Bimetallic Gold-Silver Nanoparticles Supported on Zeolitic Imidazolate Framework-8 as Highly Active Heterogenous Catalysts for Selective Oxidation of Benzyl Alcohol into Benzaldehyde
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Catalyst Preparation
2.2.1. Preparation of ZIF-8
2.2.2. Preparation of Catalyst
2.3. Characterization
2.4. Catalyst Testing
3. Results and Discussion
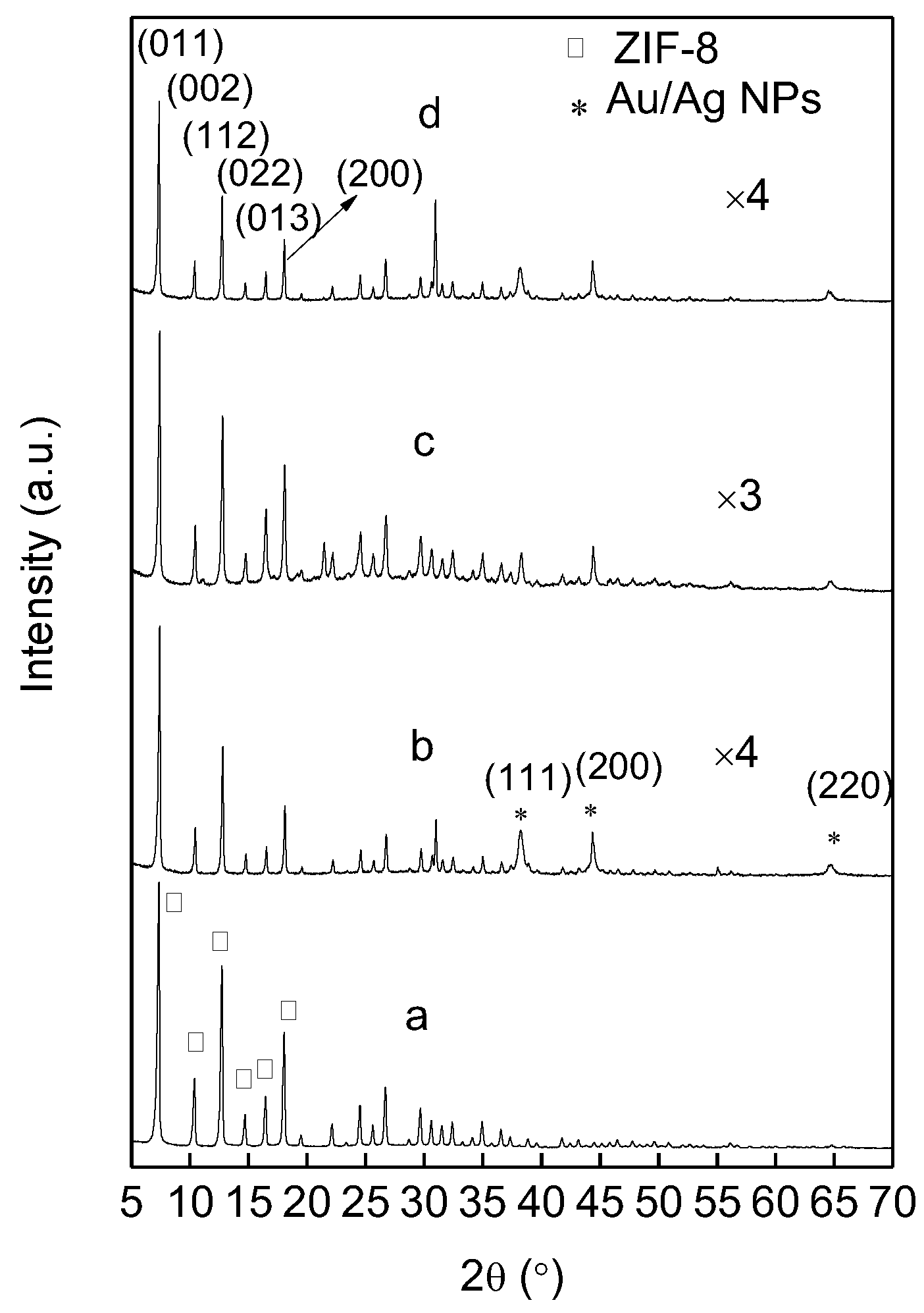
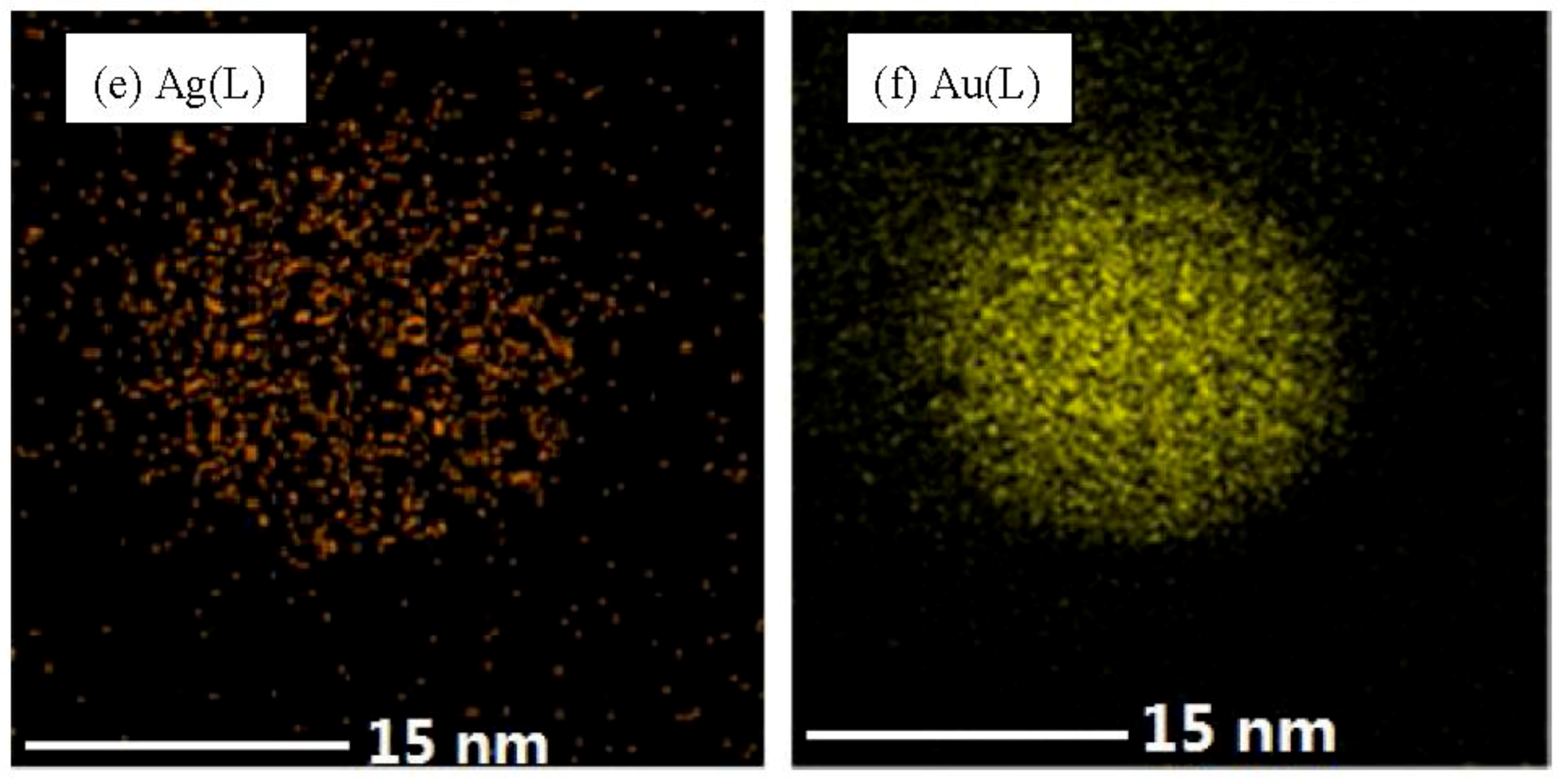
3.1. Catalyst Synthesis and Characterization
3.2. The Selective Catalytic Oxidation of Benzyl Alcohol
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, G.J.; Wang, J.S.; Jin, F.Z.; Liu, M.Y.; Zhao, C.W.; Li, Y.A.; Dong, Y.B. Pd@Cu(II)-MOF-catalyzed aerobic oxidation of benzylic alcohols in air with high conversion and selectivity. Inorg. Chem. 2016, 55, 3058–3064. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wu, X.P.; Tang, Y.; Yang, Y.; Gong, X.Q.; Fan, J. Selectivity switching resulting in the formation of benzene by surface carbonates on ceria in catalytic gas-phase oxidation of benzyl alcohol. Chem. Commun. 2016, 52, 2827–2830. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Vijaya, J.J.; Sivasanker, S.; Alam, M.; Tamizhdurai, P.; Kennedy, J. Characterization and catalytic reactivity of mordenite–investigation of selective oxidation of benzyl alcohol. Polyhedron 2015, 89, 289–296. [Google Scholar] [CrossRef]
- Nemanashi-Maumela, M.; Nongwe, I.; Motene, R.C.; Davids, B.L.; Meijboom, R. Au and Ag nanoparticles encapsulated within silica nanospheres using dendrimers as dual templating agent and their catalytic activity. Mol. Catal. 2017, 438, 184–196. [Google Scholar] [CrossRef]
- Kimi, M.; Jaidie, M.M.H.; Pang, S.C. Bimetallic Cu-Ni nanoparticles supported on activated carbon for catalytic oxidation of benzyl alcohol. J. Phys. Chem. Solids 2018, 112, 50–53. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Gautam, R.K.; Belwal, M. Synthesis and characterization of Au/γ-Al2O3 nanocatalysts for vapor-phase selective oxidation of benzyl alcohol under aerobic condition. Curr. Catal. 2018, 7, 35–42. [Google Scholar] [CrossRef]
- Chen, L.J.; Yan, J.Q.; Tong, Z.X.; Yu, S.Y.; Tang, J.T.; Ou, B.L.; Yue, L.J.; Tian, L. Nanofiber-like mesoporous alumina supported palladium nanoparticles as a highly active catalyst for base-free oxidation of benzyl alcohol. Microporous Mesoporous Mater. 2018, 266, 126–131. [Google Scholar] [CrossRef]
- Sun, J.Y.; Tong, X.L.; Liu, Z.H.; Liao, S.Y.; Zhang, X.L.; Xue, S. Gold-catalyzed selectivity-switchable oxidation of benzyl alcohol in the presence of molecular oxygen. Catal. Commun. 2016, 85, 70–74. [Google Scholar] [CrossRef]
- Song, Y.J.; Jesús, Y.M.L.D.; Fanson, P.T.; Williams, C.T. Preparation and characterization of dendrimer-derived bimetallic Ir–Au/Al2O3 catalysts for CO oxidation. J. Phys. Chem. C 2013, 117, 10999–11007. [Google Scholar] [CrossRef]
- Davis, S.E.; Ide, M.S.; Davis, R.J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles. Green Chem. 2013, 15, 17–45. [Google Scholar] [CrossRef]
- Bhattacharya, C.; Jagirdar, B.R. Monodisperse colloidal metal nanoparticles to core-shell structures and alloy nanosystems via digestive ripening in conjunction with solvated metal atom dispersion: A mechanistic study. J. Phys. Chem. C 2018, 122, 10559–10574. [Google Scholar] [CrossRef]
- Rostek, A.; Breisch, M.; Loza, K.; Garcia, P.R.A.F.; Oliveira, C.L.P.; Prymak, O.; Heggen, M.; Köller, M.; Sengstock, C.; Epple, M. Wet-chemical synthesis of Pd-Au core-shell nanoparticles (8 nm): From nanostructure to biological properties. Chem. Sel. 2018, 3, 4994–5001. [Google Scholar] [CrossRef]
- Sun, J.Y.; Han, Y.X.; Fu, H.Y.; Qu, X.L.; Xu, Z.Y.; Zheng, S.R. Au@Pd/TiO2 with atomically dispersed Pd as highly active catalyst for solvent-free aerobic oxidation of benzyl alcohol. Chem. Eng. J. 2017, 313, 1–9. [Google Scholar] [CrossRef]
- Henning, A.M.; Watt, J.; Miedziak, P.J.; Cheong, S.; Santonastaso, M.; Song, M.; Takeda, Y.; Kirkland, A.I.; Taylor, S.H.; Tilley, R.D. Gold-palladium core-shell nanocrystals with size and shape control optimized for catalytic performance. Angew. Chem. Int. Ed. 2013, 52, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, H.; Alhumaimess, M.; Alotaibi, M.H.; Alshammari, A.S. Catalytic activity of bimetallic AuPd alloys supported MgO and MnO2 nanostructures and their role in selective aerobic oxidation of alcohols. J. King Saud Univ. Sci. 2017, 29, 561–566. [Google Scholar] [CrossRef]
- Wang, Z.J.; Balkus, K.J., Jr. Wrinkled mesoporous carbon supported Pd nanoparticles for hydrogenation and aerobic oxidation reactions. J. Porous Mater. 2018, 25, 15–21. [Google Scholar] [CrossRef]
- Kumar, A.; Screedhar, B.; Chary, K.V.R. Highly dispersed gold nanoparticles supported on SBA-15 for vapor phase aerobic oxidation of benzyl alcohol. J. Nanosci. Nanotechnol. 2015, 15, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal organic frameworks as versatile hosts of Au nanoparticles in heterogeneous catalysis. ACS Catal. 2017, 7, 2896–2919. [Google Scholar] [CrossRef]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.M.; Liu, P.; Wu, Y.L.; Wei, W.; Xia, C.K.; Xie, J.M. Cage-like pores of a metal-organic framework for separations and encapsulation of Pd nanoparticles for efficient catalysis. New J. Chem. 2015, 39, 2669–2674. [Google Scholar] [CrossRef]
- To, T.A.; Tran, C.B.; Nguyen, N.T.H.; Nguyen, H.H.T.; Nguyen, A.T.; Phan, A.Q.; Phan, N.T.S. An efficient access to b-ketosulfones via bsulfonylvinylamines: Metal-organic framework catalysis for the direct C–S coupling of sodium sulfinates with oxime acetates. RSC Adv. 2018, 8, 17477–17485. [Google Scholar] [CrossRef]
- Thornburg, N.E.; Liu, Y.; Li, P.; Hupp, J.T.; Farha, O.K.; Notestein, J.M. MOFs and their grafted analogues: Regioselective epoxide ring-opening with Zr6 nodes. Catal. Sci. Technol. 2016, 6, 6480–6484. [Google Scholar] [CrossRef]
- Noori, Y.; Akhbari, K. Post-synthetic ion-exchange process in nanoporous metal-organic frameworks: An effective way for modulating their structures and properties. RSC Adv. 2017, 7, 1782–1808. [Google Scholar] [CrossRef]
- Canivet, J.; Aguado, S.; Schuurman, Y.; Farrusseng, D. MOF-supported selective ethylene dimerization single-site catalysts through one-pot postsynthetic modification. J. Am. Chem. Soc. 2013, 135, 4195–4198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Ying, J.Y. Main-chain organic frameworks with advanced catalytic functionalities. ACS Catal. 2015, 5, 2681–2691. [Google Scholar] [CrossRef]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal-organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xiao, C.; Maligal-Ganesh, R.V.; Zhou, L.; Goh, T.W.; Li, X.; Tesfagaber, D.; Thiel, A.; Huang, W. Pt nanoclusters confined within metal-organic framework cavities for chemoselective cinnamaldehyde hydrogenation. ACS Catal. 2014, 4, 1340–1348. [Google Scholar] [CrossRef]
- Li, X.; Guo, Z.; Xiao, C.; Goh, T.W.; Tesfagaber, D.; Huang, W. Tandem catalysis by palladium nanoclusters encapsulated in metal-organic frameworks. ACS Catal. 2014, 4, 3490–3497. [Google Scholar] [CrossRef]
- Neelakanda, P.; Barankova, E.; Peinemann, K.V. Polymer supported ZIF-8 membranes by conversion of sputtered zinc oxide layers. Microporous Mesoporous Mater. 2016, 220, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.W.; Zeng, Y.Y.; Wu, X.C.; Mu, M.M.; Chen, L.G. A novel Pd-Ni bimetallic synergistic catalyst on ZIF-8 for Sonogashira coupling reaction. Mater. Lett. 2018, 220, 321–324. [Google Scholar] [CrossRef]
- Xia, B.Q.; Cao, N.; Dai, H.M.; Su, J.; Wu, X.J.; Luo, W.; Cheng, G.Z. Bimetallic nickel–rhodium nanoparticles supported on ZIF-8 as highly efficient catalysts for hydrogen generation from hydrazine in alkaline solution. ChemCatChem 2014, 6, 2549–2552. [Google Scholar] [CrossRef]
- Amarante, S.F.; Freire, M.A.; Mendes, D.T.S.L.; Freitas, L.S.; Ramos, A.L.D. Evaluation of basic sites of ZIFs metal organic frameworks in the Knoevenagel condensation reaction. Appl. Catal. A Gen. 2017, 548, 47–51. [Google Scholar] [CrossRef]
- Hwang, Y.K.; Hong, D.Y.; Chang, J.S.; Jhung, S.H.; Seo, Y.K.; Kim, J.; Vimont, A.; Daturi, M.; Serre, C.; Férey, G. Amine grafting on coordinatively unsaturated metal centers of MOFs: Consequences for catalysis and metal encapsulation. Angew. Chem. Int. Ed. 2008, 47, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Mousavi, B.; Luo, Z.; Phatanasri, S.; Chaemchuen, S.; Verpoort, F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J. Mater. Chem. A 2017, 5, 952–957. [Google Scholar] [CrossRef]
- Villa, A.; Wang, D.; Su, D.S.; Prati, L. Gold sols as catalysts for glycerol oxidation: The role of stabilizer. Chem. Catal. Chem. 2009, 1, 510–514. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, X.R.; Li, C.C.; Hao, X.G.; Wang, Y.H.; Guan, G.Q. ZIF-8 incorporated polyether block amide membrane for phenol permselective pervaporation with high efficiency. Sep. Purif. Technol. 2016, 166, 252–261. [Google Scholar] [CrossRef]
- Ismail, K.; Gérald, C.; Habiba, N.; Guillaume, O.; Claire, M.; Joël, P. Assessment of the energetic performances of various ZIFs with SOD or RHO topology using high pressure water intrusion-extrusion experiments. Dalton Trans. 2016, 45, 4392–4400. [Google Scholar]
- Misra, M.; Singh, N.; Gupta, R.K. Enhanced visible-light-driven photocatalytic activity of Au@Ag core-shell bimetallic nanoparticles immobilized on electrospun TiO2 nanofibers for degradation of organic compounds. Catal. Sci. Technol. 2017, 7, 570–580. [Google Scholar] [CrossRef]
- Pramanik, S.; Chattopadhyay, S.; Das, J.K.; Manju, U.; De, G. Extremely fast Au–Ag alloy-dealloy associated reversible plasmonic modifications in SiO2 films. J. Mater. Chem. C 2016, 4, 3571–3580. [Google Scholar] [CrossRef]
- Butova, V.V.; Budnyk, A.P.; Bulanova, E.A.; Lamberti, C.; Soldatov, A.V. Hydrothermal synthesis of high surface area ZIF-8 with minimal use of TEA. Solid State Sci. 2017, 69, 13–21. [Google Scholar] [CrossRef]
- Singh, A.K.; Xu, Q. Metal-organic framework supported bimetallic Ni-Pt nanoparticles as high-performance catalysts for hydrogen generation from hydrazine in aqueous solution. ChemCatChem 2013, 5, 3000–3004. [Google Scholar] [CrossRef]
- Liu, L.L.; Tai, X.S.; Zhang, N.N.; Meng, Q.G.; Xin, C.L. Supported Au/MIL-53(Al): A reusable green solid catalyst for the three-component coupling reaction of aldehyde, alkyne, and amine. React. Kinet. Mech. Cat. 2016, 119, 335–348. [Google Scholar] [CrossRef]
- Liu, L.L.; Tai, X.S.; Liu, J.B.; Li, D.; Zhou, X.J.; Zhang, L.J.; Wei, X.F. Preparation of propargylamines catalyzed by heterogeneous catalysts with double catalytic sites. Chem. J. Chin. Univ. 2018, 39, 482–490. [Google Scholar]
- Yong, G.P.; Tian, D.; Tong, H.W.; Liu, S.M. Mesoporous SBA-15 supported silver nanoparticles as environmentally friendly catalysts for three-component reaction of aldehydes, alkynes and amines with glycol as a “green” solvent. J. Mol. Catal. A Chem. 2010, 323, 40–44. [Google Scholar] [CrossRef]
- Galvanin, F.; Sankar, M.; Cattaneo, S.; Bethell, D.; Dua, V.; Hutchings, G.; Gavriilidis, A. On the development of kinetic models for solvent-free benzyl alcohol oxidation over a gold-palladium catalyst. Chem. Eng. J. 2018, 342, 196–210. [Google Scholar] [CrossRef]
- Philip, A.; Lihavainen, J.; Keinänen, M.; Pakkanen, T.T. Gold nanoparticle-decorated halloysite nanotubes—Selective catalysts for benzyl alcohol oxidation. Appl. Clay Sci. 2017, 143, 80–88. [Google Scholar] [CrossRef]
- Miedziak, P.; Sankar, M.; Dimitratos, N.; Lopez-Sanchez, J.A.; Carley, A.F.; Knight, D.W.; Taylor, S.H.; Kiely, C.J.; Hutchings, G.J. Oxidation of benzyl alcohol using supported gold–palladium nanoparticles. Catal. Today 2011, 164, 315–319. [Google Scholar] [CrossRef]
- Cui, W.J.; Xiao, Q.; Sarina, S.; Ao, W.L.; Xie, M.X.; Zhu, H.Y.; Bao, Z. Au–Pd alloy nanoparticle catalyzed selective oxidation of benzylalcohol and tandem synthesis of imines at ambient conditions. Catal. Today 2014, 235, 152–159. [Google Scholar] [CrossRef]










| Entry | Catalyst | Solvent | T (°C) | Pressure (bar) | Conv. (%) b | S (%) c | Yield (%) d |
|---|---|---|---|---|---|---|---|
| 1 | Au@Ag/ZIF-8 | THF | 130 | 8 | 65.7 | 53.0 | 34.8 |
| 2 | Au@Ag/ZIF-8 | 1,4-dioxane | 130 | 8 | 23.7 | 72.9 | 17.3 |
| 3 | Au@Ag/ZIF-8 | DMF | 130 | 8 | 69.1 | 2.6 | 1.8 |
| 4 | Au@Ag/ZIF-8 | Acetonitrile | 130 | 8 | 27.7 | 2.0 | 0.6 |
| 5 | Au@Ag/ZIF-8 | Toluene | 130 | 8 | 8.3 | 29.3 | 2.4 |
| 6 | Au@Ag/ZIF-8 | THF | 130 | 10 | 77.0 | 24.9 | 19.2 |
| 7 | Au@Ag/ZIF-8 | THF | 130 | 6 | 25.5 | 98.0 | 25.0 |
| 8 | Au@Ag/ZIF-8 | THF | 120 | 8 | 40.1 | 66.7 | 26.7 |
| 9 | Au@Ag/ZIF-8 | THF | 140 | 8 | 80.7 | 29.9 | 24.1 |
| 10 | Ag@Au/ZIF-8 | THF | 130 | 8 | 75.3 | 53.3 | 40.1 |
| 11 | Ag@Au/ZIF-8 | 1,4-dioxane | 130 | 8 | 52.6 | 29.5 | 15.5 |
| 12 | Ag@Au/ZIF-8 | DMF | 130 | 8 | 12.3 | 16.4 | 2.0 |
| 13 | Ag@Au/ZIF-8 | Acetonitrile | 130 | 8 | 31.5 | 1.0 | 0.3 |
| 14 | Ag@Au/ZIF-8 | Toluene | 130 | 8 | 1.0 | 96.5 | 1.0 |
| 15 | Ag@Au/ZIF-8 | THF | 130 | 10 | 80.5 | 44.2 | 35.6 |
| 16 | Ag@Au/ZIF-8 | THF | 130 | 6 | 34.0 | 96.5 | 32.8 |
| 17 | Ag@Au/ZIF-8 | THF | 120 | 8 | 69.7 | 55.6 | 38.8 |
| 18 | Ag@Au/ZIF-8 | THF | 140 | 8 | 80.0 | 40.2 | 32.2 |
| 19 | AuAg/ZIF-8 | THF | 130 | 8 | 74.0 | 35.2 | 26.0 |
| 20 | AuAg/ZIF-8 | 1,4-dioxane | 130 | 8 | 51.9 | 35.0 | 18.2 |
| 21 | AuAg/ZIF-8 | DMF | 130 | 8 | 4.9 | 81.9 | 4.0 |
| 22 | AuAg/ZIF-8 | Acetonitrile | 130 | 8 | 2.0 | 95.0 | 1.9 |
| 23 | AuAg/ZIF-8 | Toluene | 130 | 8 | 4.3 | 94.6 | 4.1 |
| 24 | AuAg/ZIF-8 | THF | 130 | 10 | 80.6 | 30.6 | 24.7 |
| 25 | AuAg/ZIF-8 | THF | 130 | 6 | 32.4 | 55.6 | 18.0 |
| 26 | AuAg/ZIF-8 | THF | 120 | 8 | 34.7 | 62.3 | 21.6 |
| 27 | AuAg/ZIF-8 | THF | 140 | 8 | 89.9 | 24.8 | 22.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhou, X.; Yan, Y.; Zhou, J.; Zhang, W.; Tai, X. Bimetallic Gold-Silver Nanoparticles Supported on Zeolitic Imidazolate Framework-8 as Highly Active Heterogenous Catalysts for Selective Oxidation of Benzyl Alcohol into Benzaldehyde. Polymers 2018, 10, 1089. https://doi.org/10.3390/polym10101089
Liu L, Zhou X, Yan Y, Zhou J, Zhang W, Tai X. Bimetallic Gold-Silver Nanoparticles Supported on Zeolitic Imidazolate Framework-8 as Highly Active Heterogenous Catalysts for Selective Oxidation of Benzyl Alcohol into Benzaldehyde. Polymers. 2018; 10(10):1089. https://doi.org/10.3390/polym10101089
Chicago/Turabian StyleLiu, Lili, Xiaojing Zhou, Yongmei Yan, Jie Zhou, Wenping Zhang, and Xishi Tai. 2018. "Bimetallic Gold-Silver Nanoparticles Supported on Zeolitic Imidazolate Framework-8 as Highly Active Heterogenous Catalysts for Selective Oxidation of Benzyl Alcohol into Benzaldehyde" Polymers 10, no. 10: 1089. https://doi.org/10.3390/polym10101089





