Recent Development in ITO-free Flexible Polymer Solar Cells
Abstract
:1. Introduction
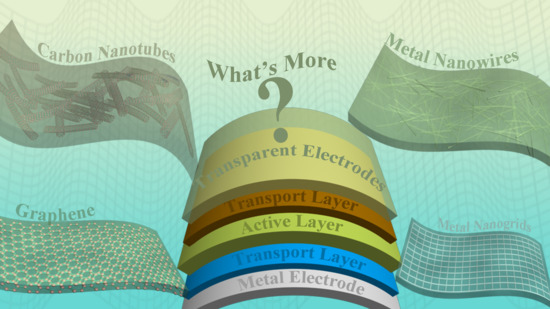
2. Alternative Transparent Electrodes to ITO
2.1. Carbon Nanotubes
2.2. Graphene
2.2.1. rGO Method
2.2.2. CVD Method
2.3. Metal Nanowires and Nanogrids
2.3.1. Metal Nanowires
2.3.2. Metal Nanogrids
2.4. Conductive Polymer
2.5. Other Transparent Electrodes
3. Stability of the Flexible Polymer Solar Cells
4. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Saga, T. Advances in crystalline silicon solar cell technology for industrial mass production. NPG Asia Mater. 2010, 2, 96–102. [Google Scholar] [CrossRef]
- Garnett, E.; Yang, P. Light trapping in silicon nanowire solar cells. Nano Lett. 2010, 10, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tan, Z.; Li, Y. Solution-processable metal oxides/chelates as electrode buffer layers for efficient and stable polymer solar cells. Energy Environ. Sci. 2015, 8, 1059–1091. [Google Scholar] [CrossRef]
- Lu, S.; Liu, K.; Chi, D.; Yue, S.; Li, Y.; Kou, Y.; Lin, X.; Wang, Z.; Qu, S.; Wang, Z. Constructing bulk heterojunction with componential gradient for enhancing the efficiency of polymer solar cells. J. Power Sources 2015, 300, 238–244. [Google Scholar] [CrossRef]
- Ye, L.; Xiong, Y.; Li, S.; Ghasemi, M.; Balar, N.; Turner, J.; Gadisa, A.; Hou, J.; O’Connor, B.T.; Ade, H. Precise manipulation of multilength scale morphology and its influence on eco-friendly printed all-polymer solar cells. Adv. Funct. Mater. 2017, 27, 1702016. [Google Scholar] [CrossRef]
- Ye, L.; Jiao, X.; Zhang, S.; Yao, H.; Qin, Y.; Ade, H.; Hou, J. Control of mesoscale morphology and photovoltaic performance in diketopyrrolopyrrole-based small band gap terpolymers. Adv. Energy Mater. 2017, 7, 1601138. [Google Scholar] [CrossRef]
- Liu, X.; Ye, L.; Zhao, W.; Zhang, S.; Li, S.; Su, G.M.; Wang, C.; Ade, H.; Hou, J. Morphology control enables thickness-insensitive efficient nonfullerene polymer solar cells. Mater. Chem. Front. 2017, 1, 2057–2064. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Cui, C.; Li, Y. Flexible and semitransparent organic solar cells. Adv. Energy Mater. 2017, 1701791. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, L.; Hou, J. Breaking the 10% efficiency barrier in organic photovoltaics: Morphology and device optimization of well-known pbdttt polymers. Adv. Energy Mater. 2016, 6, 1502529. [Google Scholar] [CrossRef]
- Ye, L.; Xiong, Y.; Yao, H.; Gadisa, A.; Zhang, H.; Li, S.; Ghasemi, M.; Balar, N.; Hunt, A.; O’Connor, B.T.; et al. High performance organic solar cells processed by blade coating in air from a benign food additive solution. Chem. Mater. 2016, 28, 7451–7458. [Google Scholar] [CrossRef]
- Bin, H.; Gao, L.; Zhang, Z.G.; Yang, Y.; Zhang, Y.; Zhang, C.; Chen, S.; Xue, L.; Yang, C.; Xiao, M.; et al. 11.4% efficiency non-fullerene polymer solar cells with trialkylsilyl substituted 2d-conjugated polymer as donor. Nat. Commun. 2016, 7, 13651. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Jiao, X.; Zhou, M.; Zhang, S.; Yao, H.; Zhao, W.; Xia, A.; Ade, H.; Hou, J. Manipulating aggregation and molecular orientation in all-polymer photovoltaic cells. Adv. Mater. 2015, 27, 6046–6054. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, S.; Ma, W.; Fan, B.; Guo, X.; Huang, Y.; Ade, H.; Hou, J. From binary to ternary solvent: Morphology fine-tuning of D/A blends in PDPP3T-based polymer solar cells. Adv. Mater. 2012, 24, 6335–6341. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xiao, B.; Liu, F.; Wu, H.; Yang, Y.; Xiao, S.; Wang, C.; Russell, T.P.; Cao, Y. Single-junction polymer solar cells with high efficiency and photovoltage. Nat. Photonics 2015, 9, 174–179. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, W.; Li, S.; Mukherjee, S.; Carpenter, J.H.; Awartani, O.; Jiao, X.; Hou, J.; Ade, H. High-efficiency nonfullerene organic solar cells: Critical factors that affect complex multi-length scale morphology and device performance. Adv. Energy Mater. 2017, 7, 1602000. [Google Scholar] [CrossRef]
- Xiao, Z.; Jia, X.; Ding, L. Ternary organic solar cells offer 14% power conversion efficiency. Sci. Bull. 2017, 62, 1562–1564. [Google Scholar] [CrossRef]
- Li, M.; Gao, K.; Wan, X.; Zhang, Q.; Kan, B.; Xia, R.; Liu, F.; Yang, X.; Feng, H.; Ni, W.; et al. Solution-processed organic tandem solar cells with power conversion efficiencies >12%. Nat. Photonics 2016, 11, 85–90. [Google Scholar] [CrossRef]
- Chen, C.C.; Chang, W.H.; Yoshimura, K.; Ohya, K.; You, J.; Gao, J.; Hong, Z.; Yang, Y. An efficient triple-junction polymer solar cell having a power conversion efficiency exceeding 11%. Adv. Mater. 2014, 26, 5670–5677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; He, Z.; Cheng, X.; Qin, D.; Yun, M.; Wang, M.; Huang, X.; Wu, J.; Wu, H.; Cao, Y. Flexible polymer solar cells with power conversion efficiency of 8.7%. J. Mater. Chem. C 2014, 2, 5077–5082. [Google Scholar] [CrossRef]
- Das, S.; Keum, J.K.; Browning, J.F.; Gu, G.; Yang, B.; Dyck, O.; Do, C.; Chen, W.; Chen, J.; Ivanov, I.N.; et al. Correlating high power conversion efficiency of PTB7:PC71BM inverted organic solar cells with nanoscale structures. Nanoscale 2015, 7, 15576–15583. [Google Scholar] [CrossRef] [PubMed]
- He, Z.C.; Zhong, C.M.; Su, S.J.; Xu, M.; Wu, H.B.; Cao, Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photonics 2012, 6, 591–595. [Google Scholar] [CrossRef]
- Angmo, D.; Krebs, F.C. Flexible ito-free polymer solar cells. J. Appl. Polym. Sci. 2013, 129, 1–14. [Google Scholar] [CrossRef]
- Basarir, F.; Irani, F.S.; Kosemen, A.; Camic, B.T.; Oytun, F.; Tunaboylu, B.; Shin, H.J.; Nam, K.Y.; Choi, H. Recent progresses on solution-processed silver nanowire based transparent conducting electrodes for organic solar cells. Mater. Today Chem. 2017, 3, 60–72. [Google Scholar] [CrossRef]
- Li, P.; Sun, C.; Jiu, T.; Wang, G.; Li, J.; Li, X.; Fang, J. High-performance inverted solar cells based on blend films of ZnO naoparticles and TiO2 nanorods as a cathode buffer layer. ACS Appl. Mater. Interfaces 2014, 6, 4074–4080. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.; Norrman, K.; Gevorgyan, S.A.; Tromholt, T.; Andreasen, B.; Krebs, F.C. Stability of polymer solar cells. Adv. Mater. 2012, 24, 580–612. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xu, Z.; Zhang, F.; Xie, S.; Xu, H.; Liu, X.Y. Recent development of transparent conducting oxide-free flexible thin-film solar cells. Adv. Funct. Mater. 2016, 26, 8855–8884. [Google Scholar] [CrossRef]
- Søndergaard, R.; Hösel, M.; Angmo, D.; Larsen-Olsen, T.T.; Krebs, F.C. Roll-to-roll fabrication of polymer solar cells. Mater. Today 2012, 15, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Pagliaro, M.; Ciriminna, R.; Palmisano, G. Flexible solar cells. ChemSusChem 2008, 1, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Pei, S.; Ma, L.; Cheng, H.M. 25th anniversary article: Carbon nanotube- and graphene-based transparent conductive films for optoelectronic devices. Adv. Mater. 2014, 26, 1958–1991. [Google Scholar] [CrossRef] [PubMed]
- Dabera, G.D.; Jayawardena, K.D.; Prabhath, M.R.; Yahya, I.; Tan, Y.Y.; Nismy, N.A.; Shiozawa, H.; Sauer, M.; Ruiz-Soria, G.; Ayala, P.; et al. Hybrid carbon nanotube networks as efficient hole extraction layers for organic photovoltaics. ACS Nano 2013, 7, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Batmunkh, M.; Biggs, M.J.; Shapter, J.G. Carbon nanotubes for dye-sensitized solar cells. Small 2015, 11, 2963–2989. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Uddin, M.J.; Dickens, T.J.; Okoli, O.I. Carbon nanotubes (CNTS) enrich the solar cells. Sol. Energy 2013, 96, 239–252. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Xu, W.; Kulkarni, S.A.; Batabyal, S.K.; Zhang, S.; Cao, A.; Wong, L.H. TiO2 nanotube arrays based flexible perovskite solar cells with transparent carbon nanotube electrode. Nano Energy 2015, 11, 728–735. [Google Scholar] [CrossRef]
- Jeon, I.; Chiba, T.; Delacou, C.; Guo, Y.; Kaskela, A.; Reynaud, O.; Kauppinen, E.I.; Maruyama, S.; Matsuo, Y. Single-walled carbon nanotube film as electrode in indium-free planar heterojunction perovskite solar cells: Investigation of electron-blocking layers and dopants. Nano Lett. 2015, 15, 6665–6671. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lim, J.; Lee, N.; Park, H.I.; Lee, K.E.; Jeon, T.; Nam, S.A.; Kim, J.; Shin, J.; Kim, S.O. Synergistic concurrent enhancement of charge generation, dissociation, and transport in organic solar cells with plasmonic metal-carbon nanotube hybrids. Adv. Mater. 2015, 27, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xu, T.; Chen, W.; Lee, J.M.; Luo, Z.; Jung, I.H.; Park, H.I.; Kim, S.O.; Yu, L. The role of n-doped multiwall carbon nanotubes in achieving highly efficient polymer bulk heterojunction solar cells. Nano Lett. 2013, 13, 2365–2369. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Park, J.S.; Lee, S.H.; Kim, H.; Yoo, S.; Kim, S.O. Selective electron- or hole-transport enhancement in bulk-heterojunction organic solar cells with n- or b-doped carbon nanotubes. Adv. Mater. 2011, 23, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Rowell, M.W.; Topinka, M.A.; McGehee, M.D.; Prall, H.-J.; Dennler, G.; Sariciftci, N.S.; Hu, L.; Gruner, G. Organic solar cells with carbon nanotube network electrodes. Appl. Phys. Lett. 2006, 88, 233506. [Google Scholar] [CrossRef]
- Cho, D.-Y.; Eun, K.; Choa, S.-H.; Kim, H.-K. Highly flexible and stretchable carbon nanotube network electrodes prepared by simple brush painting for cost-effective flexible organic solar cells. Carbon 2014, 66, 530–538. [Google Scholar] [CrossRef]
- Ma, Y.; Cheung, W.; Wei, D.; Bogozi, A.; Chiu, P.L.; Wang, L.; Pontoriero, F.; Mendelsohn, R.; He, H. Improved conductivity of carbon nanotube networks by in situ polymerization of a thin skin of conducting polymer. ACS Nano 2008, 2, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Salvatierra, R.V.; Cava, C.E.; Roman, L.S.; Zarbin, A.J.G. ITO-free and flexible organic photovoltaic device based on high transparent and conductive polyaniline/carbon nanotube thin films. Adv. Funct. Mater. 2013, 23, 1490–1499. [Google Scholar] [CrossRef]
- Jeon, I.; Cui, K.; Chiba, T.; Anisimov, A.; Nasibulin, A.G.; Kauppinen, E.I.; Maruyama, S.; Matsuo, Y. Direct and dry deposited single-walled carbon nanotube films doped with MoOx as electron-blocking transparent electrodes for flexible organic solar cells. J. Am. Chem. Soc. 2015, 137, 7982–7985. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.X.; Han, C.; Li, M.; Shen, X.Q. High performance of carbon nanotubes/silver nanowires-pet hybrid flexible transparent conductive films via facile pressing-transfer technique. Nanoscale Res. Lett. 2014, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, X.; Chen, P.; Guan, G.; Qiu, L.; Lin, H.; Yang, Z.; Bai, W.; Luo, Y.; Peng, H. Integrated polymer solar cell and electrochemical supercapacitor in a flexible and stable fiber format. Adv. Mater. 2014, 26, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, G. Flexible graphene devices related to energy conversion and storage. Energy Environ. Sci. 2015, 8, 790–823. [Google Scholar] [CrossRef]
- Vellacheri, R.; Al-Haddad, A.; Zhao, H.; Wang, W.; Wang, C.; Lei, Y. High performance supercapacitor for efficient energy storage under extreme environmental temperatures. Nano Energy 2014, 8, 231–237. [Google Scholar] [CrossRef]
- He, M.; Jung, J.; Qiu, F.; Lin, Z. Graphene-based transparent flexible electrodes for polymer solar cells. J. Mater. Chem. 2012, 22, 24254. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Fan, Z.; Liu, J.; Zhang, H. Graphene-based electrodes. Adv. Mater. 2012, 24, 5979–6004. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xiao, C.; Cheng, H.; Grote, F.; Zhang, X.; Yao, T.; Li, Z.; Wang, C.; Wei, S.; Lei, Y.; et al. Magnetocaloric effects in a freestanding and flexible graphene-based superlattice synthesized with a spatially confined reaction. Nat. Commun. 2014, 5, 3960. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Kymakis, E.; Savva, K.; Stylianakis, M.M.; Fotakis, C.; Stratakis, E. Flexible organic photovoltaic cells with in situ nonthermal photoreduction of spin-coated graphene oxide electrodes. Adv. Funct. Mater. 2013, 23, 2742–2749. [Google Scholar] [CrossRef]
- Konios, D.; Petridis, C.; Kakavelakis, G.; Sygletou, M.; Savva, K.; Stratakis, E.; Kymakis, E. Reduced graphene oxide micromesh electrodes for large area, flexible, organic photovoltaic devices. Adv. Funct. Mater. 2015, 25, 2213–2221. [Google Scholar] [CrossRef]
- Park, H.; Chang, S.; Zhou, X.; Kong, J.; Palacios, T.; Gradecak, S. Flexible graphene electrode-based organic photovoltaics with record-high efficiency. Nano Lett. 2014, 14, 5148–5154. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Yan, F. Package-free flexible organic solar cells with graphene top electrodes. Adv. Mater. 2013, 25, 4296–4301. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Chang, S.; Smith, M.; Gradecak, S.; Kong, J. Interface engineering of graphene for universal applications as both anode and cathode in organic photovoltaics. Sci. Rep. 2013, 3, 1581. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chang, S.; Gradecak, S.; Kong, J. Visibly-transparent organic solar cells on flexible substrates with all-graphene electrodes. Adv. Energy Mater. 2016, 6, 1600847. [Google Scholar] [CrossRef]
- An, C.J.; Jang, S.; Kang, K.M.; Kim, S.J.; Jin, M.L.; Jung, H.-T. A combined graphene and periodic au nanograte structure: Fundamentals and application as a flexible transparent conducting film in a flexible organic photovoltaic cell. Carbon 2016, 103, 488–496. [Google Scholar] [CrossRef]
- Lima, L.F.; Matos, C.F.; Gonçalves, L.C.; Salvatierra, R.V.; Cava, C.E.; Zarbin, A.J.G.; Roman, L.S. Water based, solution-processable, transparent and flexible graphene oxide composite as electrodes in organic solar cell application. J. Phys. D Appl. Phys. 2016, 49, 105106. [Google Scholar] [CrossRef]
- Ricciardulli, A.G.; Yang, S.; Feng, X.; Blom, P.W.M. Solution-processable high-quality graphene for organic solar cells. ACS Appl. Mater. Interfaces 2017, 9, 25412–25417. [Google Scholar] [CrossRef] [PubMed]
- An, C.J.; Kim, S.J.; Choi, H.O.; Kim, D.W.; Jang, S.W.; Jin, M.L.; Park, J.-M.; Choi, J.K.; Jung, H.-T. Ultraclean transfer of CVD-grown graphene and its application to flexible organic photovoltaic cells. J. Mater. Chem. A 2014, 2, 20474–20480. [Google Scholar] [CrossRef]
- Du, J.H.; Jin, H.; Zhang, Z.K.; Zhang, D.D.; Jia, S.; Ma, L.P.; Ren, W.C.; Cheng, H.M.; Burn, P.L. Efficient organic photovoltaic cells on a single layer graphene transparent conductive electrode using moox as an interfacial layer. Nanoscale 2017, 9, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, H.; Chen, T.; Qiu, L.; Luo, Y.; Peng, H. Photovoltaic wire derived from a graphene composite fiber achieving an 8.45% energy conversion efficiency. Angew. Chem. 2013, 52, 7545–7548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wan, X.; Xing, F.; Huang, L.; Long, G.; Yi, N.; Ni, W.; Liu, Z.; Tian, J.; Chen, Y. Solution-processable graphene mesh transparent electrodes for organic solar cells. Nano Res. 2013, 6, 478–484. [Google Scholar] [CrossRef]
- Lee, C.-P.; Lai, K.-Y.; Lin, C.-A.; Li, C.-T.; Ho, K.-C.; Wu, C.-I.; Lau, S.-P.; He, J.-H. A paper-based electrode using a graphene dot/PEDOT:PSS composite for flexible solar cells. Nano Energy 2017, 36, 260–267. [Google Scholar] [CrossRef]
- Jung, M.H.; Chu, M.J. Comparative experiments of graphene covalently and physically binding cdse quantum dots to enhance the electron transport in flexible photovoltaic devices. Nanoscale 2014, 6, 9241–9249. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, L.; Zhang, Q.; Hu, W.; Pei, Q. Silver nanowire-polymer composite electrodes for efficient polymer solar cells. Adv. Mater. 2011, 23, 4453–4457. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, X.; Luo, J.; Gan, Z.; Meng, Z.; Zhang, N. Silver nanowire/polyimide composite transparent electrodes for reliable flexible polymer solar cells operating at high and ultra-low temperature. RSC Adv. 2015, 5, 24953–24959. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Mayers, B.; Xia, Y. Shape-controlled synthesis of metal nanostructures: The case of silver. Chemistry 2005, 11, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Connor, S.T.; Cui, Y.; Peumans, P. Solution-processed metal nanowire mesh transparent electrodes. Nano Lett. 2008, 8, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Korte, K.E.; Skrabalak, S.E.; Xia, Y. Rapid synthesis of silver nanowires through a cucl- or cucl2-mediated polyol process. J. Mater. Chem. 2008, 18, 437–441. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, T.; Zhou, H.; Price, S.C.; Wiley, B.J.; You, W. Solution-processed flexible polymer solar cells with silver nanowire electrodes. ACS Appl. Mater. Interfaces 2011, 3, 4075–4084. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, W.; Liu, H.; Ma, Y.; Zhang, H. Performance improvement in flexible polymer solar cells based on modified silver nanowire electrode. Nanotechnology 2016, 27, 335203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-F.; Zou, W.-J.; Li, H.; Lu, K.; Yan, W.; Wei, Z.-X. Large-area, flexible polymer solar cell based on silver nanowires as transparent electrode by roll-to-roll printing. Chin. J. Polym. Sci. 2016, 35, 261–268. [Google Scholar] [CrossRef]
- Czolk, J.; Landerer, D.; Koppitz, M.; Nass, D.; Colsmann, A. Highly efficient, mechanically flexible, semi-transparent organic solar cells doctor bladed from non-halogenated solvents. Adv. Mater. Technol. 2016, 1, 1600184. [Google Scholar] [CrossRef]
- Wu, J.; Que, X.; Hu, Q.; Luo, D.; Liu, T.; Liu, F.; Russell, T.P.; Zhu, R.; Gong, Q. Multi-length scaled silver nanowire grid for application in efficient organic solar cells. Adv. Funct. Mater. 2016, 26, 4822–4828. [Google Scholar] [CrossRef]
- Raïssi, M.; Vedraine, S.; Garuz, R.; Trigaud, T.; Ratier, B. Solution processed cathode and interconnecting layer of silver nanowires in an efficient inverted tandem organic solar cells. Sol. Energy Mater. Sol. Cells 2017, 160, 494–502. [Google Scholar] [CrossRef]
- Maisch, P.; Tam, K.C.; Lucera, L.; Egelhaaf, H.-J.; Scheiber, H.; Maier, E.; Brabec, C.J. Inkjet printed silver nanowire percolation networks as electrodes for highly efficient semitransparent organic solar cells. Org. Electron. 2016, 38, 139–143. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Wiley, B.J. The synthesis and coating of long, thin copper nanowires to make flexible, transparent conducting films on plastic substrates. Adv. Mater. 2011, 23, 4798–4803. [Google Scholar] [CrossRef] [PubMed]
- Sachse, C.; Weiß, N.; Gaponik, N.; Müller-Meskamp, L.; Eychmüller, A.; Leo, K. Ito-free, small-molecule organic solar cells on spray-coated copper-nanowire-based transparent electrodes. Adv. Energy Mater. 2014, 4, 1300737. [Google Scholar] [CrossRef]
- Im, H.G.; Jung, S.H.; Jin, J.; Lee, D.; Lee, J.; Lee, D.; Lee, J.Y.; Kim, I.D.; Bae, B.S. Flexible transparent conducting hybrid film using a surface-embedded copper nanowire network: A highly oxidation-resistant copper nanowire electrode for flexible optoelectronics. ACS Nano 2014, 8, 10973–10979. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lin, N.; Chen, Y.; Wang, Z.; Xie, Q.; Zheng, T.; Gao, N.; Li, S.; Kang, J.; Cai, D.; et al. Copper nanowires as fully transparent conductive electrodes. Sci. Rep. 2013, 3, 2323. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhai, H.; Wang, T.; Wang, X.; Cheng, Y.; Shi, L.; Sun, J. Plasma-induced nanowelding of a copper nanowire network and its application in transparent electrodes and stretchable conductors. Nano Res. 2016, 9, 2138–2148. [Google Scholar] [CrossRef]
- Kholmanov, I.N.; Domingues, S.H.; Chou, H.; Wang, X.; Tan, C.; Kim, J.Y.; Li, H.; Piner, R.; Zarbin, A.J.; Ruoff, R.S. Reduced graphene oxide/copper nanowire hybrid films as high-performance transparent electrodes. ACS Nano 2013, 7, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, W.; Chen, J.; Fan, Y.; Zhang, Z.; Huang, Z.; Feng, X.; Mi, B.; Ma, Y.; Huang, W. Solution-processed copper nanowire flexible transparent electrodes with pedot:Pss as binder, protector and oxide-layer scavenger for polymer solar cells. Nano Res. 2015, 8, 1017–1025. [Google Scholar] [CrossRef]
- Hösel, M.; Søndergaard, R.R.; Jørgensen, M.; Krebs, F.C. Fast inline roll-to-roll printing for indium-tin-oxide-free polymer solar cells using automatic registration. Energy Technol. 2013, 1, 102–107. [Google Scholar] [CrossRef]
- Carlé, J.E.; Andersen, T.R.; Helgesen, M.; Bundgaard, E.; Jørgensen, M.; Krebs, F.C. A laboratory scale approach to polymer solar cells using one coating/printing machine, flexible substrates, no ito, no vacuum and no spincoating. Sol. Energy Mater. Sol. Cells 2013, 108, 126–128. [Google Scholar] [CrossRef]
- Carlé, J.E.; Helgesen, M.; Madsen, M.V.; Bundgaard, E.; Krebs, F.C. Upscaling from single cells to modules—fabrication of vacuum- and ito-free polymer solar cells on flexible substrates with long lifetime. J. Mater. Chem. C 2014, 2, 1290–1297. [Google Scholar] [CrossRef]
- Dam, H.F.; Andersen, T.R.; Pedersen, E.B.L.; Thydén, K.T.S.; Helgesen, M.; Carlé, J.E.; Jørgensen, P.S.; Reinhardt, J.; Søndergaard, R.R.; Jørgensen, M.; et al. Enabling flexible polymer tandem solar cells by 3d ptychographic imaging. Adv. Energy Mater. 2015, 5, 1400736. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.-S.; Jung, G.H.; Jo, J.; Kim, J.S.; Kim, J.W.; Kwak, S.-W.; Lee, J.-L.; Kim, I.; Kim, D. Transparent conductive film with printable embedded patterns for organic solar cells. Sol. Energy Mater. Sol. Cells 2013, 109, 142–147. [Google Scholar] [CrossRef]
- Seo, K.-W.; Noh, Y.-J.; Na, S.-I.; Kim, H.-K. Random mesh-like ag networks prepared via self-assembled ag nanoparticles for ito-free flexible organic solar cells. Sol. Energy Mater. Sol. Cells 2016, 155, 51–59. [Google Scholar] [CrossRef]
- Burgués-Ceballos, I.; Kehagias, N.; Sotomayor-Torres, C.M.; Campoy-Quiles, M.; Lacharmoise, P.D. Embedded inkjet printed silver grids for ito-free organic solar cells with high fill factor. Sol. Energy Mater. Sol. Cells 2014, 127, 50–57. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.; Kang, I.; Jung, M.S.; Kim, S.J.; Kim, J.K.; Cho, S.M.; Kim, J.H.; Park, J.H. Hybrid silver mesh electrode for ito-free flexible polymer solar cells with good mechanical stability. ChemSusChem 2016, 9, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Lin, J.; Liu, K.; Yue, S.; Ren, K.; Tan, F.; Wang, Z.; Jin, P.; Qu, S.; Wang, Z. Large area flexible polymer solar cells with high efficiency enabled by imprinted ag grid and modified buffer layer. Acta Mater. 2017, 130, 208–214. [Google Scholar] [CrossRef]
- Wang, J.; Fei, F.; Luo, Q.; Nie, S.; Wu, N.; Chen, X.; Su, W.; Li, Y.; Ma, C.Q. Modification of the highly conductive PEDOT:PSS layer for use in silver nanogrid electrodes for flexible inverted polymer solar cells. ACS Appl. Mater. Interfaces 2017, 9, 7834–7842. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Chen, Q.; Li, Y.; Li, Y.; Cai, J.; Su, W.; Bai, S.; Jin, Y.; Ma, C.-Q.; Cui, Z.; et al. Flexible silver grid/ PEDOT:PSS hybrid electrodes for large area inverted polymer solar cells. Nano Energy 2014, 10, 259–267. [Google Scholar] [CrossRef]
- Li, Y.; Mao, L.; Tang, F.; Chen, Q.; Wang, Y.; Ye, F.; Chen, L.; Li, Y.; Wu, D.; Cui, Z.; et al. Ambient stable large-area flexible organic solar cells using silver grid hybrid with vapor phase polymerized poly(3,4-ethylenedioxythiophene) cathode. Sol. Energy Mater. Sol. Cells 2015, 143, 354–359. [Google Scholar] [CrossRef]
- Song, M.; Kim, H.-J.; Kim, C.S.; Jeong, J.-H.; Cho, C.; Lee, J.-Y.; Jin, S.-H.; Choi, D.-G.; Kim, D.-H. ITO-free highly bendable and efficient organic solar cells with ag nanomesh/zno hybrid electrodes. J. Mater. Chem. A 2015, 3, 65–70. [Google Scholar] [CrossRef]
- Muhsin, B.; Roesch, R.; Gobsch, G.; Hoppe, H. Flexible ito-free polymer solar cells based on highly conductive PEDOT:PSS and a printed silver grid. Sol. Energy Mater. Sol. Cells 2014, 130, 551–554. [Google Scholar] [CrossRef]
- Li, Y.; Mao, L.; Gao, Y.; Zhang, P.; Li, C.; Ma, C.; Tu, Y.; Cui, Z.; Chen, L. Ito-free photovoltaic cell utilizing a high-resolution silver grid current collecting layer. Sol. Energy Mater. Sol. Cells 2013, 113, 85–89. [Google Scholar] [CrossRef]
- Guo, C.F.; Sun, T.; Liu, Q.; Suo, Z.; Ren, Z. Highly stretchable and transparent nanomesh electrodes made by grain boundary lithography. Nat. Commun. 2014, 5, 3121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Zhu, X.; Hoekstra, R.; Li, L.; Xiu, F.; Xue, M.; Zeng, B.; Wang, K.L. Metallic nanomesh electrodes with controllable optical properties for organic solar cells. Appl. Phys. Lett. 2012, 100, 143109. [Google Scholar] [CrossRef]
- Kang, M.-G.; Park, H.J.; Ahn, S.H.; Guo, L.J. Transparent Cu nanowire mesh electrode on flexible substrates fabricated by transfer printing and its application in organic solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1179–1184. [Google Scholar] [CrossRef]
- Jang, H.Y.; Lee, S.-K.; Cho, S.H.; Ahn, J.-H.; Park, S. Fabrication of metallic nanomesh: Pt nano-mesh as a proof of concept for stretchable and transparent electrodes. Chem. Mater. 2013, 25, 3535–3538. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Qiu, M.; Yu, H.; Zhang, X.; Sun, X.W.; Chen, R. Preparation of aluminum nanomesh thin films from an anodic aluminum oxide template as transparent conductive electrodes. Sci. Rep. 2016, 6, 20114. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.H.; Lee, J.; Lee, E.S.; Choi, J.H.; Jung, J.H.; Jung, J.Y.; Choi, D.G. High-durable agni nanomesh film for a transparent conducting electrode. Small 2014, 10, 3767–3774. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Isikgor, F.H.; Ouyang, J. Review on application of pedots and PEDOT:PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Stöcker, T.; Köhler, A.; Moos, R. Why does the electrical conductivity in PEDOT:PSS decrease with pss content? A study combining thermoelectric measurements with impedance spectroscopy. J. Polym. Sci. Part B 2012, 50, 976–983. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective approaches to improve the electrical conductivity of PEDOT:PSS: A review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, F.; Tvingstedt, K.; Barrau, S.; Li, F.; Tian, W.; Inganäs, O. Investigation on polymer anode design for flexible polymer solar cells. App. Phys. Lett. 2008, 92, 233308. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, F.; Tvingstedt, K.; Tian, W.; Inganäs, O. Multifolded polymer solar cells on flexible substrates. App. Phys. Lett. 2008, 93, 033302. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; White, M.S.; Glowacki, E.D.; Sekitani, T.; Someya, T.; Sariciftci, N.S.; Bauer, S. Ultrathin and lightweight organic solar cells with high flexibility. Nat. Commun. 2012, 3, 770. [Google Scholar] [CrossRef] [PubMed]
- Savagatrup, S.; Chan, E.; Renteria-Garcia, S.M.; Printz, A.D.; Zaretski, A.V.; O’Connor, T.F.; Rodriquez, D.; Valle, E.; Lipomi, D.J. Plasticization of PEDOT:PSS by common additives for mechanically robust organic solar cells and wearable sensors. Adv. Funct. Mater. 2015, 25, 427–436. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Wang, J.; Zhang, J.; Yang, Q.; Fu, Y.; Xie, Z. Highly conductive PEDOT:PSS transparent electrode prepared by a post-spin-rinsing method for efficient ITO-free polymer solar cells. Sol. Energy Mater. Sol. Cells 2016, 144, 143–149. [Google Scholar] [CrossRef]
- Kim, N.; Kee, S.; Lee, S.H.; Lee, B.H.; Kahng, Y.H.; Jo, Y.R.; Kim, B.J.; Lee, K. Highly conductive PEDOT:PSS nanofibrils induced by solution-processed crystallization. Adv. Mater. 2014, 26, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ouyang, J. Significant conductivity enhancement of conductive poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) films through a treatment with organic carboxylic acids and inorganic acids. ACS Appl. Mater. Interfaces 2010, 2, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J. Solution-processed PEDOT:PSS films with conductivities as indium tin oxide through a treatment with mild and weak organic acids. ACS Appl. Mater. Interfaces 2013, 5, 13082–13088. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, J.; Wang, H.; Liu, X.; Wang, H. Bendable ito-free organic solar cells with highly conductive and flexible PEDOT:PSS electrodes on plastic substrates. ACS Appl. Mater. Interfaces 2015, 7, 16287–16295. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xu, B.; Liu, S.; Cui, C.; Wang, J.; Yan, F. Transfer-printed PEDOT:PSS electrodes using mild acids for high conductivity and improved stability with application to flexible organic solar cells. ACS Appl. Mater. Interfaces 2016, 8, 14029–14036. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xia, Y.; Du, D.; Ouyang, J. PEDOT:PSS films with metallic conductivity through a treatment with common organic solutions of organic salts and their application as a transparent electrode of polymer solar cells. ACS Appl. Mater. Interfaces 2016, 8, 11629–11638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, B.; Qin, F.; Wu, Y.; Meng, W.; Dai, S.; Zhou, Y.; Zhan, X. Semitransparent, non-fullerene and flexible all-plastic solar cells. Polymer 2016, 107, 108–112. [Google Scholar] [CrossRef]
- Yun, J. Ultrathin metal films for transparent electrodes of flexible optoelectronic devices. Adv. Funct. Mater. 2017, 27, 1606641. [Google Scholar] [CrossRef]
- Zuo, L.; Zhang, S.; Li, H.; Chen, H. Toward highly efficient large-area ito-free organic solar cells with a conductance-gradient transparent electrode. Adv. Mater. 2015, 27, 6983–6989. [Google Scholar] [CrossRef] [PubMed]
- Stec, H.M.; Williams, R.J.; Jones, T.S.; Hatton, R.A. Ultrathin transparent au electrodes for organic photovoltaics fabricated using a mixed mono-molecular nucleation layer. Adv. Funct. Mater. 2011, 21, 1709–1716. [Google Scholar] [CrossRef]
- Kang, H.; Jung, S.; Jeong, S.; Kim, G.; Lee, K. Polymer-metal hybrid transparent electrodes for flexible electronics. Nat. Commun. 2015, 6, 6503. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Liu, X.; Zhang, N.; Chen, H.; Zheng, X.; Wang, H.; Guo, X. High-performance NiO/Ag/ NiO transparent electrodes for flexible organic photovoltaic cells. ACS Appl. Mater. Interfaces 2014, 6, 16403–16408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, B.; Wang, H.; Xu, J.; Ta, T.; Li, W.; Wang, Q.; Yang, S.; Tang, Y.; Zou, B. Transparent WO3/Ag/WO3 electrode for flexible organic solar cells. Mater. Lett. 2017, 188, 107–110. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, L.; Yu, W.; Long, Y.; Guo, W.; Chen, W.; Ruan, S. Highly efficient ito-free polymer solar cells based on metal resonant microcavity using WO3/Au/WO3 as transparent electrodes. Org. Electron. 2014, 15, 1545–1551. [Google Scholar] [CrossRef]
- Ping, S.; Liang, S.; Yongbing, L.; Geheng, C. Indium tin oxide-free polymer solar cells: Microcavity enhancing the performance using WO3/Au/WO3 as transparent electrode. IEEE Electron. Device Lett. 2014, 35, 1109–1111. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Lin, F.; Li, H.; Fan, Y.; Zhang, N. Highly conductive transparent organic electrodes with multilayer structures for rigid and flexible optoelectronics. Sci. Rep. 2015, 5, 10569. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Li, C.Z.; Chang, C.Y.; Yip, H.L.; Jen, A.K. Interfacial engineering of ultrathin metal film transparent electrode for flexible organic photovoltaic cells. Adv. Mater. 2014, 26, 3618–3623. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Song, M.; Bae, T.-S.; Park, Y.H.; Kang, Y.-C.; Lee, S.-G.; Kim, S.-Y.; Kim, D.H.; Lee, S.; Min, G.; et al. Transparent ultrathin oxygen-doped silver electrodes for flexible organic solar cells. Adv. Funct. Mater. 2014, 24, 1551–1561. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, J.W.; Hong, S.H.; Song, S.H.; Park, S.J. MgxZn1−xO/Ag/MgxZn1−xO multilayers as high-performance transparent conductive electrodes. ACS Appl. Mater. Interfaces 2016, 8, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Kim, S.M.; Lee, S.-G.; Bae, T.-S.; Mun, C.; Lee, S.; Yu, H.; Lee, G.-H.; Lee, H.-S.; Song, M.; et al. Bendable solar cells from stable, flexible, and transparent conducting electrodes fabricated using a nitrogen-doped ultrathin copper film. Adv. Funct. Mater. 2016, 26, 4180–4191. [Google Scholar] [CrossRef]
- Chen, S.; Dai, Y.; Zhao, D.; Zhang, H. Ito-free flexible organic photovoltaics with multilayer MoO3/LiF/ MoO3/Ag/ MoO3 as the transparent electrode. Semicond. Sci. Technol. 2016, 31, 055013. [Google Scholar] [CrossRef]
- Wen, X.-M.; Ma, R.; Yin, D.; Bi, Y.-G. Efficient inverted flexible polymer solar cells with transparent top MoO3/Au/Ag/NPB electrodes. Opt. Mater. Express 2017, 7, 2188. [Google Scholar] [CrossRef]
- Hambsch, M.; Jin, H.; Clulow, A.J.; Nelson, A.; Yamada, N.L.; Velusamy, M.; Yang, Q.; Zhu, F.; Burn, P.L.; Gentle, I.R.; et al. Improved stability of non-ito stacked electrodes for large area flexible organic solar cells. Sol. Energy Mater. Sol. Cells 2014, 130, 182–190. [Google Scholar] [CrossRef]
- Bag, M.; Banerjee, S.; Faust, R.; Venkataraman, D. Self-healing polymer sealant for encapsulating flexible solar cells. Sol. Energy Mater. Sol. Cells 2016, 145, 418–422. [Google Scholar] [CrossRef]
- Son, H.J.; Kim, S.H.; Kim, D.H. Critical impact of hole transporting layers and back electrode on the stability of flexible organic photovoltaic module. Adv. Energy Mater. 2017, 7, 1601289. [Google Scholar] [CrossRef]
- Beliatis, M.J.; Helgesen, M.; García-Valverde, R.; Corazza, M.; Roth, B.; Carlé, J.E.; Jørgensen, M.; Krebs, F.C.; Gevorgyan, S.A. Slot-die-coated V2O5 as hole transport layer for flexible organic solar cells and optoelectronic devices. Adv. Eng. Mater. 2016, 18, 1494–1503. [Google Scholar] [CrossRef]
- O’Connor, T.F.; Zaretski, A.V.; Savagatrup, S.; Printz, A.D.; Wilkes, C.D.; Diaz, M.I.; Sawyer, E.J.; Lipomi, D.J. Wearable organic solar cells with high cyclic bending stability: Materials selection criteria. Sol. Energy Mater. Sol. Cells 2016, 144, 438–444. [Google Scholar] [CrossRef]
- Sapkota, S.B.; Spies, A.; Zimmermann, B.; Dürr, I.; Würfel, U. Promising long-term stability of encapsulated ITO-free bulk-heterojunction organic solar cells under different aging conditions. Sol. Energy Mater. Sol. Cells 2014, 130, 144–150. [Google Scholar] [CrossRef]
- Roesch, R.; Eberhardt, K.-R.; Engmann, S.; Gobsch, G.; Hoppe, H. Polymer solar cells with enhanced lifetime by improved electrode stability and sealing. Sol. Energy Mater. Sol. Cells 2013, 117, 59–66. [Google Scholar] [CrossRef]
- Gevorgyan, S.A.; Madsen, M.V.; Dam, H.F.; Jørgensen, M.; Fell, C.J.; Anderson, K.F.; Duck, B.C.; Mescheloff, A.; Katz, E.A.; Elschner, A.; et al. Interlaboratory outdoor stability studies of flexible roll-to-roll coated organic photovoltaic modules: Stability over 10,000 h. Sol. Energy Mater. Sol. Cells 2013, 116, 187–196. [Google Scholar] [CrossRef]
- Tanenbaum, D.M.; Dam, H.F.; Rösch, R.; Jørgensen, M.; Hoppe, H.; Krebs, F.C. Edge sealing for low cost stability enhancement of roll-to-roll processed flexible polymer solar cell modules. Sol. Energy Mater. Sol. Cells 2012, 97, 157–163. [Google Scholar] [CrossRef]
- Mao, L.; Tong, J.; Xiong, S.; Jiang, F.; Qin, F.; Meng, W.; Luo, B.; Liu, Y.; Li, Z.; Jiang, Y.; et al. Flexible large-area organic tandem solar cells with high defect tolerance and device yield. J. Mater. Chem. A 2017, 5, 3186–3192. [Google Scholar] [CrossRef]
- Gu, X.; Zhou, Y.; Gu, K.; Kurosawa, T.; Guo, Y.; Li, Y.; Lin, H.; Schroeder, B.C.; Yan, H.; Molina-Lopez, F.; et al. Roll-to-roll printed large-area all-polymer solar cells with 5% efficiency based on a low crystallinity conjugated polymer blend. Adv. Energy Mater. 2017, 1602742. [Google Scholar] [CrossRef]
- Zhen, H.; Li, K.; Chen, C.; Yu, Y.; Zheng, Z.; Ling, Q. Water-borne foldable polymer solar cells: One-step transferring free-standing polymer films onto woven fabric electrodes. J. Mater. Chem. A 2017, 5, 782–788. [Google Scholar] [CrossRef]
- Jinno, H.; Fukuda, K.; Xu, X.; Park, S.; Suzuki, Y.; Koizumi, M.; Yokota, T.; Osaka, I.; Takimiya, K.; Someya, T. Stretchable and waterproof elastomer-coated organic photovoltaics for washable electronic textile applications. Nat. Energy 2017, 2, 780–785. [Google Scholar] [CrossRef]














| Electrode | Rs of electrode (Ω/sq) | Device structure | Device area (cm2) | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Ag NWs-polymer | 10 | Ag NWs-polymer/PEDOT:PSS/P3HT:PCBM/LiF/Al | 0.14 | 9.71 | 0.52 | 65 | 3.28 | [69] |
| PI/Ag NWs | 20 | PI/Ag NWs/PEDOT:PSS/PBDTTT-C:PC70BM/LiF/Al | 0.12 | 13.01 | 0.69 | 51 | 4.58 | [70] |
| Ag NWs | 30.8 | PET/Ag NWs/PEDOT:PSS/PBnDT-DTffBT:PCBM/Ca/Al | 0.12 | 8.58 | 0.75 | 38.72 | 2.5 | [74] |
| Ag NWs | 36.5 | PET/Ag NWs/PEDOT:PSS/P3HT:PCBM/LiF/Al | 0.11 | 8.4 | 0.58 | 56.91 | 2.77 | [75] |
| 36.5 | PET/Ag NWs/MoO3/P3HT:PCBM/LiF/Al | 0.11 | 8.4 | 0.58 | 56.07 | 2.73 | [75] | |
| Ag NWs | 73.9–6.4 | PET-Ag NWs/ZnO/PPDT2FBT:PC71BM/MoOx/Ag | 7 | 10.45 | 0.71 | 40.6 | 3.04 | [76] |
| Ag mesh and Ag NWs | 16 | PET/Ag mesh/PEDOT:PSS/ZnO/PffBT4T-2OD:PC61BM:PC71BM/PEDOT:PSS-Ag NWs | 106–126 | 13.7 | 0.764 | 56 | 5.8 | [77] |
| Ag NWs grids | 28 | Glass/Ag NWs grids/ZnO/PTB7-Th:PC71BM/MoOx/Ag | 0.0863 | 17.8 | 0.78 | 65 | 9.02 | [78] |
| Ag NWs | 10 | Glass/Ag NWs/ZnO/P3HT:ICBA/PEDOT:PSS/Ag NWs/ZnO/PTB7:PC71BM/MoO3/Ag | 0.18 | 11.23 | 1.47 | 58 | 9.24 | [79] |
| Ag NWs | 18 | Glass/Ag NWs/ZnO/PV2000:PC70BM/PEDOT:PSS/Ag NWs | 1 | 10.7 | 0.76 | 52.8 | 4.3 | [80] |
| Cu NWs | 19 | PEA/Cu NWs/TiO2/P3HT:PCBM/MoO3/Ag | NA | 9.51 | 0.54 | 52 | 2.67 | [85] |
| Cu NWs | 15 | PET/Cu NWs-PEDOT:PSS/P3HT:PCBM/Al | NA | 6.05 | 0.58 | 40 | 1.4 | [87] |
| Ag-grid | NA | PET/Ag-grid/HCPEDOT:PSS/ZnO/PDTSTTz-4:PCBM/PEDOT:PSS/Ag | 8 | 2.1 | 2.72 | 55.5 | 3.2 | [90] |
| Ag network | 3.95 | PET/Ag network/PEDOT:PSS/PTB7:PC70BM/Ca/Al | NA | 14.11 | 0.718 | 68.60 | 6.95 | [93] |
| Ag-mesh | 13.26 | PET/Ag mesh/H-PEDOT:PSS/PVP AI4083/PTB7:PC71BM/TiOx/Al | 0.1143 | 14.21 | 0.73 | 67.11 | 6.94 | [95] |
| Ag-grid | NA | PET/Ag-grid/ZnO/PFN/PTB7-Th:PC71BM/MoO3/Ag | 2.25 | 14.30 | 0.773 | 58.14 | 6.43 | [96] |
| Ag-grid | NA | Ag-grid/PEDOT:PSS/ZnO/PTB7-Th: PC71BM/MoO3/Al | NA | 14.29 | 0.78 | 59 | 6.58 | [97] |
| Ag-mesh | 15 | PES/Ag nanomesh/ZnO/PTB7:PC71BM/PEDOT:PSS/Ag | 0.38 | 16.03 | 0.73 | 60.89 | 7.15 | [100] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Sun, Y.; Ren, K.; Liu, K.; Wang, Z.; Qu, S. Recent Development in ITO-free Flexible Polymer Solar Cells. Polymers 2018, 10, 5. https://doi.org/10.3390/polym10010005
Lu S, Sun Y, Ren K, Liu K, Wang Z, Qu S. Recent Development in ITO-free Flexible Polymer Solar Cells. Polymers. 2018; 10(1):5. https://doi.org/10.3390/polym10010005
Chicago/Turabian StyleLu, Shudi, Yang Sun, Kuankuan Ren, Kong Liu, Zhijie Wang, and Shengchun Qu. 2018. "Recent Development in ITO-free Flexible Polymer Solar Cells" Polymers 10, no. 1: 5. https://doi.org/10.3390/polym10010005