Kinetic, Isotherm, and Thermodynamic Studies for Ag(I) Adsorption Using Carboxymethyl Functionalized Poly(glycidyl methacrylate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Synthesis Process of Carboxymehyl Functionalized PGMA
2.3. Characterization
2.4. Adsorption Experiment
3. Results and Discussion
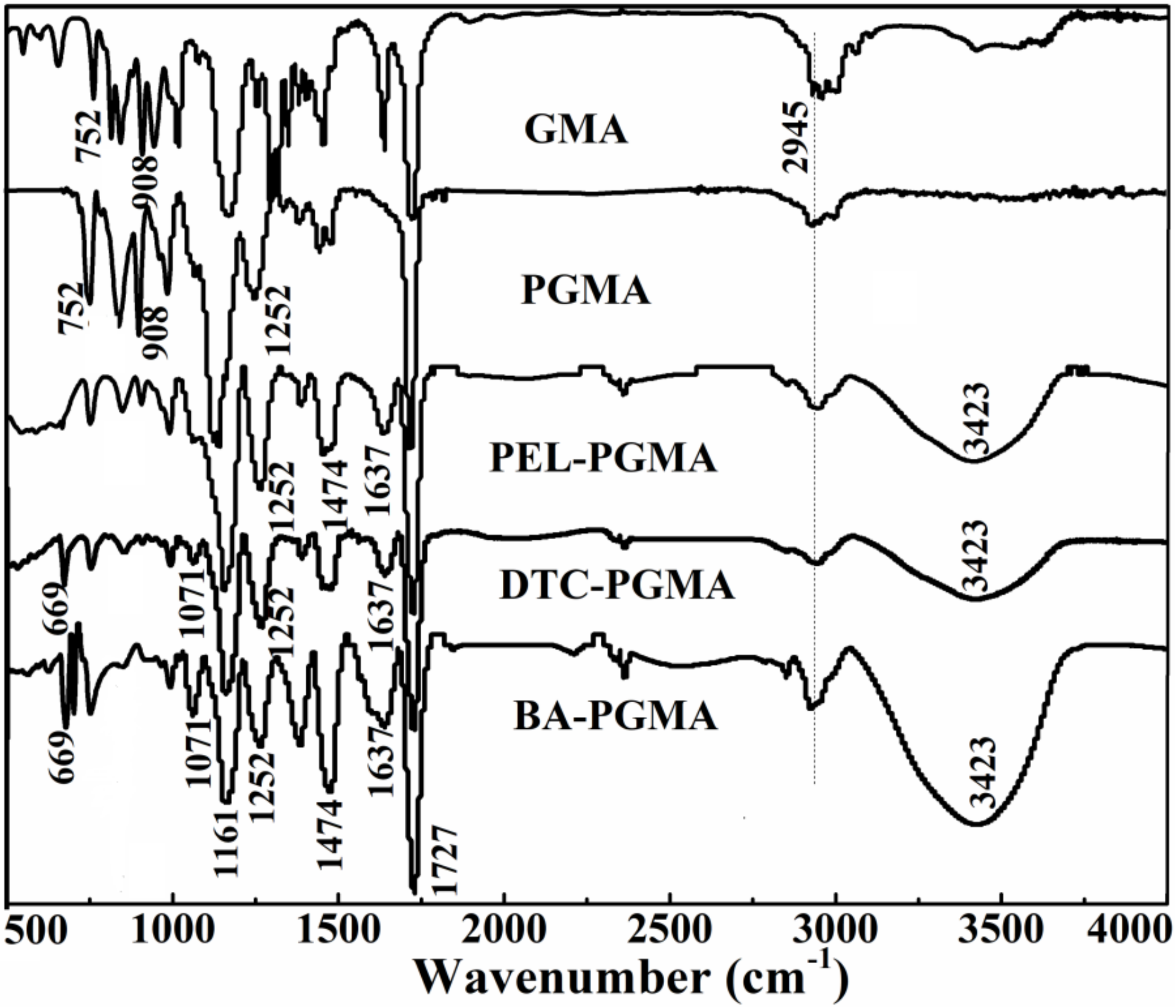
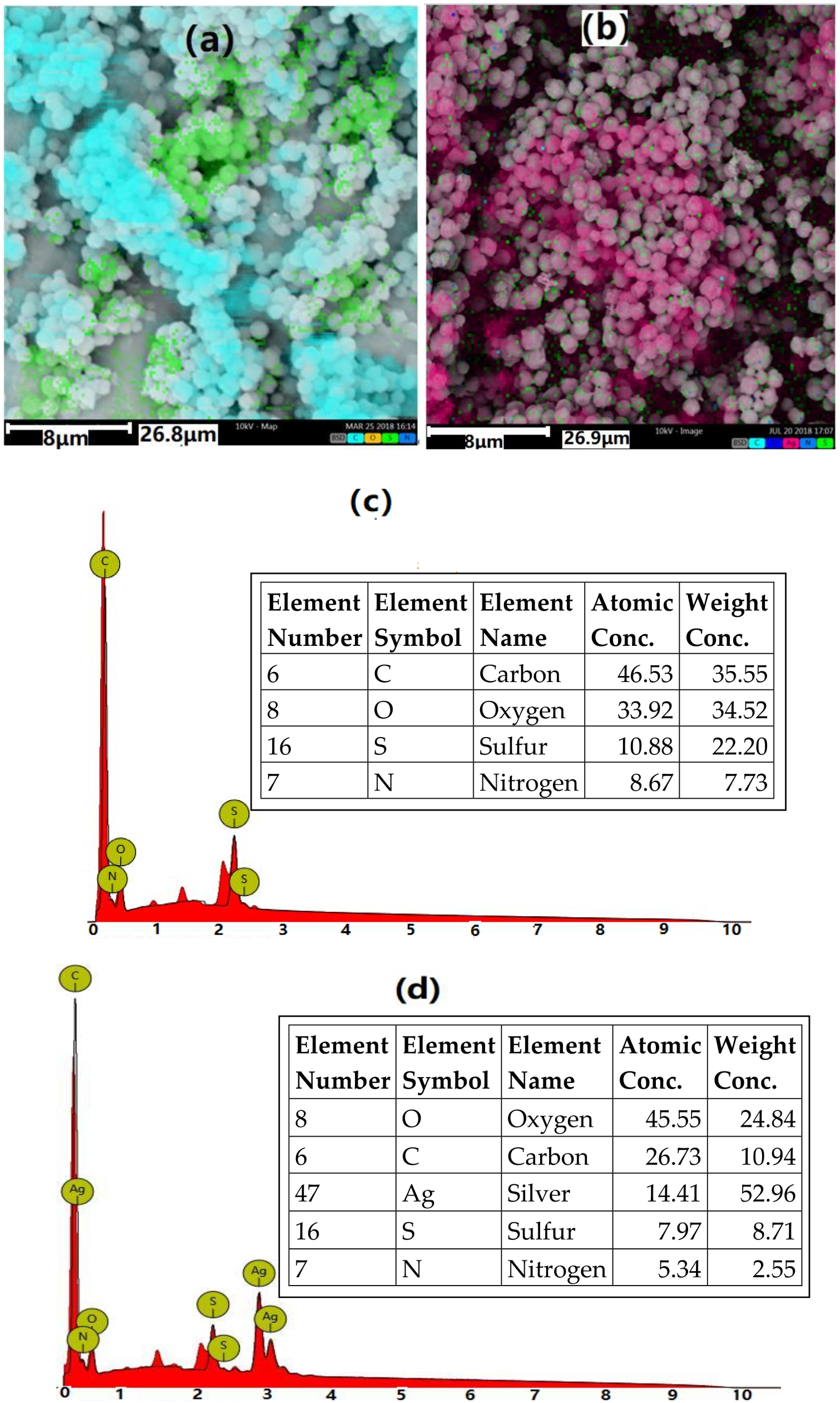
3.1. Characterization of BA-PGMA
3.2. Effect of Experimental Parameters on Adsorption of Ag(I)
3.2.1. Effect of pH
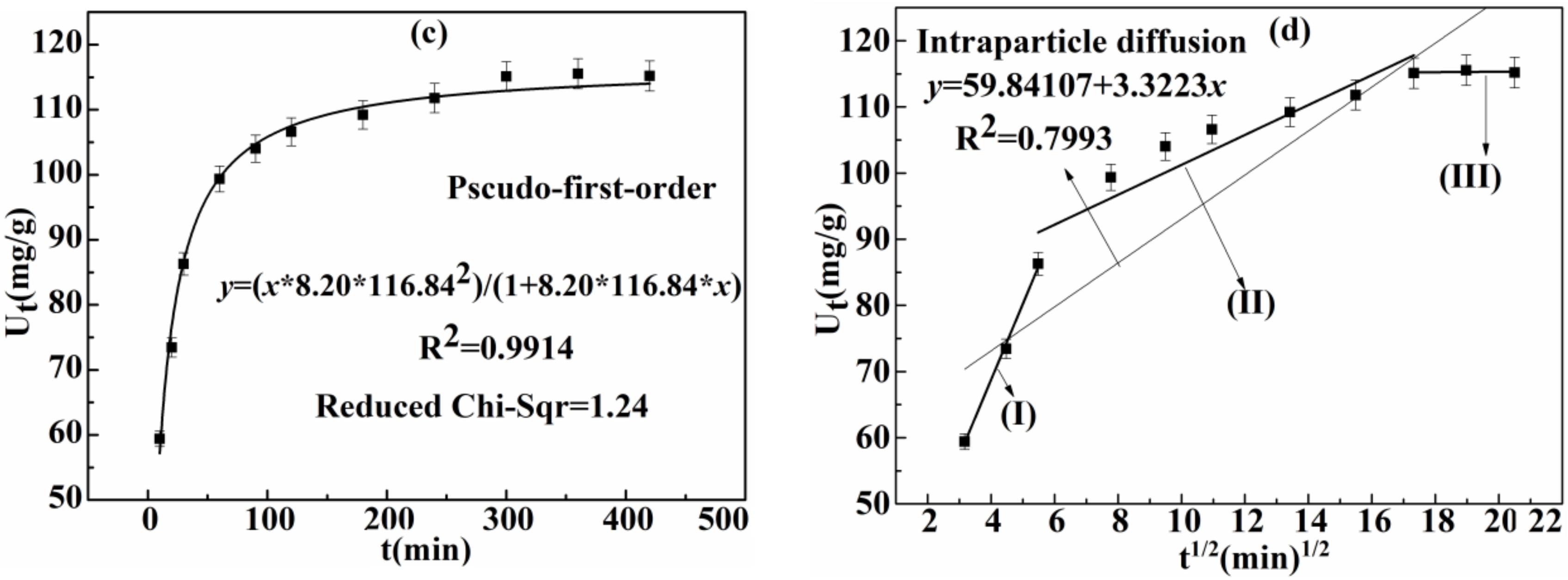
3.2.2. Effect of Time on Adsorption Capacity of Ag(I) and Kinetics
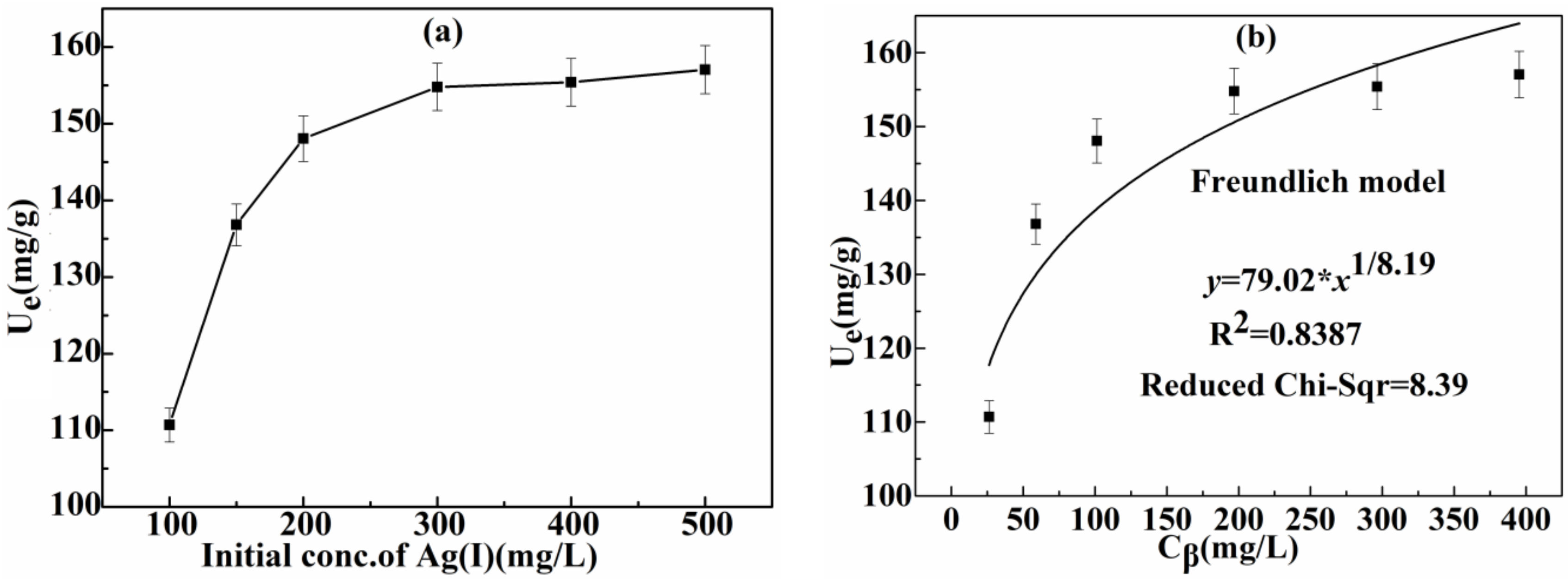
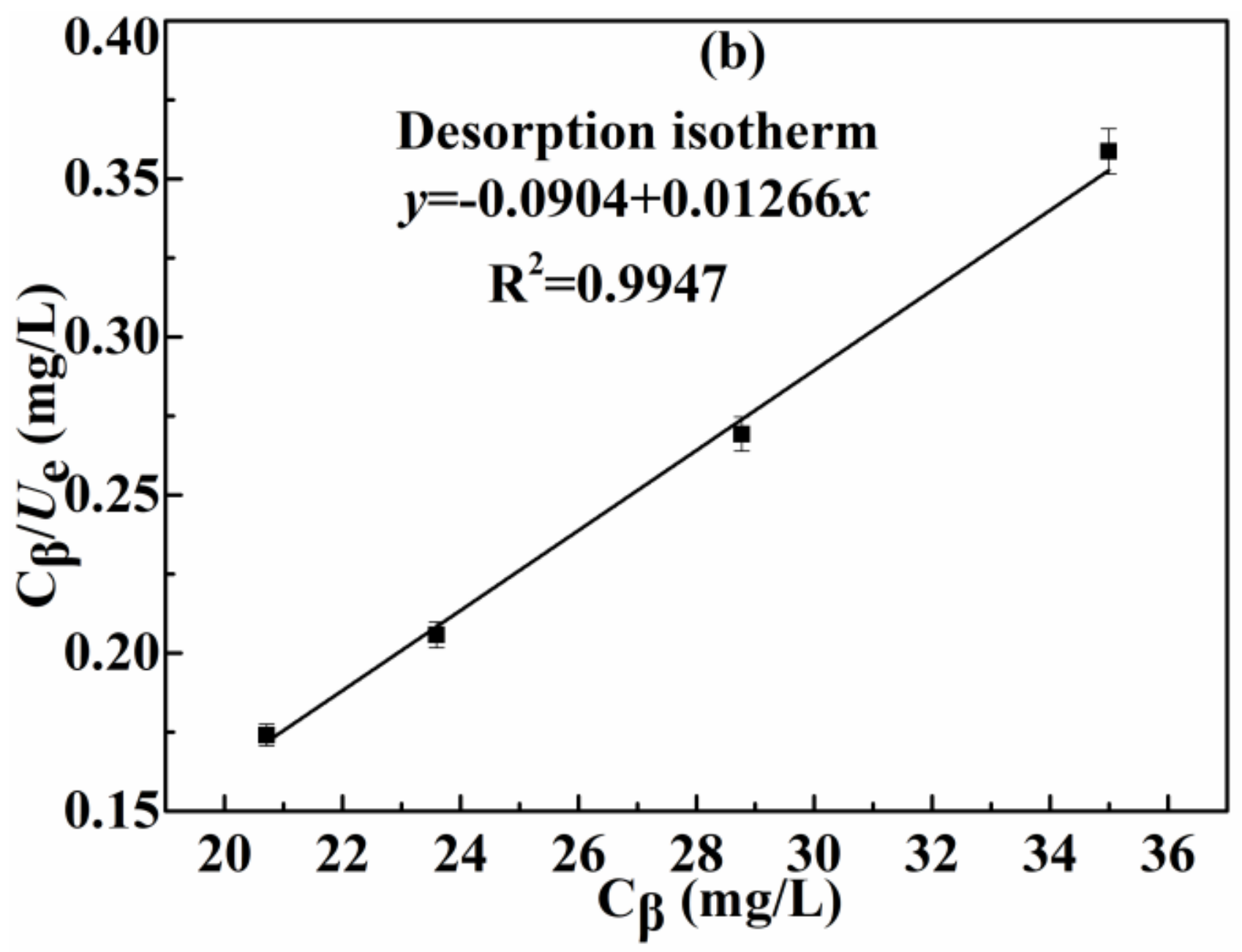
3.2.3. Effect of Initial Concentration of Ag(I) and Adsorption Isotherm
3.2.4. Selectivity of BA-PGMA for Ag(I)
3.2.5. Reuse Performance of BA-PGMA
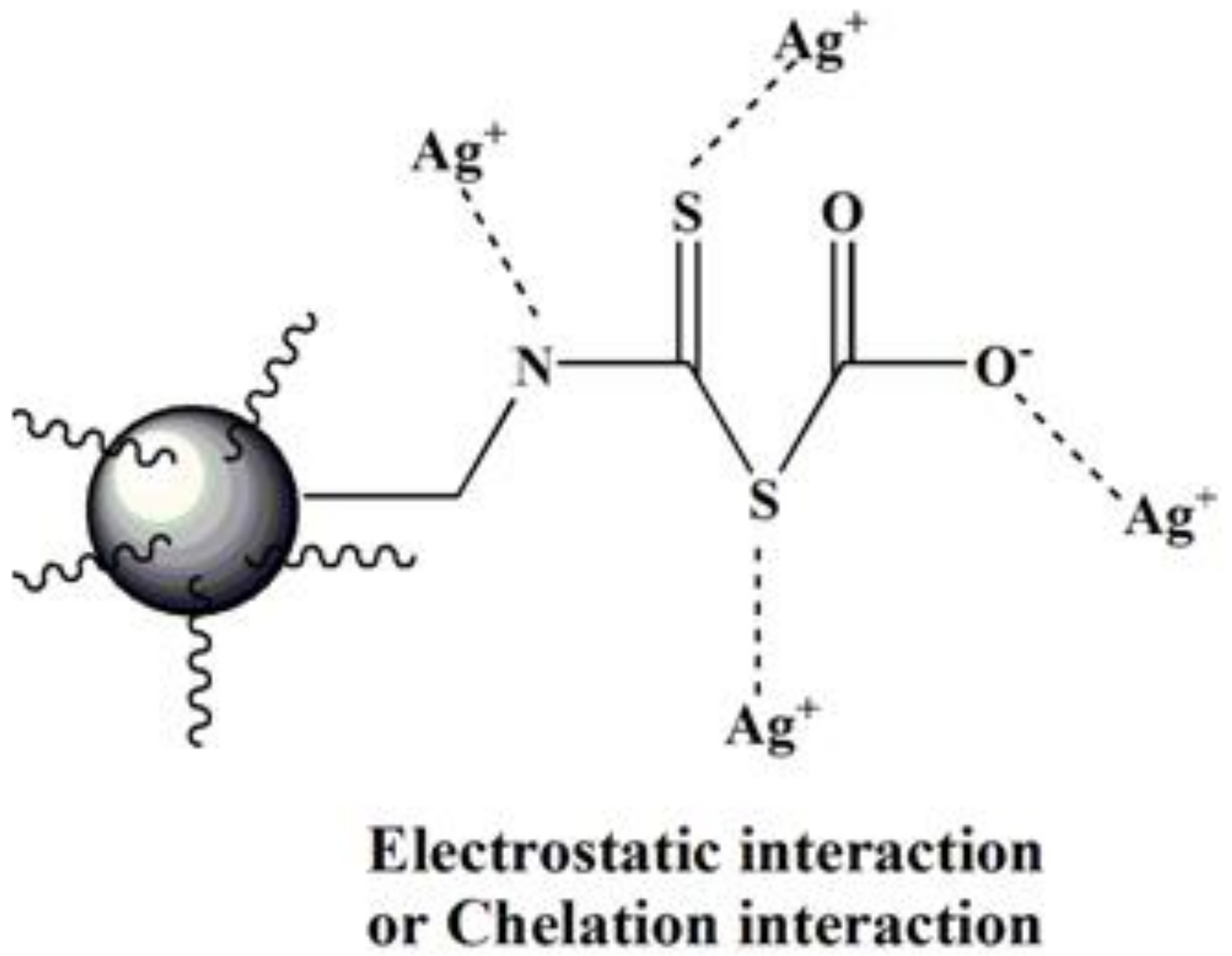
3.2.6. Adsorption Mechanism of Ag(I) by BA-PGMA
3.2.7. Thermodynamic Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farahi, R.H.; Passian, A.; Tetard, L.; Thundat, T. Critical issues in sensor science to aid food and water safety. ACS Nano 2012, 6, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, I.; Tripathi, B.N. Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment. Chemosphere 2011, 82, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.P.; Lu, C.; Su, F. Sorption of divalent metal ions from aqueous solution by carbon nanotubes: A review. Sep. Purif. Technol. 2007, 58, 224–231. [Google Scholar] [CrossRef]
- Blaser, S.A.; Scheringer, M.; Macleod, M.; Hungerbühler, K. Estimation of cumulative aquatic exposure and risk due to silver: Contribution of nano-functionalized plastics and textiles. Sci. Total Environ. 2008, 390, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.C.; Gao, Z.Y.; Ding, H.; Xu, N.; Wu, W.M. Recovery of silver from silver(I)-containing solutions in bioelectrochemical reactors. Bioresour. Technol. 2012, 111, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Li, W.; Jing, X.; Wang, Y.; Xing, Z.; Shan, W.; Lou, Z. Selective recovery of Ag(I) coordination anion from simulate nickel electrolyte using corn stalk based adsorbent modified by ammonia–thiosemicarbazide. J. Hazard. Mater. 2016, 301, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.Y.; Hong, J.Y.; Jang, J. Heavy metal ion adsorption behavior in nitrogen-doped magnetic carbon nanoparticles: Isotherms and kinetic study. J. Hazard. Mater. 2011, 190, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C. Adsorption of silver ions from industrial wastewater using waste coffee grounds. Korean J. Chem. Eng. 2016, 34, 1–8. [Google Scholar] [CrossRef]
- Wajima, T. Synthesis of zeolitic material from green tuff stone cake and its adsorption properties of silver (I) from aqueous solution. Microporous Mesoporous Mater. 2016, 233, 154–162. [Google Scholar] [CrossRef]
- Bianchini, A.; Wood, C.M. Mechanism of acute silver toxicity in Daphnia magna. Environ. Toxicol. Chem. 2003, 22, 1361–1367. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Hubicki, Z. Equilibrium and kinetic studies on the sorption of acidic dye by macro porous anion exchange. Chem. Eng. J. 2010, 157, 29–34. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Han, T.; Zhong, L.; Ma, C. Improvement of Ag(I) adsorption onto chitosan/triethanolamine composite sorbent by an ion-imprinted technology. Appl. Surf. Sci. 2012, 263, 696–703. [Google Scholar] [CrossRef]
- Tang, B.; Yu, G.; Fang, J.; Shi, T. Recovery of high-purity silver directly from dilute effluents by an emulsion liquid membrane-crystallization process. J. Hazard. Mater. 2010, 177, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Virolainen, S.; Tyster, M.; Haapalainen, M.; Sainio, T. Ion exchange recovery of silver from concentrated base metal-chloride solutions. Hydrometallurgy 2015, 152, 100–106. [Google Scholar] [CrossRef]
- Das, N. Recovery of precious metals through biosorption—A review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Ngah, W.S.; Fatinathan, S. Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: Kinetic, equilibrium and thermodynamic studies. J. Environ. Manag. 2010, 91, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Dang, Q.; Liu, C.; Cha, D.; Zhang, H.; Zhu, W.; Zhang, Q.; Fan, B. Preparation and characterization of poly(maleic acid)-grafted cross-linked chitosan microspheres for Cd(II) adsorption. Carbohydr. Polym. 2017, 172, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.Q.; Lee, J.E.; Kim, J.K.; You, N.K.; Shao, G.N.; Kim, H.T. A gentle method to graft thiol-functional groups onto silica gel for adsorption of silver ions and immobilization of silver nanoparticles. Powder Technol. 2013, 235, 221–227. [Google Scholar]
- Liu, P.; Sehaqui, H.; Tingaut, P.; Wichser, A.; Oksman, K.; Mathew, A.P. Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose 2014, 21, 449–461. [Google Scholar] [CrossRef]
- Wang, L.; Xing, R.; Liu, S.; Yu, H.; Qin, Y.; Li, K.; Feng, J.; Li, R.; Li, P. Recovery of silver (I) using a thiourea-modified chitosan resin. J. Hazard. Mater. 2010, 180, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Madrakian, T.; Afkhami, A.; Zolfigol, M.A.; Solgi, M. Separation, preconcentration and determination of silver ion from water samples using silica gel modified with 2,4,6-trimorpholino-1,3,5-triazin. J. Hazard. Mater. 2006, 128, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Duranoğlu, D.; Kaya, İ.G.B.; Beker, U.; Şenkal, B.F. Synthesis and adsorption properties of polymeric and polymer-based hybrid adsorbent for hexavalent chromium removal. Chem. Eng. J. 2012, s181–182, 103–112. [Google Scholar] [CrossRef]
- Niu, L.; Deng, S.B.; Yu, G.; Huang, J. Efficient removal of Cu(II), Pb(II), Cr(VI) and As(V) from aqueous solution using an aminated resin prepared by surface-initiated atom transfer radical polymerization. Chem. Eng. J. 2010, 165, 751–757. [Google Scholar] [CrossRef]
- Gupta, A.; Jain, R.; Gupta, D.C. Studies on uptake behaviour of Hg (II) and Pb(II) by amine modified glycidyl methacrylate-styrene-N, N’-methylene bis-acrylamide terpolymer. React. Funct. Polym. 2015, 93, 22–29. [Google Scholar] [CrossRef]
- Dou, X.B.; Chai, M.Y.; Zhu, Y.; Yang, W.T.; Xu, F.J. Aminated Poly(glycidyl methacrylate)s for Constructing Efficient Gene Carriers. ACS Appl. Mater. Interfaces 2013, 5, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bai, R. Extended study of DETA-functionalized PGMA adsorbent in the selective adsorption behaviors and mechanisms for heavy metal ions of Cu, Co, Ni, Zn, and Cd. J. Colloid Interface Sci. 2010, 350, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, F.; Yao, M.; Qiu, T.; Jiang, W.; Fan, L.J. Atom transfer radical polymerization of glycidyl methacrylate followed by amination on the surface of monodispersed highly crosslinked polymer microspheres and the study of cation adsorption. React. Funct. Polym. 2014, 82, 66–71. [Google Scholar] [CrossRef]
- Liu, C.; Bai, R.; Hong, L. Diethylenetriamine-grafted poly(glycidyl methacrylate) adsorbent for effective copper ion adsorption. J. Colloid Interface Sci. 2006, 303, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Huš, S.; Kolar, M.; Krajnc, P. Separation of heavy metals from water by functionalized glycidyl methacrylate poly (high internal phase emulsions). J. Chromatogr. A 2016, 1437, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, L.; Xing, H.; Zhao, J.; Li, X.; Huang, Y.; Liu, H. High capacity adsorption of Cr(VI) from aqueous solution using polyethylenimine-functionalized poly(glycidyl methacrylate) microspheres. Colloid Surf. A 2014, 457, 160–168. [Google Scholar] [CrossRef]
- Yang, W.; Yun, Z.; Chen, H.; He, X.; Liu, M. Preparation of a novel TETA functionalized magnetic PGMA nano-absorbent by ATRP method and used for highly effective adsorption of Hg(II). J. Taiwan Inst. Chem. Eng. 2015, 58, 283–289. [Google Scholar]
- Liu, W.; Yin, P.; Liu, X.; Dong, X.; Zhang, J.; Xu, Q. Thermodynamics, kinetics, and isotherms studies for gold(III) adsorption using silica functionalized by diethylenetriamine methylene phosphonic acid. Chem. Eng. Res. Des. 2013, 91, 2748–2758. [Google Scholar] [CrossRef]
- Hong, S.G. The thermal-oxidative degradation of an epoxy adhesive on metal substrates: XPS and RAIR analyses. Polym. Degrad. Stab. 1995, 48, 211–218. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Wang, S.; Liu, B.; Peng, J. Selective removal of cationic dyes from aqueous solutions by an activated carbon-based multicarboxyl adsorbent. RSC Adv. 2015, 5, 99618–99626. [Google Scholar] [CrossRef]
- Castillo, M.; Criado, A.; Guzmán, R.; Criado, J.J.; Macias, B. Chemistry of dithiocarbamate derivatives of amino acids. Part III. X-ray photoelectron spectroscopy of Ba(S2CNHCH2CO2)·3H2O. I.r. and e.s.r. studies of α-amino acid-dithiocarbamate complexes of copper(II). Transit. Metal Chem. 1987, 12, 225–229. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Chang, Y.Y.; Yang, J.K.; Singh, J.; Choi, E.H.; Shiratani, M.; Koduru, J.R.; Attri, P. Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem. Eng. J. 2017, 307, 74–84. [Google Scholar] [CrossRef]
- Tanzifi, M.; Hosseini, S.H.; Kiadehi, A.D.; Olazar, M.; Karimipour, K.; Rezaiemehr, R.; Ali, I. Artificial neural network optimization for methyl orange adsorption onto polyaniline nano-adsorbent: Kinetic, isotherm and thermodynamic studies. J. Mol. Liq. 2017, 244, 189–200. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Moon, H.; Lee, W.K. Intraparticle diffusion in liquid-phase adsorption of phenols with activated carbon in finite batch adsorber. J. Colloid Interface Sci. 1983, 96, 162–171. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Naiya, T.K.; Mandal, S.N.; Das, S.K. Adsorption, kinetics and equilibrium studies on removal of Cr(VI) from aqueous solutions using different low-cost adsorbents. Chem. Eng. J. 2008, 137, 529–541. [Google Scholar] [CrossRef]
- Wang, C.; Boithias, L.; Ning, Z.; Han, Y.; Sauvage, S.; Sánchez-Pérez, J.M.; Kuramochi, K. Comparison of Langmuir and Freundlich adsorption equations within the SWAT-K model for assessing potassium environmental losses at basin scale. Agric. Water Manag. 2016, 180, 205–211. [Google Scholar] [CrossRef]
- Park, H.S.; Koduru, J.R.; Choo, K.H.; Lee, B. Activated carbons impregnated with iron oxide nanoparticles for enhanced removal of bisphenol A and natural organic matter. J. Hazard. Mater. 2015, 286, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Koduru, J.R.; Lingamdinne, L.P.; Singh, J.; Choo, K.H. Effective removal of bisphenol A (BPA) from water using a goethite/activated carbon composite. Process Saf. Environ. 2016, 103, 87–96. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Padmesh, T.V.N.; Palanivelu, K.; Velan, M. Biosorption of nickel(II) ions onto Sargassum wightii: Application of two-parameter and three-parameter isotherm models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Zhao, Z.; Li, Y.; Shan, W.; Xiong, Y.; Fang, D.; Yue, S. Contribution of tertiary amino groups to Re(VII) biosorption on modified corn stalk: Competitiveness and regularity. Bioresour. Technol. 2013, 133, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Choi, Y.L.; Kim, I.S.; Yang, J.K.; Koduru, J.R.; Chang, Y.Y. Preparation and characterization of porous reduced graphene oxide based inverse spinel nickel ferrite nanocomposite for adsorption removal of radionuclides. J. Hazard. Mater. 2016, 326, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Iranpoor, N.; Saghir, N. Directly silica bonded analytical reagents: Synthesis of 2-mercaptobenzothiazole–silica gel and its application as a new sorbent for preconcentration and determination of silver ion using solid-phase extraction method. Sep. Purif. Technol. 2004, 40, 303–308. [Google Scholar] [CrossRef]
- Coruh, S.; Senel, G.; Ergun, O.N. A comparison of the properties of natural clinoptilolites and their ion-exchange capacities for silver removal. J. Hazard. Mater. 2010, 180, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Borrell, P.F.; Kokol, V.; Oksman, K.; Mathew, A.P. Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+ and Fe3+ from industrial effluents. J. Hazard. Mater. 2015, 294, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Gülfen, M. Separation and Recovery of Silver(I) Ions from Base Metal Ions by Melamine-formaldehyde-thiourea (MFT) Chelating Resin. Sep. Sci. Technol. 2008, 43, 376–388. [Google Scholar]
- Gülfen, M. Separation and Recovery of Silver(I) Ions from Base Metal Ions by Thiourea- or Urea-Formaldehyde Chelating Resin. Sep. Sci. Technol. 2009, 44, 1869–1883. [Google Scholar]
- Hou, H.; Yu, D.; Hu, G. Preparation and properties of ion-imprinted hollow particles for the selective adsorption of silver ions. Langmuir 2015, 31, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Helleur, R.; Zhang, Y. Ion-imprinted chitosan gel beads for selective adsorption of Ag+ from aqueous solutions. Carbohydr. Polym. 2015, 130, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Yurtsever, M.; Şengil, A. Adsorption and desorption behavior of silver ions onto valonia tannin resin. Trans. Nonferrous Metals Soc. China 2012, 22, 2846–2854. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, S.; Zhang, L.; Zhou, Y.; Peng, J. Selective recovery of silver from aqueous solutions by poly (glycidyl methacrylate) microsphere modified with trithiocyanuric acid. J. Mol. Liq. 2018, 254, 340–348. [Google Scholar] [CrossRef]
- Khan, M.A.; Wallace, W.T.; Islam, S.Z.; Nagpure, S.; Strzalka, J.; Littleton, J.M.; Rankin, S.E. Adsorption and Recovery of Polyphenolic Flavonoids Using TiO2 Functionalized Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 32114–32125. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, A.; Oleszczuk, P.; Dobrowolski, R. Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water. Bioresour. Technol. 2015, 196, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, G.; Wang, S.; Peng, J.; Cui, W. Sulfoethyl functionalized silica nanoparticle as an adsorbent to selectively adsorb silver ions from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 71, 330–337. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, L.; Wang, S.; Zhang, G.; Peng, J. Selective recovery of Au(III) from aqueous solutions by nano-silica grafted with 4-(aminomethyl) pyridine. J. Sol-Gel Sci. Technol. 2017, 83, 467–477. [Google Scholar] [CrossRef]
- Gavioli, E.; Maier, N.M.; Haupt, K.; Mosbach, K.; Lindner, W. Analyte templating: Enhancing the enantioselectivity of chiral selectors upon incorporation into organic polymer environments. Anal. Chem. 2005, 77, 5009–5018. [Google Scholar] [CrossRef] [PubMed]
- Lingamdinne, L.P.; Koduru, J.R.; Roh, H.; Choi, Y.L.; Chang, Y.Y.; Yang, J.K. Adsorption removal of Co (II) from waste-water using graphene oxide. Hydrometallurgy 2016, 165, 90–96. [Google Scholar] [CrossRef]
- Pang, S.K.; Yung, K.C. Prerequisites for achieving gold adsorption by multiwalled carbon nanotubes in gold recovery. Chem. Eng. Sci. 2014, 107, 58–65. [Google Scholar] [CrossRef]




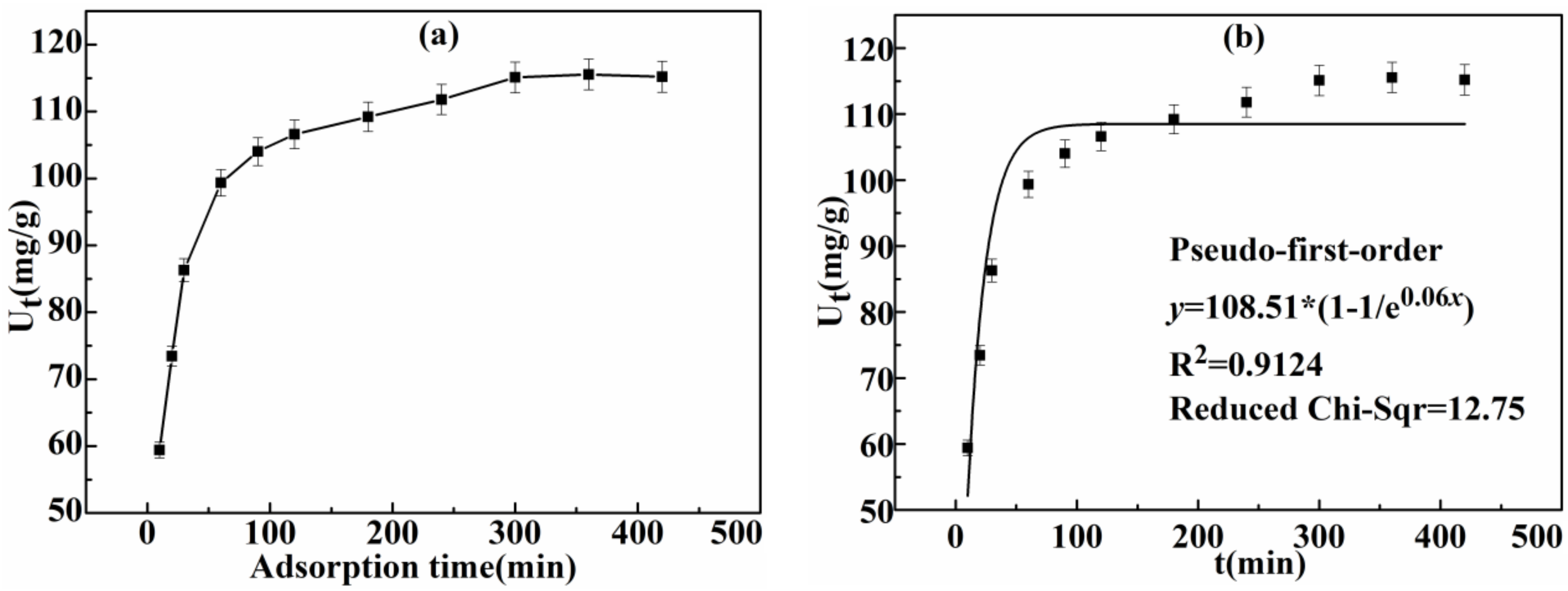






| First-Order Model | Ka (g/(mg·min)) | Ue (mg/g) | R2 Reduced Chi-Square |
| 0.06 | 108.51 | 0.9124 12.75 | |
| Second-Order Model | Kb (g/(mg·min)) | Ue (mg/g) | R2 Reduced Chi-Square |
| 8.20 | 116.84 | 0.9914 1.24 | |
| Intra-Particle Diffusion Model | Kc (mg/(g·min1/2) | R2 | |
| 3.32 | 0.7993 | ||
| Stage | Kc | R2 | C |
| I | 11.46 | 0.9953 | 22.95 |
| II | 2.16 | 0.8583 | 78.62 |
| III | 0.03 | −0.8881 | 114.61 |
| Freundlich | Kh | n | R2 Reduced Chi-Sqr |
| 79.02 | 8.19 | 0.8387 8.39 | |
| Langmuir | Um (mg/g) | Ki | R2 Reduced Chi-Sqr |
| 163.46 | 0.08 | 0.9927 0.37 | |
| Temkin | Kj | VT | R2 Reduced Chi-Sqr |
| 29.22 | 17.49 | 0.8747 6.52 | |
| Hill | KL | N | R2 Reduced Chi-Sqr |
| 12.14 | 0.91 | 0.9848 0.78 | |
| D-R | ω | E (kj/mol) | R2 Reduced Chi-Sqr |
| 0.0041 | 11.04 | 0.9974 0.13 |
| Cα (mg/L) | RL |
|---|---|
| 100 | 0.0808 |
| 150 | 0.0554 |
| 200 | 0.0421 |
| 300 | 0.0285 |
| 400 | 0.0215 |
| 500 | 0.0172 |
| Sorbent | Um (mg/g) | Reference |
|---|---|---|
| Natural Clinoptilolites | 31.44 | [48] |
| Active carbon | 32.80 | [49] |
| Nanocelluloses and their phosphorylated derivatives | 56 | [50] |
| MFT chelating resin | 60.05 | [51] |
| Bentonite clay | 61.48 | [52] |
| Ion-imprinted chitosan gel beads | 80.50 | [53] |
| Ag+-imprinted chitosan gel bead | 89.20 | [54] |
| Valonia tannin resin | 97.08 | [55] |
| BA-PGMA | 157.05 | This work |
| Coexisting Ions | Kf (mL/g) | K |
|---|---|---|
| Ag+ | 4097.0 | - |
| Ni2+ | 81.3 | 50.4 |
| Co2+ | 41.9 | 97.8 |
| Zn2+ | 97.4 | 42.1 |
| Cu2+ | 409.1 | 10.0 |
| Initial Ag(I) Concentration | T (K) | ΔSθ (J∙K−1∙moL−1) | ΔHθ (kJ∙moL−1) | ΔGθ (kJ∙moL−1) |
|---|---|---|---|---|
| 100 (mg/L) | 25 °C (298 K) | 163.95 | 28.22 | −20.68 |
| 35 °C (303 K) | −21.79 | |||
| 45 °C (313 K) | - | −23.19 | ||
| 200 (mg/L) | 25 °C (298 K) | 159.7 | 29.60 | −18.05 |
| 35 °C (303 K) | −19.25 | |||
| 45 °C (313 K) | - | −20.48 | ||
| 300 (mg/L) | 25 °C (298 K) | 147.73 | 27.52 | −16.50 |
| 35 °C (303 K) | −17.71 | |||
| 45 °C (313 K) | - | −18.74 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Wang, S.; Zhang, L.; Wang, C.; Zhang, B. Kinetic, Isotherm, and Thermodynamic Studies for Ag(I) Adsorption Using Carboxymethyl Functionalized Poly(glycidyl methacrylate). Polymers 2018, 10, 1090. https://doi.org/10.3390/polym10101090
Zhao J, Wang S, Zhang L, Wang C, Zhang B. Kinetic, Isotherm, and Thermodynamic Studies for Ag(I) Adsorption Using Carboxymethyl Functionalized Poly(glycidyl methacrylate). Polymers. 2018; 10(10):1090. https://doi.org/10.3390/polym10101090
Chicago/Turabian StyleZhao, Jiling, Shixing Wang, Libo Zhang, Chen Wang, and Bing Zhang. 2018. "Kinetic, Isotherm, and Thermodynamic Studies for Ag(I) Adsorption Using Carboxymethyl Functionalized Poly(glycidyl methacrylate)" Polymers 10, no. 10: 1090. https://doi.org/10.3390/polym10101090






