Sustainable, Low Flammability, Mechanically-Strong Poly(vinyl alcohol) Aerogels
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Aerogels
2.3. Characterizations
3. Results and Discussion
3.1. Mechanical Properties
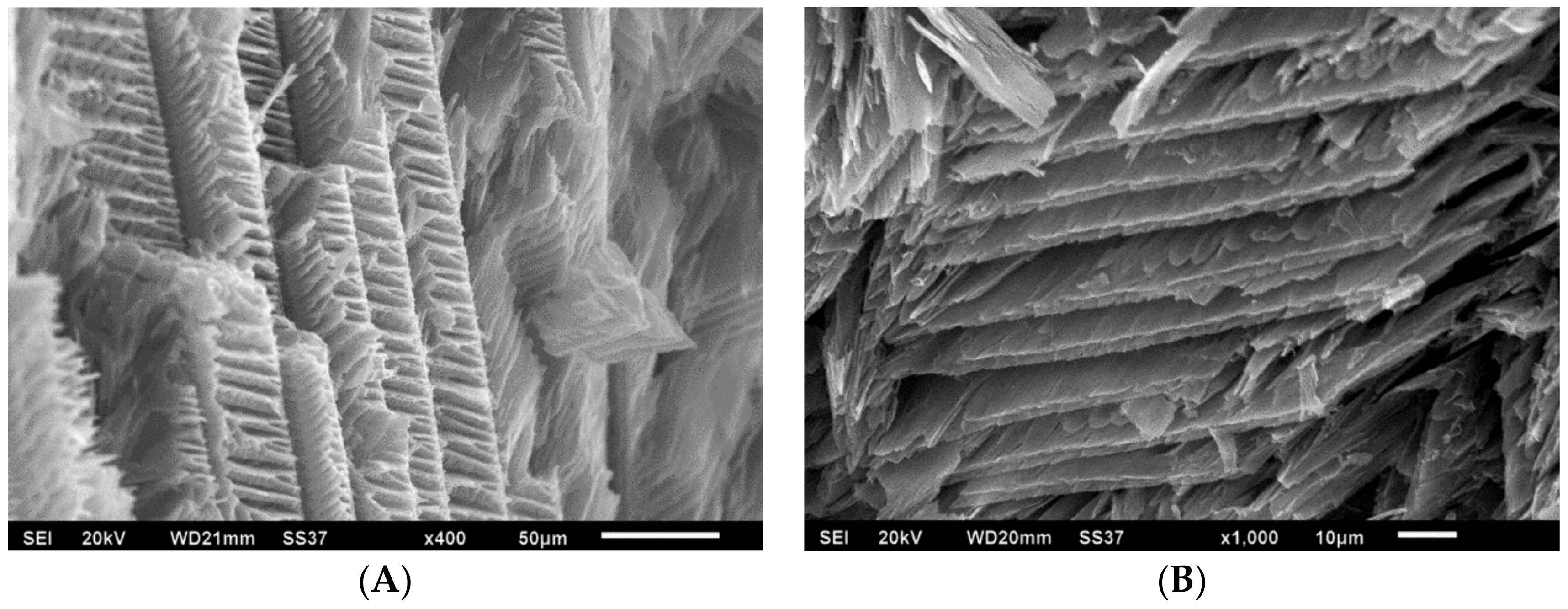
3.2. Morphology
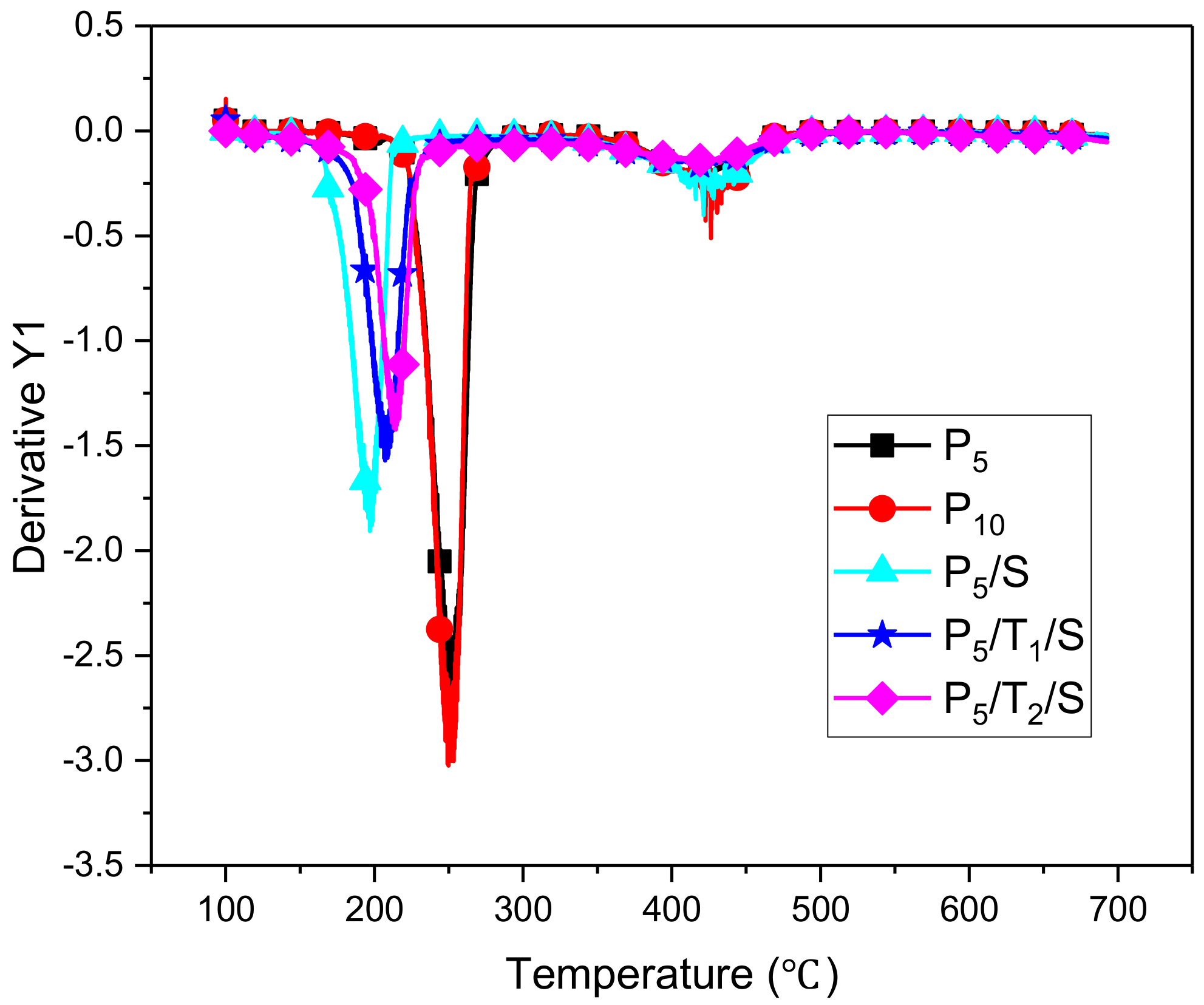
3.3. Thermal Stability
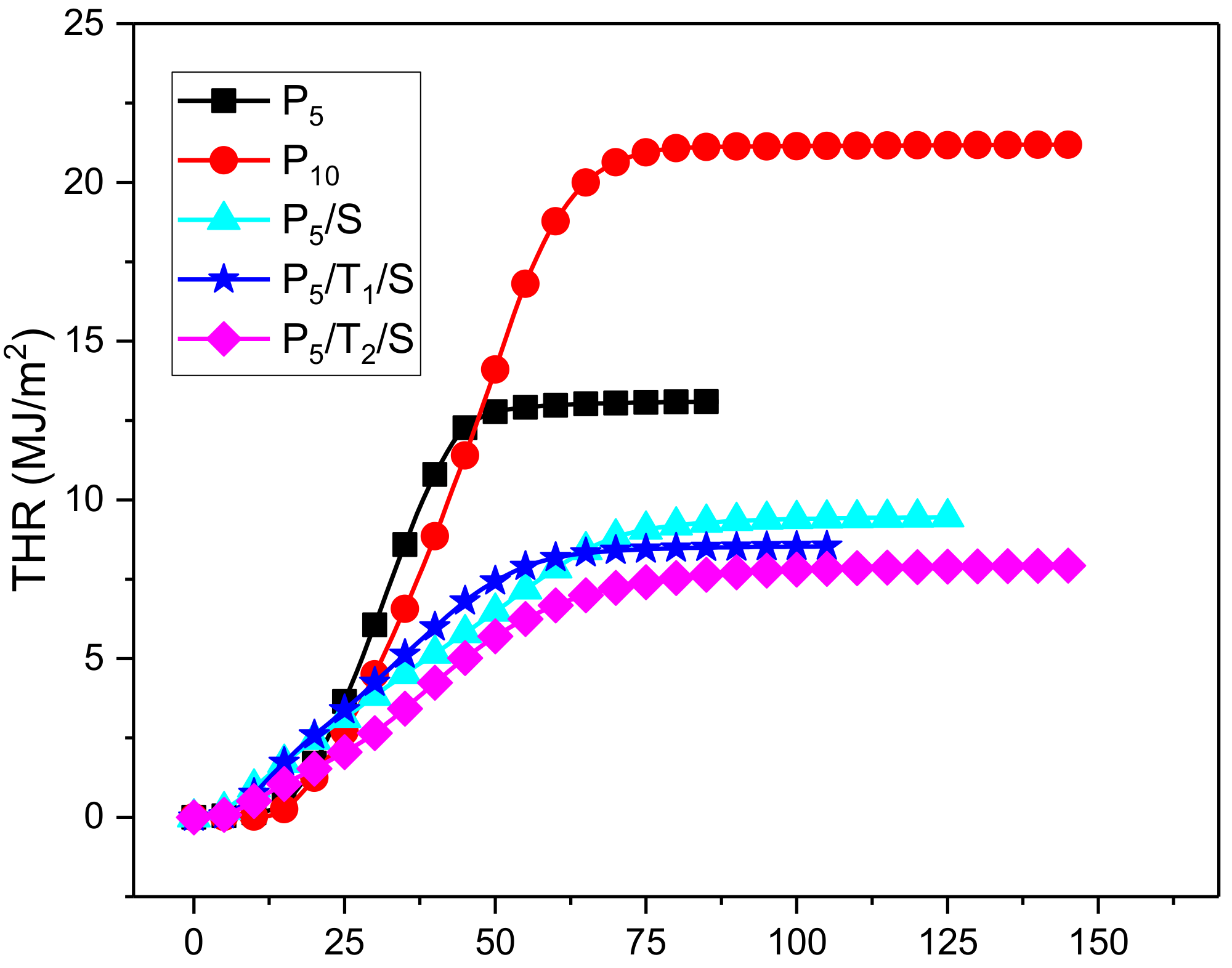
3.4. Combustion Behavior
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Bandi, S.; Schiraldi, D.A. Glass Transition Behavior of Clay Aerogel/Poly (Vinyl Alcohol) Composites. Macromolecules 2006, 39, 6537–6545. [Google Scholar] [CrossRef]
- Aaltonen, O.; Jauhiainen, O. The Preparation of Lignocellulosic Aerogels from Ionic Liquid Solutions. Carbohydr. Polym. 2009, 75, 125–129. [Google Scholar] [CrossRef]
- Zou, J.; Liu, J.; Karakoti, A.S.; Kumar, A.; Joung, D.; Li, Q.; Khondaker, S.I.; Seal, S.; Zhai, L. Ultralight Multiwalled Carbon Nanotube Aerogel. ACS Nano 2010, 4, 7293–7302. [Google Scholar] [CrossRef] [PubMed]
- Haq, E.U.; Zaidi, S.F.A.; Zubair, M.; Karim, M.R.A.; Padmanabhan, S.K.; Licciulli, A. Hydrophobic Silica Aerogel Glass-Fibre Composite with Higher Strength and Thermal Insulation Based on Methyltrimethoxysilane (MTMS) Precursor. Energy Build. 2017, 151, 494–500. [Google Scholar] [CrossRef]
- Gawryla, M.D.; Schiraldi, D.A. Novel Absorbent Materials Created Via Ice Templating. Macromol. Mater. Eng. 2009, 294, 570–574. [Google Scholar] [CrossRef]
- Xia, W.; Qu, C.; Liang, Z.; Zhao, B.; Dai, S.; Qiu, B.; Jiao, Y.; Zhang, Q.; Huang, X.; Guo, W. High-Performance Energy Storage and Conversion Materials Derived from a Single Metal–organic Framework/Graphene Aerogel Composite. Nano Letters 2017, 17, 2788–2795. [Google Scholar] [CrossRef] [PubMed]
- Pajonk, G.M. Aerogel Catalysts. Appl. Catal. 1991, 72, 217–266. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, L.; Huang, Y.; Zhang, L.; Lai, F.; Fan, W.; Liu, T. In-Situ Growth of Few-Layered MoS2 Nanosheets on Highly Porous Carbon Aerogel as Advanced Electrocatalysts for Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2015, 3, 3140–3148. [Google Scholar] [CrossRef]
- Yu, Z.; McInnis, M.; Calderon, J.; Seal, S.; Zhai, L.; Thomas, J. Functionalized Graphene Aerogel Composites for High-Performance Asymmetric Supercapacitors. Nano Energy 2015, 11, 611–620. [Google Scholar] [CrossRef]
- Arndt, E.M.; Gawryla, M.D.; Schiraldi, D.A. Elastic, Low Density Epoxy/Clay Aerogel Composites. J. Mater. Chem. 2007, 17, 3525–3529. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Li, P.; Gao, C. Strong, Conductive, Lightweight, Neat Graphene Aerogel Fibers with Aligned Pores. ACS Nano 2012, 6, 7103–7113. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Du, B.; Wang, E. Self-Crosslink Method for a Straightforward Synthesis of Poly (Vinyl Alcohol)-Based Aerogel Assisted by Carbon Nanotube. Adv. Funct. Mater. 2017, 27, 1604423. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Schiraldi, D.A. Preparation and Flammability of Poly (Vinyl Alcohol) Composite Aerogels. ACS Appl. Mater. Interfaces 2014, 6, 6790–6796. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.; Degracia, K.; Han, L.; Sun, H.; Sun, M.; Wang, Y.; Schiraldi, D.A. Green Approach to Improving the Strength and Flame Retardancy of Poly(Vinyl Alcohol)/Clay Aerogels: Incorporating Biobased Gelatin. ACS Appl. Mater. Interfaces 2017, 9, 42258–42265. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.S.; Peppas, N.A. Mesh Size and Diffusive Characteristics of Semicrystalline Poly (Vinyl Alcohol) Membranes Prepared by Freezing/Thawing Techniques. J. Membr. Sci. 1995, 107, 229–237. [Google Scholar] [CrossRef]
- Chen, H.; Hollinger, E.; Wang, Y.; Schiraldi, D.A. Facile Fabrication of Poly (Vinyl Alcohol) Gels and Derivative Aerogels. Polymer 2014, 55, 380–384. [Google Scholar] [CrossRef]
- Chen, H.; Liu, B.; Huang, W.; Wang, J.; Zeng, G.; Wu, W.; Schiraldi, D.A. Fabrication and Properties of Irradiation-Cross-Linked Poly (Vinyl Alcohol)/Clay Aerogel Composites. ACS Appl. Mater. Interfaces 2014, 6, 16227–16236. [Google Scholar] [CrossRef] [PubMed]
- Immelman, E.; Bezuidenhout, D.; Sanderson, R.D.; Jacobs, E.P.; van Reenen, A.J. Poly(Vinyl Alcohol) Gel Sub-Layers for Reverse Osmosis Membranes. III. Insolubilization by Crosslinking with Potassium Peroxydisulphate. Desalination 1993, 94, 115–132. [Google Scholar] [CrossRef]
- Trochimczuk, A.W.; Kabay, N.; Arda, M.; Streat, M. Stabilization of Solvent Impregnated Resins (SIRs) by Coating with Water Soluble Polymers and Chemical Crosslinking. React. Funct. Polym. 2004, 59, 1–7. [Google Scholar] [CrossRef]
- Liu, A.; Medina, L.; Berglund, L.A. High-Strength Nanocomposite Aerogels of Ternary Composition: Poly (Vinyl Alcohol), Clay, and Cellulose Nanofibrils. ACS Appl. Mater. Interfaces 2017, 9, 6453–6461. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sanchez-Soto, M.; Maspoch, M.L. Polymer/Clay Aerogel Composites with Flame Retardant Agents: Mechanical, Thermal and Fire Behavior. Mater. Des. 2013, 52, 609–614. [Google Scholar] [CrossRef]
- Gilman, J.W.; Ritchie, S.J.; Kashiwagi, T.; Lomakin, S.M. Fire-retardant Additives for Polymeric materials—I. Char Formation from Silica Gel–potassium Carbonate. Fire Mater. 1997, 21, 23–32. [Google Scholar] [CrossRef]
- Tributsch, H.; Fiechter, S. The Material Strategy of Fire-Resistant Tree Barks. High Performance Structures and Materials IV. WIT Trans. Built Environ. 2008, 97, 43–52. [Google Scholar]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- Xia, Z.; Singh, A.; Kiratitanavit, W.; Mosurkal, R.; Kumar, J.; Nagarajan, R. Unraveling the Mechanism of Thermal and Thermo-Oxidative Degradation of Tannic Acid. Thermochim. Acta 2015, 605, 77–85. [Google Scholar] [CrossRef]
- Darnerud, P.O. Toxic Effects of Brominated Flame Retardants in Man and in Wildlife. Environ. Int. 2003, 29, 841–853. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, L.; Liu, T.; Wang, Y.; Shi, S.; Wang, H. Poly (Vinyl Alcohol)–tannic Acid Hydrogels with Excellent Mechanical Properties and Shape Memory Behaviors. ACS Appl. Mater. Interfaces 2016, 8, 27199–27206. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H. Polyvinyl Alcohol/Tannic Acid Hydrogel Prepared by a Freeze-Thawing Process for Wound Dressing Applications. Polym. Bull. 2017, 74, 2861–2872. [Google Scholar] [CrossRef]
- Alexy, P.; Kachova, D.; Kršiak, M.; Bakoš, D.; Šimková, B. Poly (Vinyl Alcohol) Stabilisation in Thermoplastic Processing. Polym. Degrad. Stab. 2002, 78, 413–421. [Google Scholar] [CrossRef]
- Chu, W.; Yang, J.; Liu, T.; Tiu, C.; Guo, J. The Effects of pH, Molecular Weight and Degree of Hydrolysis of Poly (Vinyl Alcohol) on Slot Die Coating of PVA Suspensions of TiO2 and SiO2. Colloids Surf. Physicochem. Eng. Aspects 2007, 302, 1–10. [Google Scholar] [CrossRef]
- Nam, S.; Condon, B.D.; Xia, Z.; Nagarajan, R.; Hinchliffe, D.J.; Madison, C.A. Intumescent Flame-Retardant Cotton Produced by Tannic Acid and Sodium Hydroxide. J. Anal. Appl. Pyrolysis 2017, 126, 239–246. [Google Scholar] [CrossRef]
- Shi, S.; Peng, X.; Liu, T.; Chen, Y.; He, C.; Wang, H. Facile Preparation of Hydrogen-Bonded Supramolecular Polyvinyl Alcohol-Glycerol Gels with Excellent Thermoplasticity and Mechanical Properties. Polymer 2017, 111, 168–176. [Google Scholar] [CrossRef]
- Van Olphen, H. Polyelectrolyte Reinforced Aerogels of Clays---Application as Chromatographic Adsorbents. Clays Clay Miner. 1967, 15, 423–435. [Google Scholar] [CrossRef]
- Nam, S.; Kim, H.J.; Condon, B.D.; Hinchliffe, D.J.; Chang, S.; McCarty, J.C.; Madison, C.A. High Resistance to Thermal Decomposition in Brown Cotton is Linked to Tannins and Sodium Content. Cellulose 2016, 23, 1137–1152. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of Fire-retarded Materials—Interpretation of Cone Calorimeter Data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]





| Sample | PVA (g/%total) | Tannic acid (g/%total) | NaOH (g/%total) | DI water (g) |
|---|---|---|---|---|
| P5 | 5/100% | 0/0% | 0/0% | 100 |
| P10 | 10/100% | 0/0% | 0/0% | 100 |
| P5/S | 5/90% | 0/0% | 0.587/10% | 100 |
| P5/T1/S | 5/76% | 1/15% | 0.587/9% | 100 |
| P5/T2/S | 5/61% | 2/25% | 1.174/14% | 100 |
| Sample | Density (g/cm3) | Modulus (MPa) | Specific modulus (MPa cm3/g) |
|---|---|---|---|
| P5 | 0.065±0.003 | 0.5±0.0 | 7.7±0.4 |
| P10 | 0.124±0.002 | 8.6±2.3 | 69±18 |
| P5/S | 0.087±0.001 | 1.4±0.4 | 16±5 |
| P5/T1/S | 0.088±0.001 | 4.3±0.6 | 49±7 |
| P5/T2/S | 0.141±0.003 | 13±3 | 90±21 |
| Sample | Td10% (°C) | Td20% (°C) | Tdmax (°C) | Dw/dt (%/°C) | Weight at 100(%) | Residue (%) |
|---|---|---|---|---|---|---|
| P5 | 228 | 239 | 250 | 2.67 | 94.4 | 6.4 |
| P10 | 228 | 238 | 250 | 3.03 | 93.6 | 2.6 |
| P5/S | 170 | 186 | 198 | 1.88 | 92.6 | 22 |
| P5/T1/S | 156 | 197 | 208 | 1.57 | 91.4 | 26 |
| P5/T2/S | 193 | 209 | 214 | 1.41 | 95.4 | 35 |
| Sample | Weight (g) | TTI (s) | PHRR (kW/m2) | TTPHRR (s) | FIGRA (kW/(s m2)) | THR (MJ/m2) | THR/mass (MJ/(m2 g)) |
|---|---|---|---|---|---|---|---|
| P5 | 5.3 | 8 | 534 | 32 | 16.7 | 12.9 | 2.4 |
| P10 | 8.9 | 8 | 574 | 51 | 11.3 | 21.1 | 2.4 |
| P5/S | 5.9 | 3 | 160 | 12 | 13.4 | 9.4 | 1.6 |
| P5/T1/S | 5.8 | 6 | 201 | 16 | 12.6 | 8.3 | 1.4 |
| P5/T2/S | 6.7 | 7 | 166 | 37 | 4.5 | 7.5 | 1.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; DeGracia, K.; Schiraldi, D.A. Sustainable, Low Flammability, Mechanically-Strong Poly(vinyl alcohol) Aerogels. Polymers 2018, 10, 1102. https://doi.org/10.3390/polym10101102
Cheng Z, DeGracia K, Schiraldi DA. Sustainable, Low Flammability, Mechanically-Strong Poly(vinyl alcohol) Aerogels. Polymers. 2018; 10(10):1102. https://doi.org/10.3390/polym10101102
Chicago/Turabian StyleCheng, Zhihan, Kimberly DeGracia, and David A. Schiraldi. 2018. "Sustainable, Low Flammability, Mechanically-Strong Poly(vinyl alcohol) Aerogels" Polymers 10, no. 10: 1102. https://doi.org/10.3390/polym10101102






