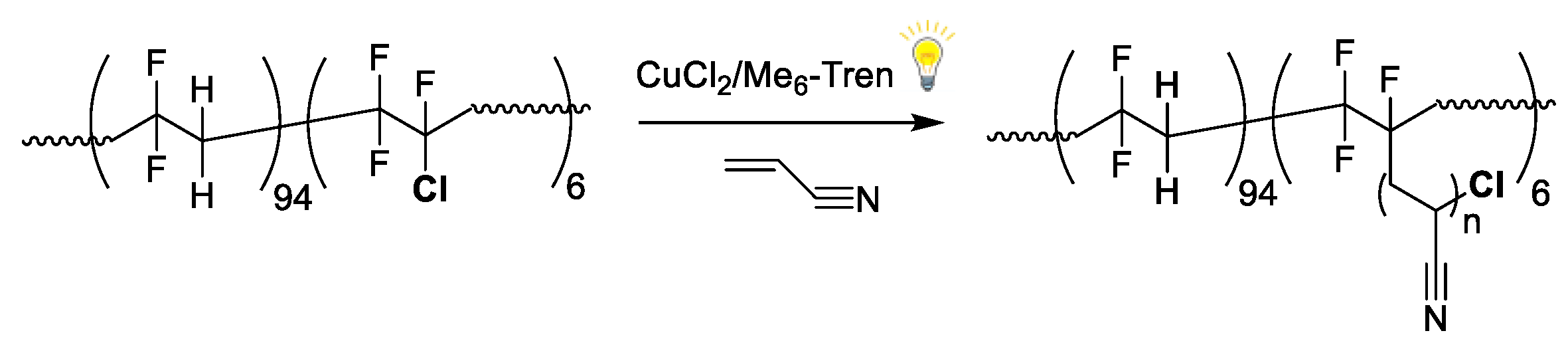
Photoinduced Cu(II)-Mediated RDRP to P(VDF-co-CTFE)-g-PAN
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis Procedure
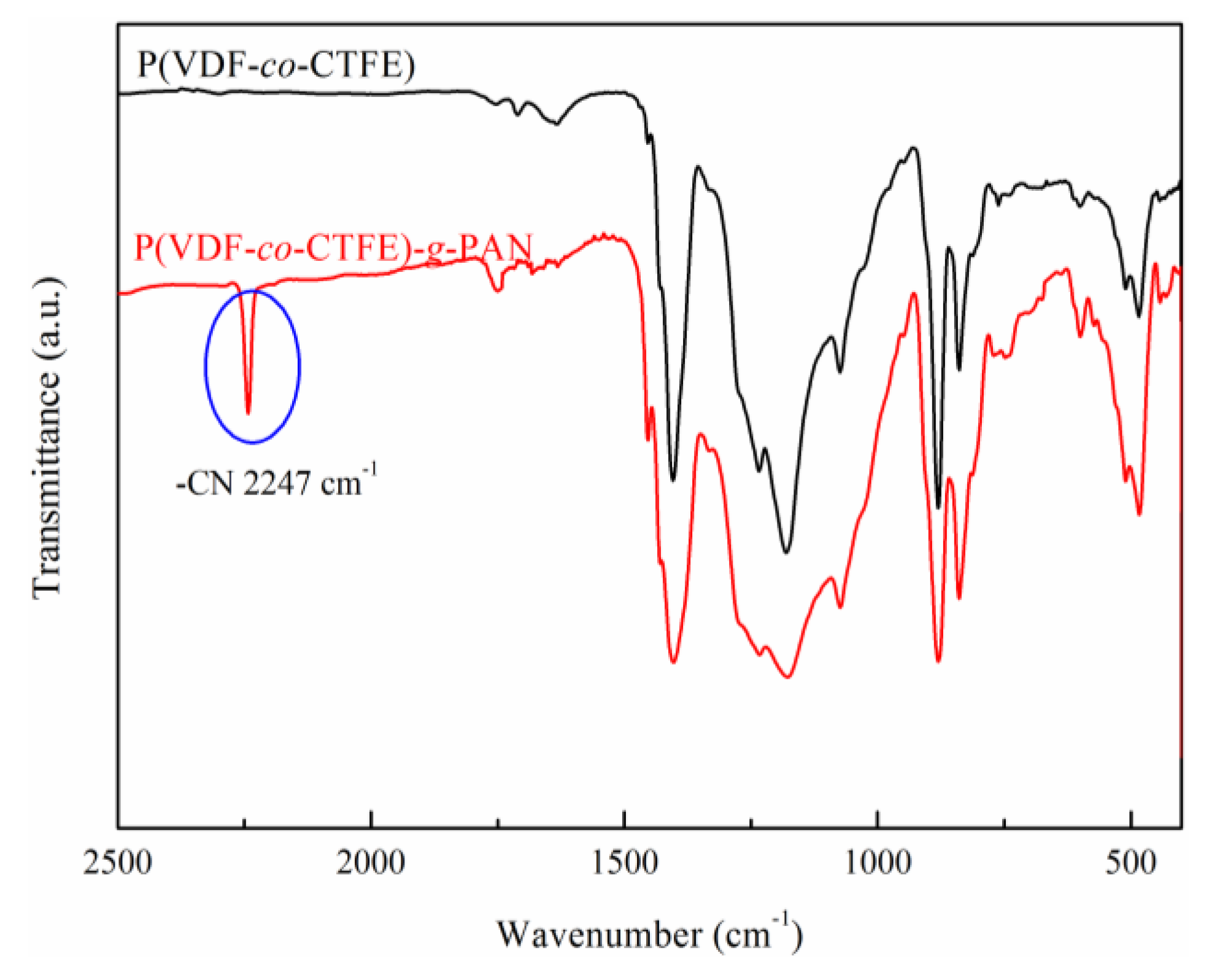
2.3. Characterization
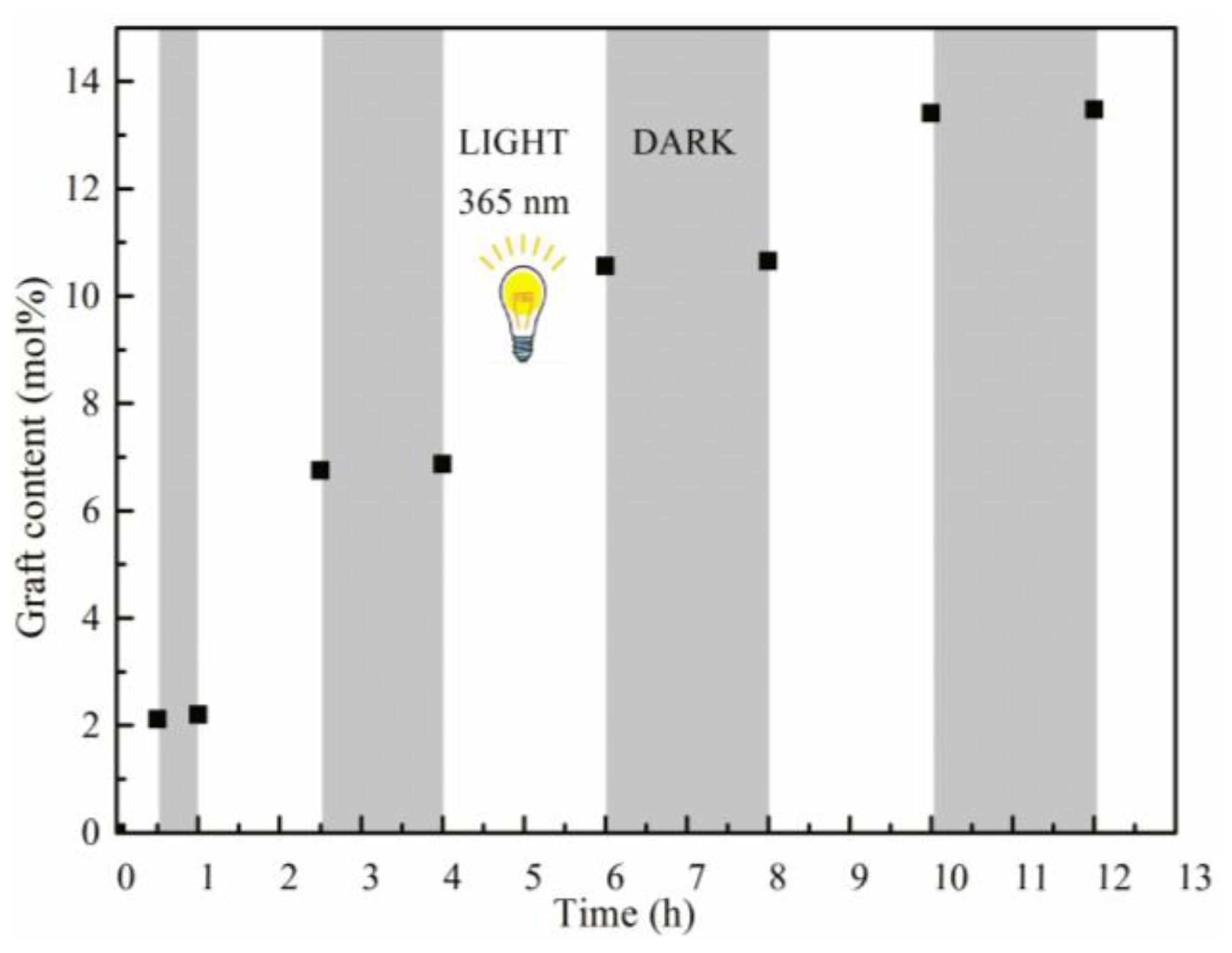
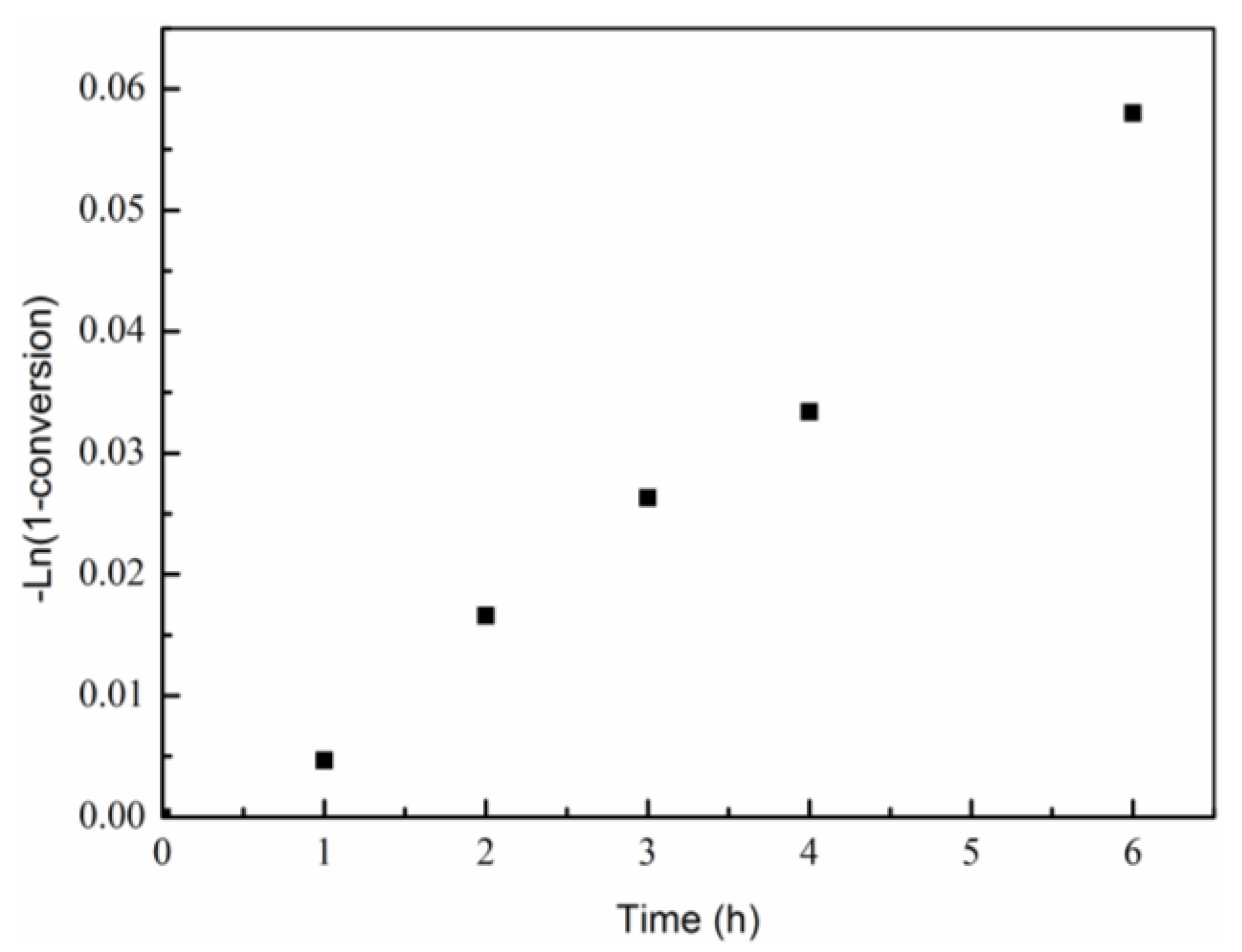
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J.; Matyjaszewski, K. Atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of Methyl Methacrylate with the Carbon Tetrachloride/Dichlorotris-(triphenylphosphine)ruthenium(II)/Methylaluminum Bis(2,6-di-tert-butylphenoxide) Initiating System: Possibility of Living Radical Polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.; Mayadunne, R.T.; Meijs, G.F.; Moad, C.L.; Moad, G. Living Free-Radical Polymerization by Reversible Addition-Fragmentation Chain Transfer: The RAFT Process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Boyer, C.; Bulmus, V.; Davis, T.P.; Ladmiral, V.; Liu, J.; Perrier, S. Bioapplications of RAFT Polymerization. Chem. Rev. 2009, 109, 5402–5436. [Google Scholar] [CrossRef] [PubMed]
- Benot, D.; Chaplinski, V.; Raslau, R.B.; Hawker, C.J. Development of a Universal Alkoxyamine for “Living” Free Radical Polymerizations. J. Am. Chem. Soc. 1999, 121, 3904–3920. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New Polymer Synthesis by Nitroxide Mediated Living Radical Polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Gao, H.; Matyjaszewski, K. Use of Ascorbic Acid as Reducing Agent for Synthesis of Well-Defined Polymers by ARGET ATRP. Macromolecules 2007, 40, 1789–1791. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Jakubowski, W.; Min, K.; Tang, W.; Huang, J.Y.; Braunecker, W.A.; Tsarevsky, N.V. Diminishing catalyst concentration in atom transfer radical polymerization with reducing agents. Proc. Natl. Acad. Sci. USA 2006, 103, 15309–15314. [Google Scholar] [CrossRef] [PubMed]
- Magenau, A.J.D.; Strandwitz, N.C.; Gennaro, A.; Matyjaszewski, K. Electrochemically Mediated Atom Transfer Radical Polymerization. Science 2011, 332, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Matyjaszewski, K. ATRP of Methyl Acrylate with Metallic Zinc, Magnesium, and Iron as Reducing Agents and Supplemental Activators. Macromolecules 2011, 44, 683–685. [Google Scholar] [CrossRef]
- Percec, V.; Guliashvili, T.; Ladislaw, J.S.; Wistrand, A.; Stjerndahl, A.; Sienkowska, M.J.; Monteiro, M.J.; Sahoo, S. Ultrafast Synthesis of Ultrahigh Molar Mass Polymers by Metal-Catalyzed Living Radical Polymerization of Acrylates, Methacrylates, and Vinyl Chloride Mediated by SET at 25 °C. J. Am. Chem. Soc. 2006, 128, 14156–14165. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, A.; Nikolaou, V.; Nurumbetov, G.; Wilson, P.; Kempe, K.; Quinn, J.F.; Davis, T.P.; Whittaker, M.R.; Haddleton, D.M. Cu(0)-Mediated Living Radical Polymerization: A Versatile Tool for Materials Synthesis. Chem. Rev. 2016, 116, 835–877. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, A.; Nikolaou, V.; Haddleton, D.M. Cu(0)-mediated living radical polymerization: recent highlights and applications; a perspective. Polym. Chem. 2016, 7, 1002–1026. [Google Scholar] [CrossRef]
- Shanmugam, S.; Boyer, C. Organic photocatalysts for cleaner polymer synthesis. Science 2016, 352, 1053–1054. [Google Scholar] [CrossRef] [PubMed]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced Electron Transfer Reactions for Macromolecular Syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-Controlled Radical Polymerization: Mechanisms, Methods, and Applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef] [PubMed]
- Zivic, N.; Bouzrati-Zerelli, M.; Kermagoret, A.; Dumur, F.; Fouassier, J.; Gigmes, D.; Lalevée, J. Photocatalysts in Polymerization Reactions. Chem. Cat. Chem. 2016, 8, 1617–1631. [Google Scholar] [CrossRef]
- Pan, X.; Tasdelen, M.A.; Laun, J.; Junkers, T.; Yagci, Y.; Matyjaszewski, K. Photomediated controlled radical polymerization. Prog. Polym. Sci. 2016, 62, 73–125. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Uygun, M.; Yagci, Y. Photoinduced Controlled Radical Polymerization in Methanol. Macromol. Chem. Phys. 2010, 211, 2271–2275. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Uygun, M.; Yagci, Y. Photoinduced Controlled Radical Polymerization. Macromol. Rapid Commun. 2011, 32, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Konkolewicz, D.; Schroder, K.; Buback, J.; Bernhard, S.; Matyjaszewski, K. Visible Light and Sunlight Photoinduced ATRP with ppm of Cu Catalyst. ACS Macro Lett. 2012, 1, 1219–1223. [Google Scholar] [CrossRef]
- Ribelli, T.G.; Konkolewicz, D.; Bernhard, S.; Matyjaszewski, K. How are Radicals (Re)Generated in Photochemical ATRP? J. Am. Chem. Soc. 2014, 136, 13303–13312. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, A.; Nikolaou, V.; Zhang, Q.; Burns, J.; Samanta, S.R.; Wladron, C.; Haddleton, A.J.; McHale, R.; Fox, D.; Percec, V.; et al. Copper(II)/Tertiary Amine Synergy in Photoinduced Living Radical Polymerization: Accelerated Synthesis of ω-Functional and α,ω-Heterofunctional Poly(acrylates). J. Am. Chem. Soc. 2014, 136, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.R.; Whitfield, R.; Anastasaki, A.; Haddleton, D.M. Aqueous Copper(II) Photoinduced Polymerization of Acrylates: Low Copper Concentration and the Importance of Sodium Halide Salts. J. Am. Chem. Soc. 2016, 138, 7346–7352. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, A.; Nkkolaou, V.; McCaul, N.W.; Simular, A.; Godfrey, J.; Waldron, C.; Wilson, P.; Kempe, K.; Haddleton, D.M. Photoinduced Synthesis of α,ω-Telechelic Sequence-Controlled Multiblock Copolymers. Macromolecules 2015, 48, 1404–1411. [Google Scholar] [CrossRef]
- Anastasaki, A.; Nikolaou, V.; Simular, A.; Godfrey, J.; Li, M.; Nurumbetov, G.; Wilson, P.; Haddleton, D.M. Expanding the Scope of the Photoinduced Living Radical Polymerization of Acrylates in the Presence of CuBr2 and Me6-Tren. Macromolecules 2014, 47, 3852–3859. [Google Scholar] [CrossRef]
- Anastasaki, A.; Nikolaou, V.; Pappas, G.S.; Zhang, Q.; Wan, C.Y.; Wilson, P.; Davis, T.P.; Whittaker, M.R.; Haddleton, D.M. Photoinduced sequence-control via one pot living radical polymerization of acrylates. Chem. Sci. 2014, 5, 3536–3542. [Google Scholar] [CrossRef]
- Chuang, Y.M.; Ethirajan, A.; Junkers, T. Photoinduced Sequence-Controlled Copper-Mediated Polymerization: Synthesis of Decablock Copolymers. ACS Macro Lett. 2014, 3, 732–737. [Google Scholar] [CrossRef]
- Chuang, Y.M.; Wenn, B.; Gielen, S.; Ethirajan, A.; Junkers, T. Ligand switch in photoinduced copper-mediated polymerization: synthesis of methacrylate-acrylate block copolymers. Polym. Chem. 2015, 6, 6488–6497. [Google Scholar] [CrossRef]
- Vandenbergh, J.; Reekmans, G.; Adriaensens, P.; Junkers, T. Synthesis of sequence-defined acrylate oligomers via photo-induced copper-mediated radical monomer insertions. Chem. Sci. 2015, 6, 5753–5761. [Google Scholar] [CrossRef]
- Frick, E.; Anastasaki, A.; Haddleton, D.M.; Barner-Kowollik, C. Enlightening the Mechanism of Copper Mediated PhotoRDRP via High-Resolution Mass Spectrometry. J. Am. Chem. Soc. 2015, 137, 6889–6896. [Google Scholar] [CrossRef] [PubMed]
- Discekici, E.H.; Anastasaki, A.; Kaminker, R.; Willenbacher, J.; Truong, N.P.; Fleischmann, C.; Oschmann, B.; Lunn, D.J.; de Alaniz, J.R.; Davis, T.P.; et al. Light-Mediated Atom Transfer Radical Polymerization of Semi-Fluorinated (Meth)acrylates: Facile Access to Functional Materials. J. Am. Chem. Soc. 2017, 139, 5939–5945. [Google Scholar] [CrossRef] [PubMed]
- Laun, J.; Vorobii, M.; Pereira, A.D.; Pop-Georgievski, O.; Trouillet, V.; Welle, A.; Barner-Kowollik, C.; Rodriguez-Emmenegger, C.; Junkers, T. Surface Grafting via Photo-Induced Copper-Mediated Radical Polymerization at Extremely Low Catalyst Concentrations. Macromol. Rapid Commun. 2015, 36, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Fors, B.P.; Hawker, C.J. Control of a Living Radical Polymerization of Methacrylates by Light. Angew. Chem. Int. Ed. 2012, 51, 8850–8853. [Google Scholar] [CrossRef] [PubMed]
- Treat, N.J.; Fors, B.P.; Kramer, J.W.; Christianson, M.; Chiu, C.; Alaniz, J.R.; Hawker, C.J. Controlled Radical Polymerization of Acrylates Regulated by Visible Light. ACS Macro Lett. 2014, 3, 580–584. [Google Scholar] [CrossRef]
- Poelma, J.E.; Fors, B.P.; Meyers, G.F.; Kramer, J.W.; Hawker, C.J. Fabrication of Complex Three-Dimensional Polymer Brush Nanostructures through Light-Mediated Living Radical Polymerization. Angew. Chem. Int. Ed. 2013, 52, 6844–6848. [Google Scholar] [CrossRef] [PubMed]
- Melker, A.; Fors, B.P.; Hawker, C.J.; Poelma, E.J. Continuous flow synthesis of poly(methyl methacrylate) via a light-mediated controlled radical polymerization. J. Polym. Sci. A 2015, 53, 2693–2698. [Google Scholar] [CrossRef]
- Zhang, G.; Song, I.Y.; Ahn, K.H.; Park, T.; Choi, W. Free Radical Polymerization Initiated and Controlled by Visible Light Photocatalysis at Ambient Temperature. Macromolecules 2011, 44, 7594–7599. [Google Scholar] [CrossRef]
- Treat, T.J.; Sprafke, H.; Kramer, J.W.; Clark, P.G.; Barton, B.E.; de Alaniz, J.R.; Fors, B.P.; Hawker, C.J. Metal-Free Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 2014, 136, 16096–16101. [Google Scholar] [CrossRef] [PubMed]
- Miyake, G.M.; Theriot, J.C. Perylene as an Organic Photocatalyst for the Radical Polymerization of Functionalized Vinyl Monomers through Oxidative Quenching with Alkyl Bromides and Visible Light. Macromolecules 2014, 47, 8255–8261. [Google Scholar] [CrossRef]
- Pan, X.; Lamson, M.; Yan, J.; Matyjaszewski, K. Photoinduced Metal-Free Atom Transfer Radical Polymerization of Acrylonitrile. ACS Macro Lett. 2015, 4, 192–196. [Google Scholar] [CrossRef]
- Pan, X.; Fang, C.; Fantin, M.; Malhotra, N.; So, W.Y.; Peteanu, L.A.; Isse, A.A.; Gennaro, A.; Liu, P.; Matyjaszewski, K. Mechanism of Photoinduced Metal-Free Atom Transfer Radical Polymerization: Experimental and Computational Studies. J. Am. Chem. Soc. 2016, 138, 2411–2452. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Cheng, Z.; Zhu, X. Metal-free photoinduced electron transfer-atom transfer radical polymerization (PET-ATRP) via a visible light organic photocatalyst. Polym. Chem. 2016, 7, 689–700. [Google Scholar] [CrossRef]
- Theriot, J.C.; Lim, C.; Yang, H.; Ryan, M.D.; Musgrave, C.B.; Miyake, G.M. Organocatalyzed atom transfer radical polymerization driven by visible light. Science 2016, 352, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.M.; Lim, C.; McCarthy, B.G.; Musgrave, C.B.; Miyake, G.M. Organocatalyzed Atom Transfer Radical Polymerization Using N-Aryl Phenoxazines as Photoredox Catalysts. J. Am. Chem. Soc. 2016, 138, 11399–11407. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Ryan, M.D.; McCarthy, B.G.; Theriot, J.C.; Sartor, S.M.; Damrauer, N.H.; Musgrave, C.B.; Miyake, G.M. Intramolecular Charge Transfer and Ion Pairing in N,N-Diaryl Dihydrophenazine Photoredox Catalysts for Efficient Organocatalyzed Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 2017, 139, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Dadashi-Silab, S.; Pan, X.; Matyjaszewski, K. Phenyl Benzo[b]phenothiazine as a Visible Light Photoredox Catalyst for Metal-Free Atom Transfer Radical Polymerization. Chem. Eur. J. 2017, 23, 5972–5977. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.D.; Pearson, R.M.; French, T.A.; Miyake, G.M. Impact of Light Intensity on Control in Photoinduced Organocatalyzed Atom Transfer Radical Polymerization. Macromolecules 2017, 50, 4616–4622. [Google Scholar] [CrossRef]
- Ramsey, B.L.; Pearson, R.M.; Beck, L.R.; Miyake, G.M. Photoinduced Organocatalyzed Atom Transfer Radical Polymerization Using Continuous Flow. Macromolecules 2017, 50, 2668–2674. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Zhan, X.; Ren, K.; Neese, B.; Lin, M.; Wang, Q.; Bauer, F.; Zhang, Q. A Dielectric Polymer with High Electric Energy Density and Fast Discharge Speed. Science 2006, 313, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Pan, J.; Wang, J.; Wang, Q.; Zhu, L. Crystal Orientation Effect on Electric Energy Storage in Poly(vinylidene fluoride-co-hexafluoropropylene) Copolymers. Macromolecules 2010, 43, 384–392. [Google Scholar] [CrossRef]
- Li, J.; Hu, X.; Gao, G.; Ding, S.; Li, H.; Yang, L.; Zhang, Z. Tuning phase transition and ferroelectric properties of poly(vinylidene fluoride-co-trifluoroethylene) via grafting with desired poly(methacrylic ester)s as side chains. J. Mater. Chem. C 2013, 1, 1111–1121. [Google Scholar] [CrossRef]
- Li, J.; Tan, S.; Ding, S.; Li, H.; Yang, L.; Zhang, Z. High-field antiferroelectric behaviour and minimized energy loss in poly(vinylidene-co-trifluoroethylene)-graft-poly(ethyl methacrylate) for energy storage application. J. Mater. Chem. 2012, 22, 23468–23476. [Google Scholar] [CrossRef]
- Tan, S.; Liu, E.; Zhang, Q.; Zhang, Z. Controlled hydrogenation of P(VDF-co-CTFE) to prepare P(VDF-co-TrFE-co-CTFE) in the presence of CuX (X = Cl, Br) complexes. Chem. Commun. 2011, 47, 4544–4546. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Li, H.; Zhang, Z. Synthesis and characterization of poly(vinylidene fluoride-co-chlorotrifluoroethylene)-grafted-poly(acrylonitrile) via single electron transfer-living radical polymerization process. J. Polym. Sci. A 2012, 50, 3126–3134. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Li, H.; Zhang, Z. Cu(0)/2,6-bis(imino)pyridines catalyzed single-electron transfer-living radical polymerization of methyl methacrylate initiated with poly(vinylidene fluoride-co-chlorotrifluoroethylene). J. Polym. Sci. A 2013, 51, 4378–4388. [Google Scholar] [CrossRef]
- Hu, X.; Tan, S.; Gao, G.; Xie, Y.; Wang, Q.; Li, N.; Zhang, Z. ynthesis of unsaturation containing P(VDF-co-TrFE-co-CTFE) from P(VDF-co-CTFE) in one-pot catalyzed with Cu(0)-based single electron transfer living radical polymerization system. J. Polym. Sci. A 2014, 52, 3429–3440. [Google Scholar] [CrossRef]
- Zhu, N.; Hu, X.; Zhang, Y.; Zhang, K.; Li, Z.; Guo, K. Continuous flow SET-LRP in the presence of P(VDF-co-CTFE) as macroinitiator in a copper tubular reactor. Polym. Chem. 2016, 7, 474–480. [Google Scholar] [CrossRef]
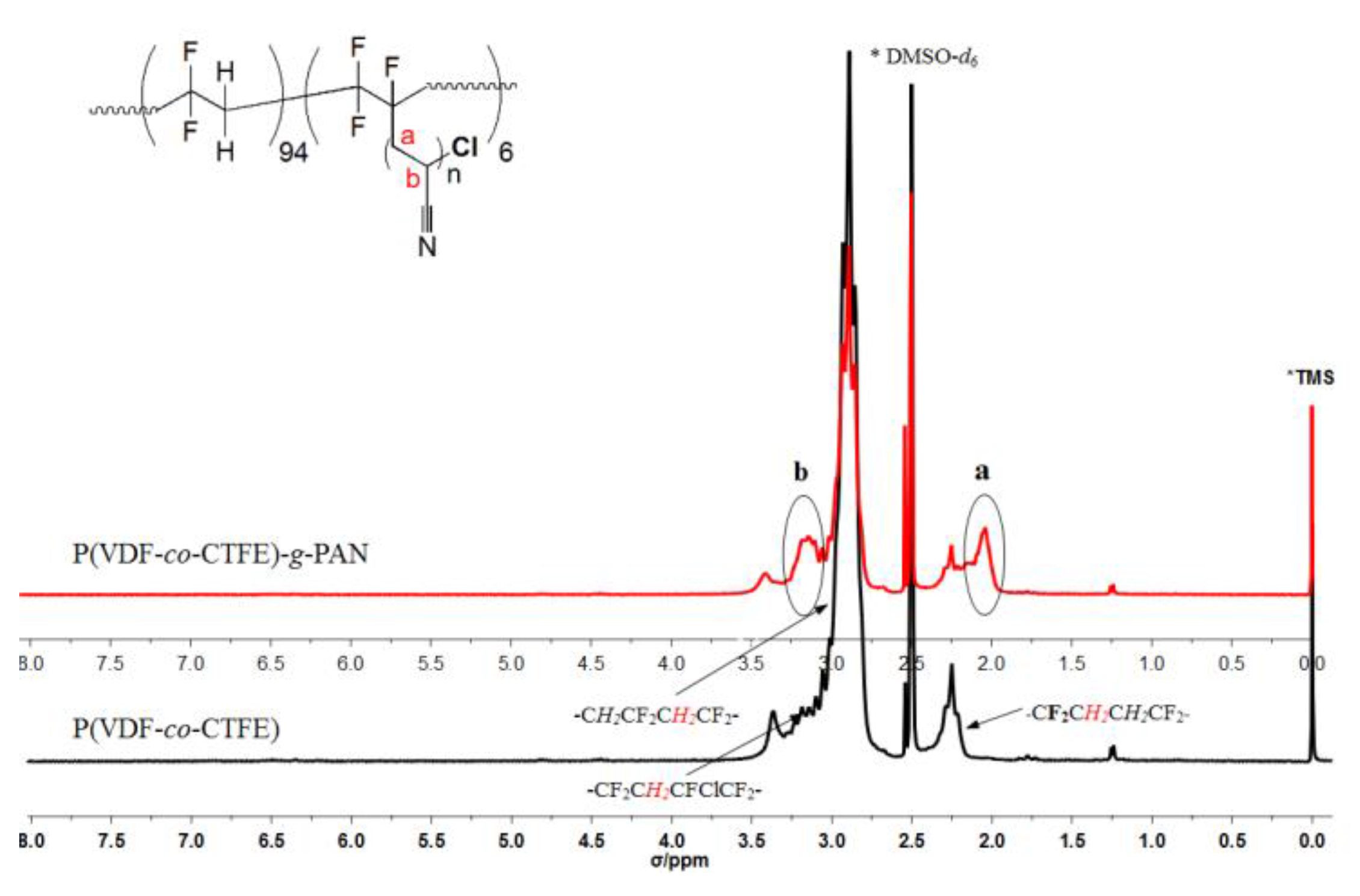
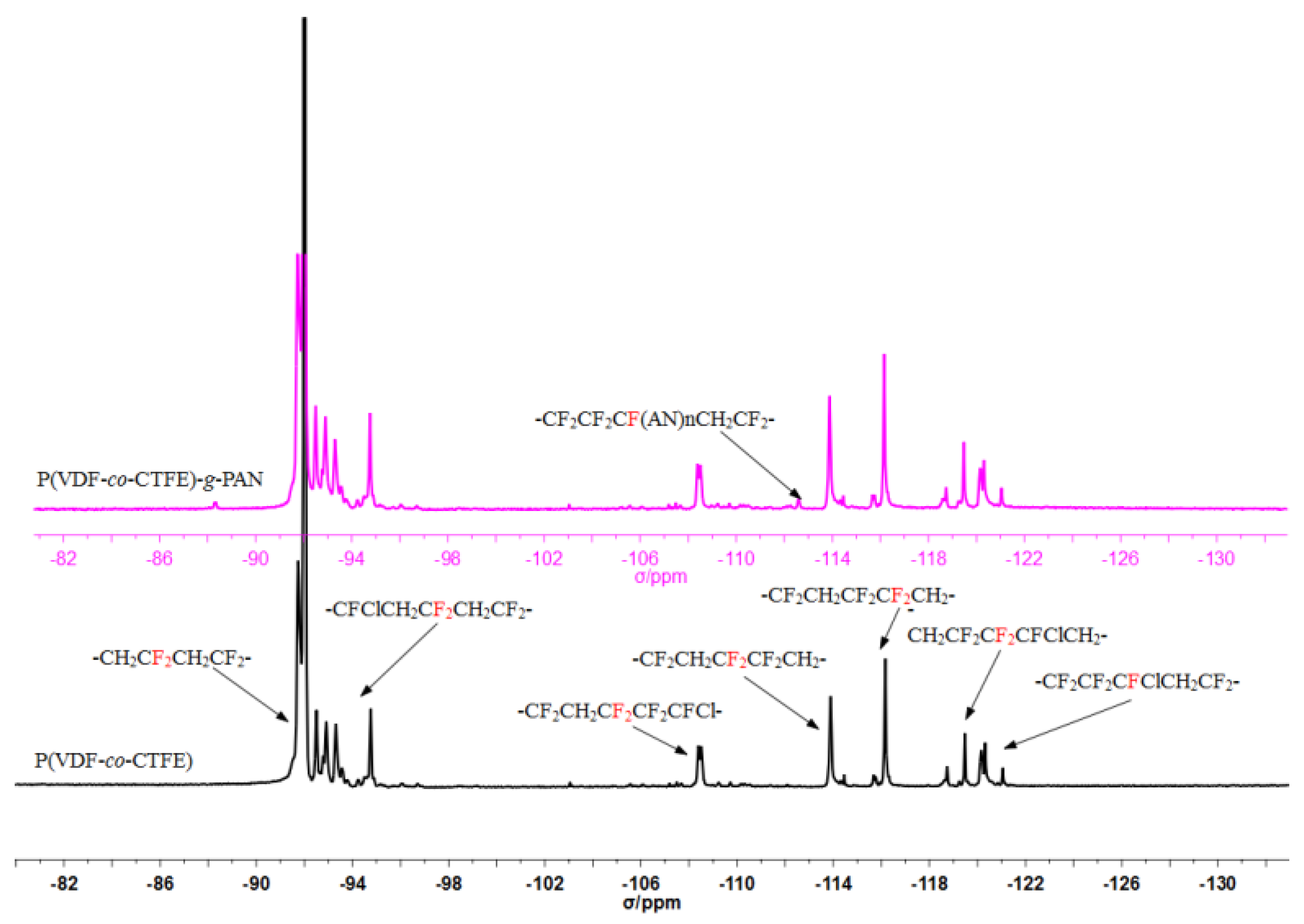
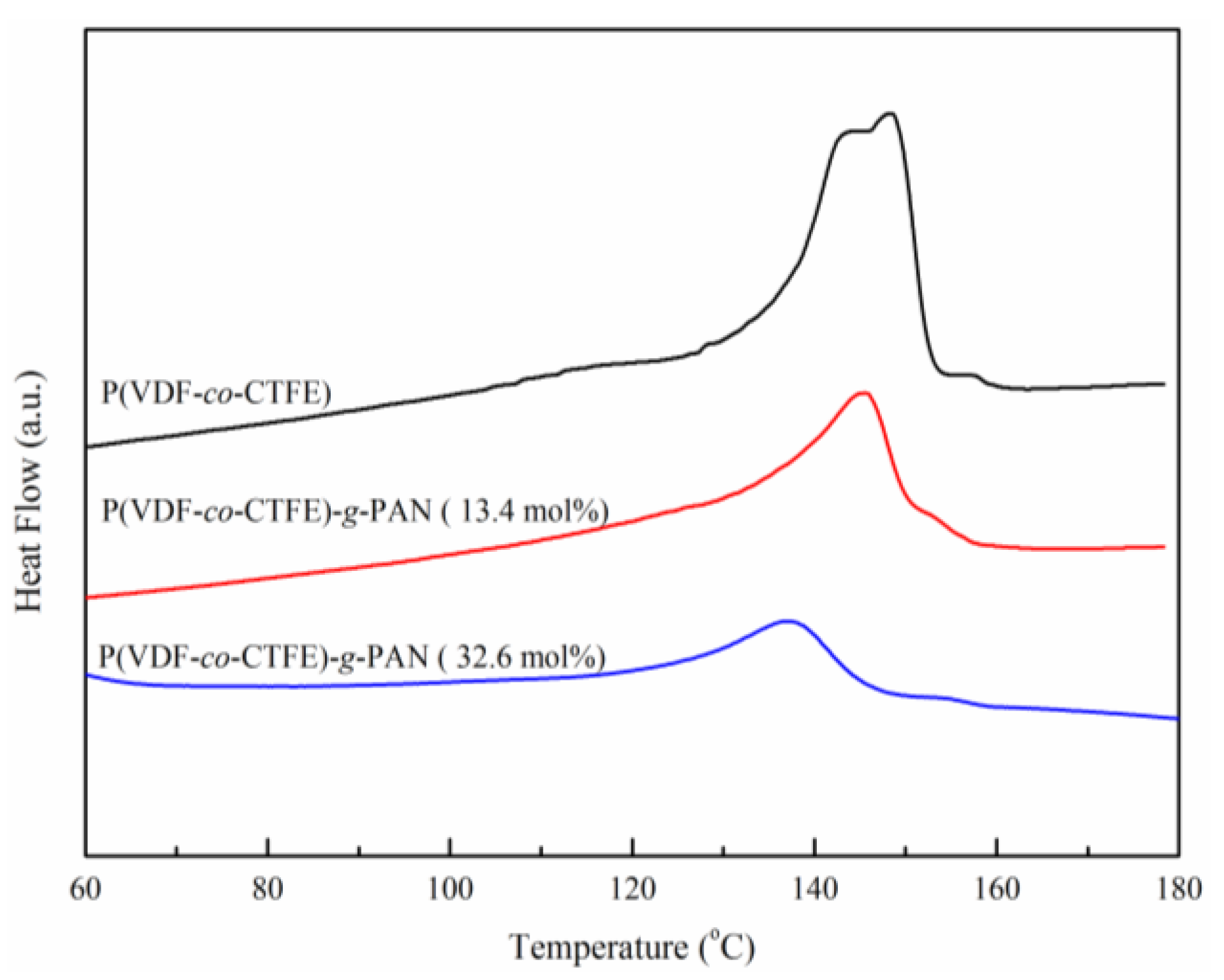
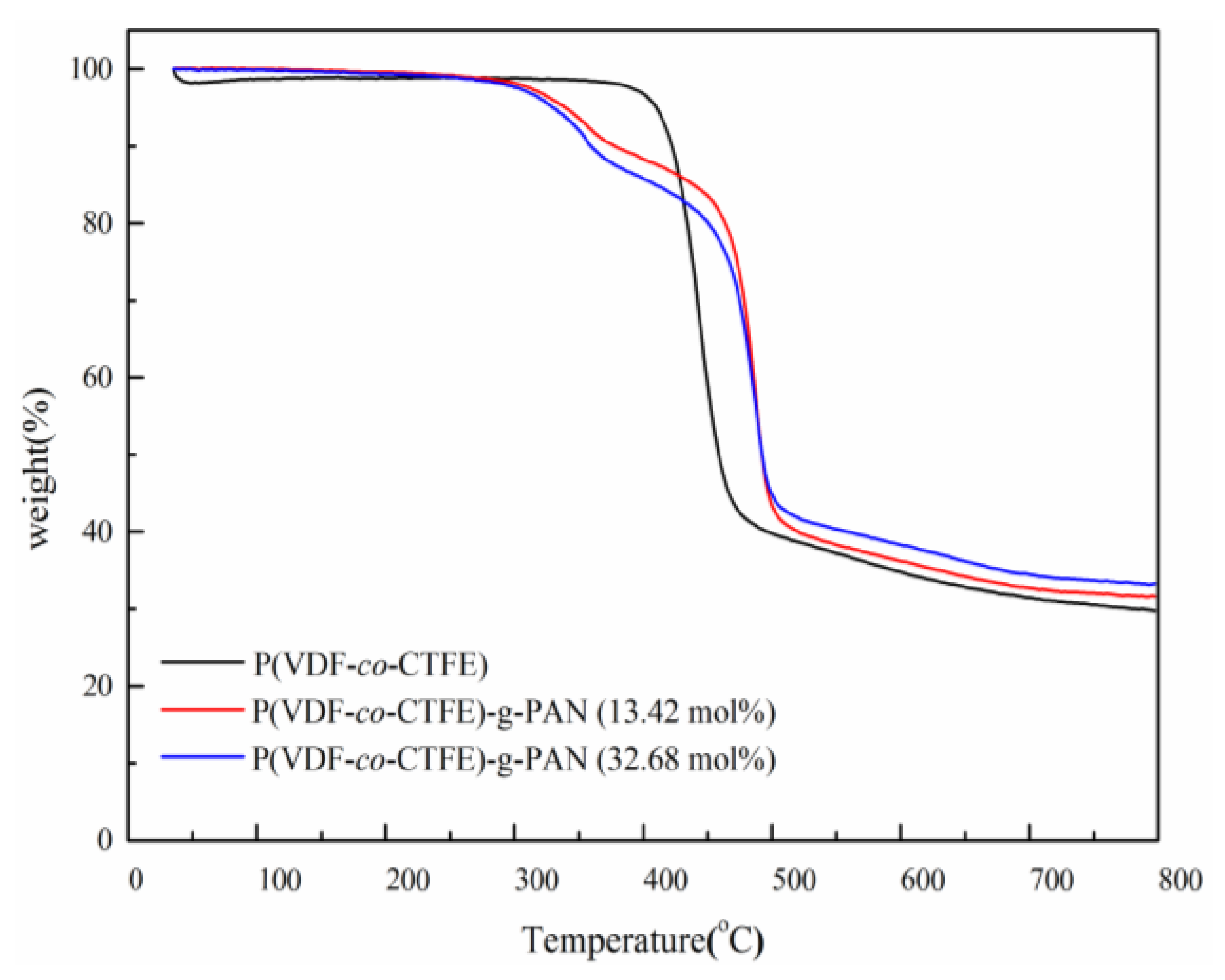
| Run | [Cl]:[Cu]:[L]:[AN] | Conversion% | Graft content [a]mol % | Graft length [b] |
|---|---|---|---|---|
| 1[c] | 1:(1/32):(6/32):30 | 0 | 0 | 0 |
| 2 | 0:(1/32):(6/32):30 | 0 | - | - |
| 3 | 1:(0):(0):30 | 0 | 0 | 0 |
| 4 | 1:(1/32):(4/32):30 | 10.0 | 17.2 | 2.9 |
| 5 | 1:(1/32):(6/32):30 | 12.3 | 20.8 | 3.5 |
| 6 | 1:(1/32):(8/32):30 | 8.1 | 13.7 | 2.3 |
| 7 | 1:(1/64):(6/64):30 | 7.8 | 13.4 | 2.2 |
| 8 | 1:(1/32):(6/32):50 | 9.9 | 27.5 | 4.6 |
| 9 | 1:(1/32):(6/32):80 | 7.5 | 32.6 | 5.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Cui, G.; Zhu, N.; Zhai, J.; Guo, K. Photoinduced Cu(II)-Mediated RDRP to P(VDF-co-CTFE)-g-PAN. Polymers 2018, 10, 68. https://doi.org/10.3390/polym10010068
Hu X, Cui G, Zhu N, Zhai J, Guo K. Photoinduced Cu(II)-Mediated RDRP to P(VDF-co-CTFE)-g-PAN. Polymers. 2018; 10(1):68. https://doi.org/10.3390/polym10010068
Chicago/Turabian StyleHu, Xin, Guopeng Cui, Ning Zhu, Jinglin Zhai, and Kai Guo. 2018. "Photoinduced Cu(II)-Mediated RDRP to P(VDF-co-CTFE)-g-PAN" Polymers 10, no. 1: 68. https://doi.org/10.3390/polym10010068






