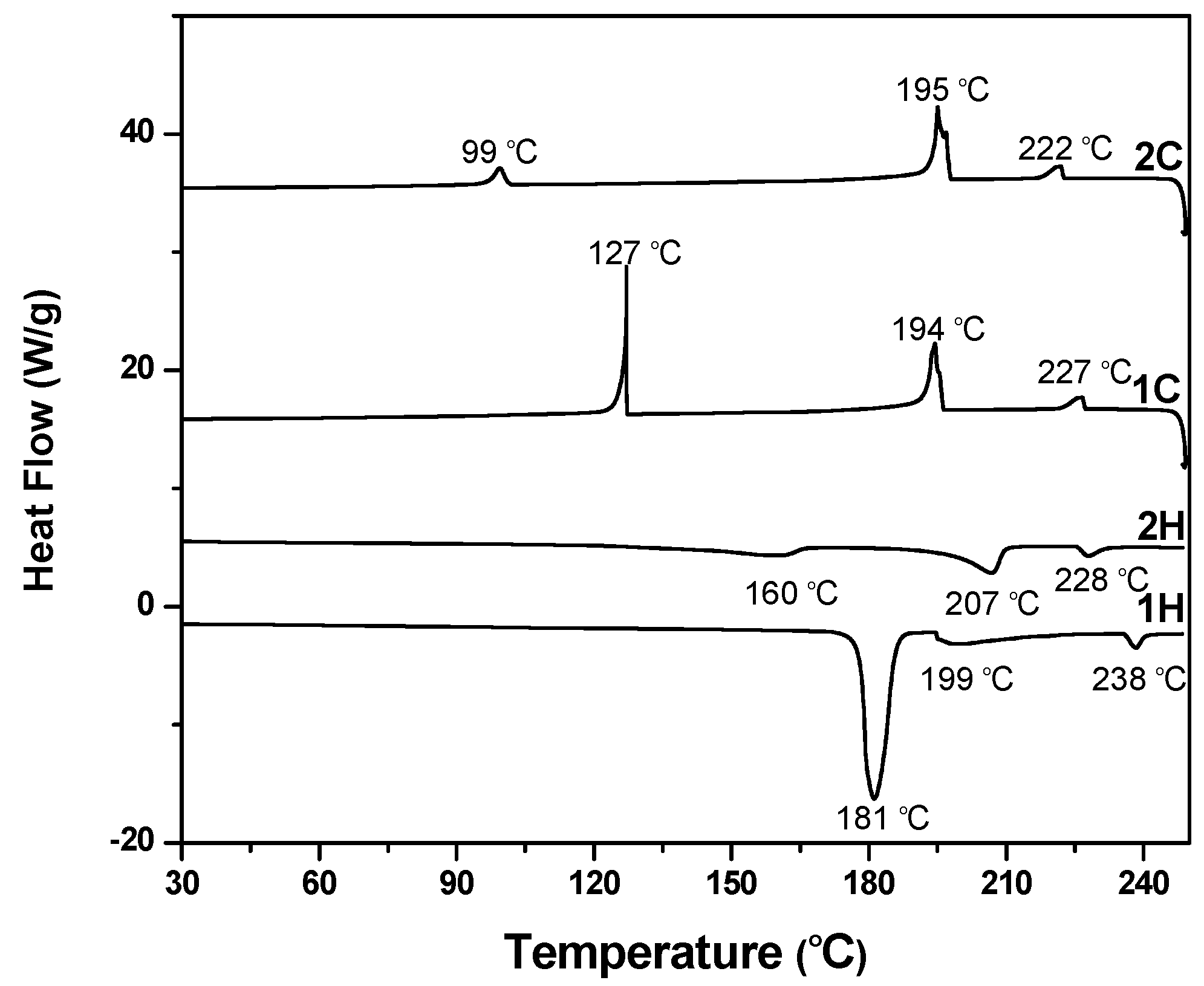
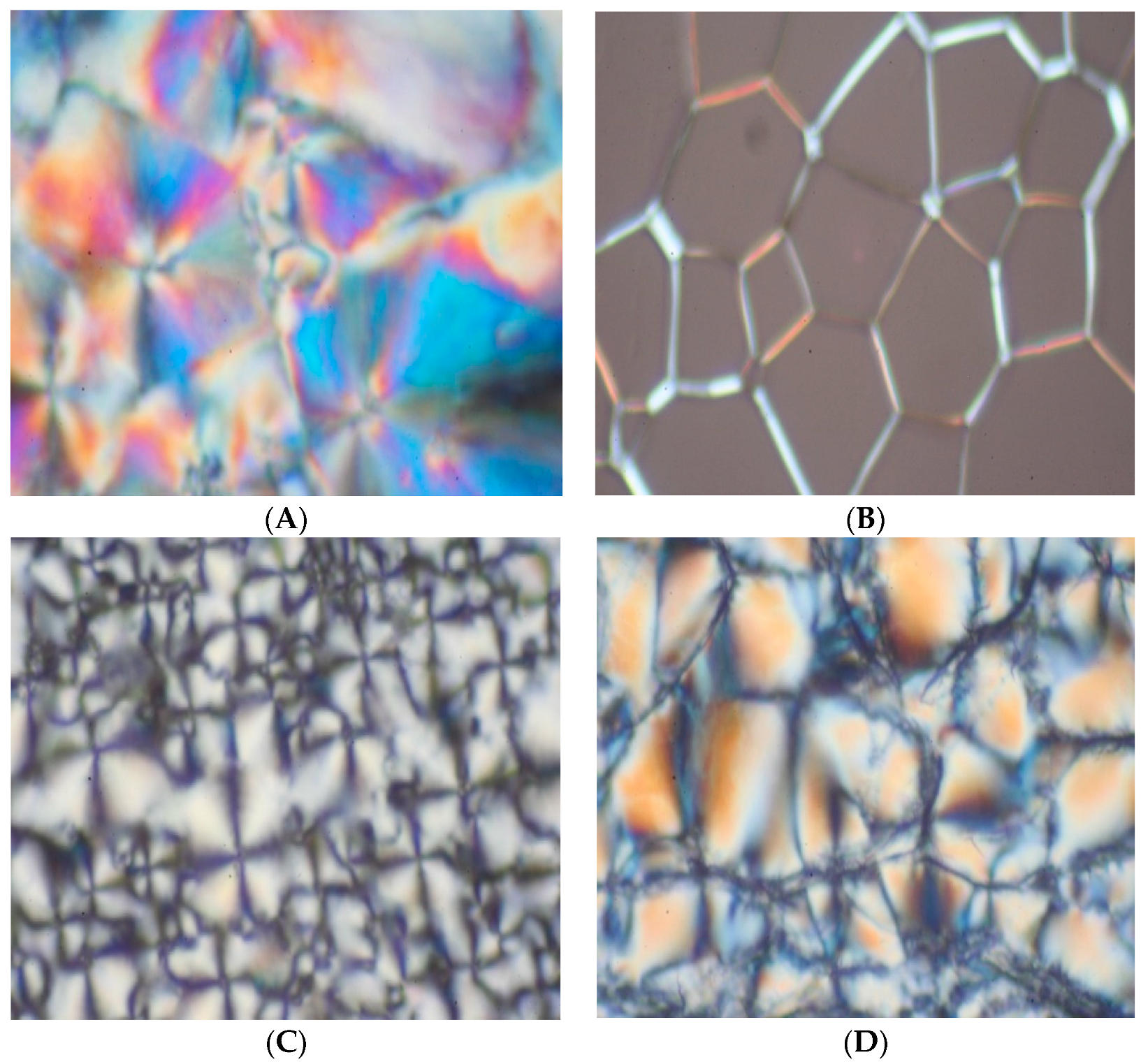
3.3. Thermotropic LC Properties of (V6-V18) by DSC and POM [36,37,38,39,40]
3.4. Thermal Stabilities of Viologen Salts (V6–V18)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Monk, P.M.S. The Viologens Physiochemical Properties, Synthesis and Applications of the Salts of 4,4′-Bipyridine; Wiley: New York, NY, USA, 1998; pp. 1–311. [Google Scholar]
- Striepe, L.; Baumgartner, T. Viologens and Their Application as Functional Materials. Chem. Eur. J. 2017, 23, 16924–16940. [Google Scholar] [CrossRef] [PubMed]
- Rigby, C.R.; Han, H.; Bhowmik, P.K.; Bahari, M.; Chang, A.; Harb, J.N.; Lewis, R.S.; Watt, G.D. Soluble Viologen Polymers as Carbohydrate Oxidation Catalysts for Alkaline Carbohydrate Fuel Cells. J. Electroanal. Chem. 2018, 823, 416–421. [Google Scholar] [CrossRef]
- Hatazawa, T.; Terrill, R.H.; Murray, R.W. Microelectrode Voltammetry and Electron Transport in an Undiluted Room Temperature Melt of an Oligo(ethylene glycol)-Tailed Viologen. Anal. Chem. 1996, 68, 597–603. [Google Scholar] [CrossRef]
- Ito-Akita, K.; Ohno, H. Low Temperature Molten Viologens-Phase Transitions and Electrochemical Properties. Electrochem. Soc. Procced. 1999, 99-14, 193–201. [Google Scholar]
- Causin, V.; Saielli, G. Effect of a structural modification of the bipyridinium core on the phase behaviour of viologen-based bistriflimide salts. J. Mol. Liq. 2009, 145, 41–47. [Google Scholar] [CrossRef]
- Causin, V.; Saielli, G. Effect of asymmetric substitution on the mesomorphic behaviour of low-melting viologen salts of bis(trifluoromethanesulfonyl)amide. J. Mater. Chem. 2009, 19, 9153–9162. [Google Scholar] [CrossRef]
- Bonchio, M.; Carraro, M.; Casella, G.; Causin, V.; Rastrelli, F.; Saielli, G. Thermal behaviour and electrochemical properties of bis(trifluoromethanesulfonyl)amide and dodecatungstosilicate viologen dimers. Phys. Chem. Chem. Phys. 2012, 14, 2710–2717. [Google Scholar] [CrossRef]
- Gunaratne, H.Q.N.; Nockemann, P.; Olejarz, S.; Reid, S.M.; Seddon, K.R.; Srinivasan, G. Ionic Liquids with Solvatochromic and Charge-Transfer Functionalities Incorporating the Viologen Moiety. Aust. J. Chem. 2013, 66, 607–611. [Google Scholar] [CrossRef]
- Jordäo, N.; Cabrita, L.; Pina, F.; Branco, L.C. Novel Bipyridinium Ionic Liquids as Liquid Electrochromic Devices. Chem. Eur. J. 2014, 20, 3982–3988. [Google Scholar] [CrossRef]
- Tahara, H.; Furue, Y.; Suenaga, C.; Sagara, T. A Dialkyl Viologen Ionic Liquid: X-ray Crystal Structure Analysis of Bis(trifluoromethanesulfonyl)imide Salts. Cryst. Growth Des. 2015, 15, 4735–4740. [Google Scholar] [CrossRef]
- Jordäo, N.; Cruz, H.; Branco, A.; Pina, F.; Branco, L.C. Electrochromic Devices Based on Disubstituted Oxo-Bipyridinium Ionic Liquids. ChemPlusChem. 2015, 80, 202–208. [Google Scholar] [CrossRef]
- Yu, L.P.; Samulski, E.T. Ionomeric Liquid Crystals. In Oriented Fluids and Liquid Crystals; Griffin, A.C., Johnson, J.F., Eds.; Plenum: New York, NY, USA, 1984; Volume 4, pp. 697–704. [Google Scholar]
- Tabushi, I.; Yamamura, K.; Kominami, K. Electric Stimulus-Response Behavior of Liquid-Crystalline Viologen. J. Am. Chem. Soc. 1986, 108, 6409–6410. [Google Scholar] [CrossRef]
- Yamamura, K.; Okada, Y.; Ono, S.; Kominami, K.; Tabushi, I. New Liquid Crystalline Viologens Exhibiting Electric Stimulus-Response Behavior. Tetrahedron Lett. 1987, 28, 6475–6478. [Google Scholar] [CrossRef]
- Haramoto, Y.; Yin, M.; Matukawa, Y.; Ujiie, S.; Nanasawa, M. A new ionic liquid crystal compound with viologen group in the principal structure. Liq. Cryst. 1995, 19, 319–320. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.; Cebe, J.J.; Burchett, R.A.; Acharya, A.; Kumar, S. Ambient temperature thermotropic liquid crystalline viologen bis(triflimide) salts. Liq. Cryst. 2003, 30, 1433–1440. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.; Nedeltchev, I.K.; Cebe, J.J. Room-Temperature Thermotropic Ionic Liquid Crystals: Viologen Bis(triflimide) Salts. Mol. Cryst. Liq. Cryst. 2004, 419, 27–46. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Killarney, S.T.; Li, J.R.A.; Koh, J.J.; Han, H.; Sharpnack, L.; Agra-Kooijman, D.M.; Fisch, M.R.; Kumar, S. Thermotropic liquid-crystalline properties of extended viologen bis(triflimide) salts. Liq. Cryst. 2018, 45, 872–885. [Google Scholar] [CrossRef]
- Casella, G.; Causin, V.; Rastrelli, F.; Saielli, G. Viologen-based ionic liquid crystals: Induction of a smectic A phase by dimerisation. Phys. Chem. Chem. Phys. 2014, 16, 5048–5051. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.; Causin, V.; Rastrelli, F.; Saielli, G. Ionic liquid crystals based on viologen dimers: Tuning the mesomorphism by varying the conformational freedom of the ionic layer. Liq. Cryst. 2016, 43, 1161–1173. [Google Scholar] [CrossRef]
- Tanabe, K.; Yasuda, T.; Yoshio, M.; Kato, T. Viologen-Based Redox-Active Ionic Liquid Crystals Forming Columnar Phases. Org. Lett. 2007, 9, 4271–4274. [Google Scholar] [CrossRef] [PubMed]
- Asaftei, S.; Ciobanu, M.; Lepadatu, A.M.; Song, E.; Beginn, U. Thermotropic ionic liquid crystals by molecular assembly and ion pairing of 4,4′-bipyridinium derivatives and tris(dodecyloxy)benzenesulfonates in a non-polar solvent. J. Mater. Chem. 2012, 22, 14426–14437. [Google Scholar] [CrossRef]
- Wang, R.-T.; Lee, G.-H.; Lai, C.K. Anion-induced ionic liquid crystals of diphenylviologens. J. Mater. Sci. C 2018, 6, 9430–9444. [Google Scholar] [CrossRef]
- Binnemans, K. Ionic Liquid Crystals. Chem. Rev. 2005, 105, 4148–4204. [Google Scholar] [CrossRef] [PubMed]
- Axenov, K.; Laschat, S. Thermotropic ionic liquid crystals. Materials 2011, 4, 206–259. [Google Scholar] [CrossRef] [PubMed]
- Causin, V.; Saielli, G. Ionic liquid crystals. In Green Solvents II. Properties and Applications of Ionic Liquids; Mohammad, A., Inamuddin, D., Eds.; Springer: London, UK, 2012; pp. 79–118. [Google Scholar]
- Mansueto, M.; Laschat, S. Ionic Liquid Crystals. In Handbook of Liquid Crystals. Vol. 6: Nanostructured and Amphiphilic Liquid Crystals, 2nd ed.; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H., Raynes, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; pp. 231–280. [Google Scholar]
- Fernandez, A.A.; Kouwer, P.H.J. Key Developments in Ionic Liquid Crystals. Int. J. Mol. Sci. 2016, 17, 731. [Google Scholar] [CrossRef] [PubMed]
- Goossens, K.; Lava, K.; Bielawski, C.W.; Binnemans, K. Ionic Liquid Crystals: Versatile Materials. Chem. Rev. 2016, 116, 4643–4807. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yoshio, M.; Ichikawa, T.; Soberats, B.; Ohno, H.; Funahashi, M. Transport of ions and electrons in nanostructured liquid crystals. Nat. Rev. Mater. 2017, 2, 17001. [Google Scholar] [CrossRef]
- Gilbert, E.E. Recent Developments in Preparative Sulfonation and Sulfation. Synthesis 1969, 1969, 3–10. [Google Scholar] [CrossRef]
- Martin, S.M.; Yonezawa, J.; Horner, M.J.; Macosko, C.W.; Ward, M.D. Structure and rheology of hydrogen bond reinforced liquid crystals. Chem. Mater. 2004, 16, 3045–3055. [Google Scholar] [CrossRef]
- Mathevet, F.; Masson, P.; Nicoud, J.-F.; Skoulios, A. Smectic liquid crystals from supramolecular guanidinium alkylbenzenesulfonates. Chem. Eur. J. 2002, 8, 2248–2254. [Google Scholar] [CrossRef]
- Nanasawa, M.; Matsukawa, Y.; Jin, J.J.; Haramoto, Y. Redox photochemistry of viologen in organized solid state. J. Photochem. Photobiol. A Chem. 1997, 109, 35–38. [Google Scholar] [CrossRef]
- Gray, G.W.; Goodby, J.W.G. Smectic Liquid Crystals: Textures and Structures; Leonard Hill: Glasgow, UK, 1984. [Google Scholar]
- Collins, P.J.; Hird, M. Introduction to Liquid Crystals Chemistry and Physics; Taylor & Francis: Bristol, PA, USA, 1997. [Google Scholar]
- Demus, D.; Goodby, J.W.; Gray, G.W.; Spiess, H.-W.; Vill, V. (Eds.) Handbook of Liquid Crystals; Wiley-VCH: Weinheim, Germany, 1998; Volumes 1–3. [Google Scholar]
- Dierking, I. Textures of Liquid Crystals; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Goodby, J.W.; Collings, P.J.; Kato, T.; Tschierske, C.; Gleeson, H.F.; Raynes, P. (Eds.) Handbook of Liquid Crystals: 8 Volume Set, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]


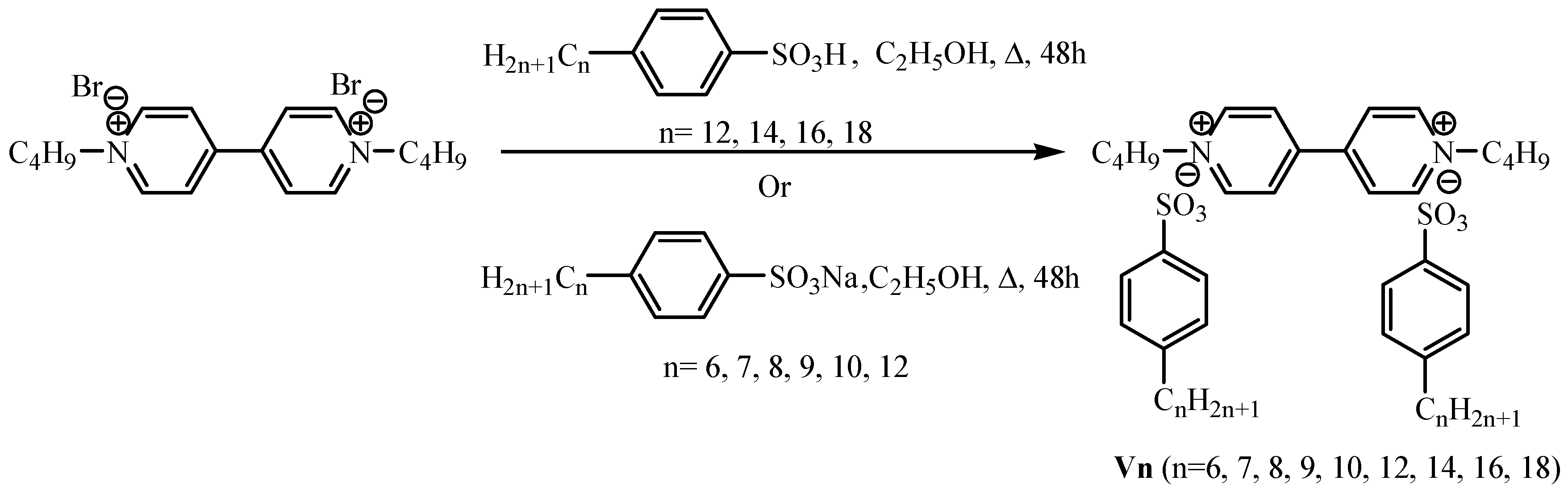




| Identification | Phase Transition Temperature (Enthalpy Change) °C (J/g) |
|---|---|
| V6 | Cr 185 (85.4) SmA 202 (14.3) I |
| I 199 (−26.9) SmA | |
| V7 | Cr 185 (84.0) SmA 199 (24.1) I |
| I 204 (−27.7) SmA | |
| V8 | Cr 181 (83.0) SmX 199 (12.7) SmA 238 (3.2) I |
| I 227 (−3.3) SmA 194 (−17.9) SmX 127 (−12.4) Cr | |
| V9 | Cr 178 (85.8) SmX 202 (1.2) SmX 245 (10.6) SmA268 (1.7) I |
| I 250 (−5.0) SmA 161 (−7.0) Cr 39 (−7.6) Cr | |
| V10 | Cr 174 (84.2) SmA 202 (1.2) I |
| I − SmA 148 (−71.8) Cr | |
| V12 | Cr 56 * Cr 169 (79.5) 206 (0.8) I |
| I − SmA 145 (−58.3) Cr 54 Cr | |
| V14 | Cr 68 * Cr 164 (81.5) SmA 195 (20.9) I |
| I – SmA 159 (−3.4) SmX 126 (−37.2) Cr 88 (−1.0) Cr 66 (−8.2) Cr | |
| V16 | Cr 75 * Cr 157 (81.3) SmA 205 (2.3) I |
| I – SmA 160 (− 4. 2) SmX 113 (−29.4) Cr 73 (−20.0) Cr | |
| V18 | Cr 80 * Cr 160 (82.9) SmA 206 (2.2) I |
| I – SmA 134 (−60.5) Cr 62 ( −20.2) Cr |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhowmik, P.K.; Chang, A.; Kim, J.; Dizon, E.J.; Principe, R.C.G.; Han, H. Thermotropic Liquid-Crystalline Properties of Viologens Containing 4-n-alkylbenzenesulfonates. Crystals 2019, 9, 77. https://doi.org/10.3390/cryst9020077
Bhowmik PK, Chang A, Kim J, Dizon EJ, Principe RCG, Han H. Thermotropic Liquid-Crystalline Properties of Viologens Containing 4-n-alkylbenzenesulfonates. Crystals. 2019; 9(2):77. https://doi.org/10.3390/cryst9020077
Chicago/Turabian StyleBhowmik, Pradip K., Anthony Chang, Jongin Kim, Erenz J. Dizon, Ronald Carlo G. Principe, and Haesook Han. 2019. "Thermotropic Liquid-Crystalline Properties of Viologens Containing 4-n-alkylbenzenesulfonates" Crystals 9, no. 2: 77. https://doi.org/10.3390/cryst9020077