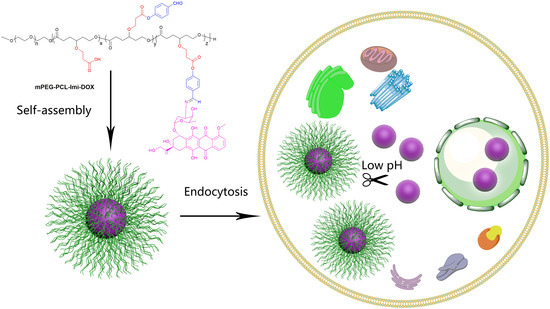
Design, Synthesis, and Characterization of Schiff Base Bond-Linked pH-Responsive Doxorubicin Prodrug Based on Functionalized mPEG-PCL for Targeted Cancer Therapy
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of mPEG-PCL with Substituted Carboxylic Groups (mPEG-CPCL)
2.2.1. Synthesis of mPEG-BuPCL Block Copolymer
2.2.2. Synthesis of mPEG-CPCL
2.3. Synthesis of Imine-Linked DOX Prodrugs Based on PEG-PCL di-Block Copolymer (mPEG-PCL-Imi-DOX)
2.3.1. Synthesis of Aldehyde-Functionalized mPEG-PCL (mPEG-APCL)
2.3.2. Synthesis of mPEG-PCL-Imi-DOX
2.4. Structural Characterization
2.5. Preparation and Characterization of mPEG-PCL-Imi-DOX Micelles
2.6. In Vitro Drug Release
2.7. In Vitro Cellular Uptake
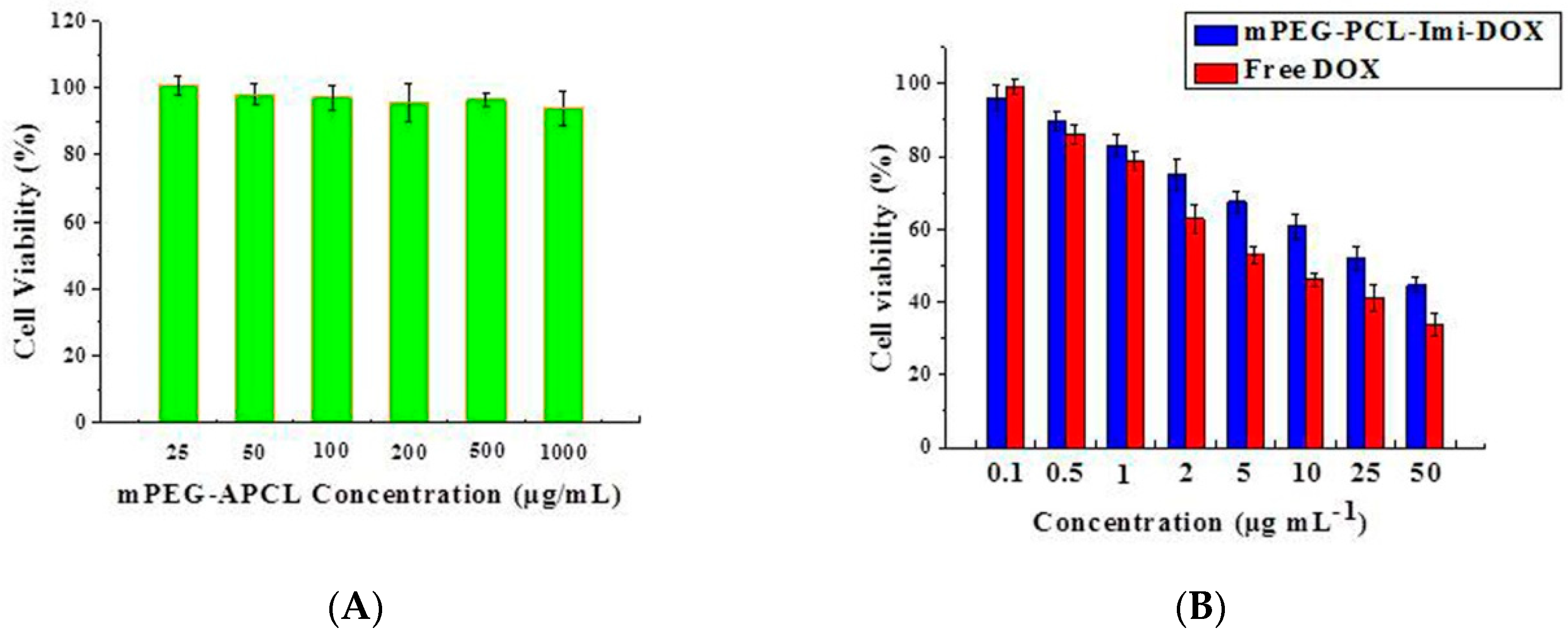
2.8. In Vitro Cytotoxicity Assay
3. Results and Discussion
3.1. Polymer Synthesis and Characterization
3.2. Characterization of mPEG-PCL-Imi-DOX Micelles
3.3. In Vitro Drug Release of mPEG-PCL-Imi-DOX Micelles
3.4. In Vitro Cellular Uptake and Cytotoxicity Studies of mPEG-PCL-Imi-DOX Micelles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Blum, R.H.; Carter, S.K. Adriamycin. A new anticancer drug with significant clinical activity. Ann. Intern. Med. 1974, 80, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, Y.; Chen, Z.; Chen, T.; Xu, Z.; Zhang, X.; Zhang, H. Mesoporous silica nanoparticles combining Au particles as glutathione and pH dual-sensitive nanocarriers for doxorubicin. Mater. Sci. Eng. 2016, 59, 258–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Y.; Tao, L.; Bligh, S.W.A.; Yang, H.; Pan, Q.; Zhu, L. Targeted delivery and controlled release of doxorubicin into cancer cells using a multifunctional graphene oxide. Mater. Sci. Eng. 2016, 59, 652–660. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.E.R.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, F.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX (TM)/Doxil (R)) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Injac, R.; Perse, M.; Cerne, M.; Potocnik, N.; Radic, N.; Govedarica, B.; Djordjevic, A.; Cerar, A.; Strukelj, B. Protective effects of fullerenol C-60(OH)(24) against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats with colorectal cancer. Biomaterials 2009, 30, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Arola, O.J.; Saraste, A.; Pulkki, K.; Kallajoki, M.; Parvinen, M.; Voipio-Pulkki, L.M. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000, 60, 1789–1792. [Google Scholar] [PubMed]
- Huttunen, K.M.; Raunio, H.; Rautio, J. Prodrugs-from serendipity to rational design. Pharmacol. Rev. 2011, 63, 750–771. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Lyu, Y.; Ding, D.; Pu, K. Semiconducting oligomer nanoparticles as an activatable photoacoustic probe with amplified brightness for in vivo imaging of pH. Adv. Mater. 2016, 28, 3662–3668. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Mao, D.; Sung, S.H.P.; Kwok, R.T.K.; Lam, J.W.Y.; Kong, D.; Ding, D.; Tang, B.Z. Activatable fluorescent nanoprobe with aggregation-induced emission characteristics for selective in vivo imaging of elevated peroxynitrite generation. Adv. Mater. 2016, 28, 7249–7256. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.-T.; Lei, Q.; Yang, B.; Jia, H.-Z.; Cheng, H.; Liu, L.-H.; Zeng, X.; Feng, J.; Zhuo, R.-X.; Zhang, X.-Z. Utilization of H-bond interaction of nucleobase uralic with antitumor methotrexate to design drug carrier with ultrahigh loading efficiency and pH-responsive drug release. Regen. Biomater. 2014, 1, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, Z.; Wang, Y.; He, T.; Yang, C.; Ren, C.; Ma, L.; Gong, C.; Li, X.; Yang, Z. Enzyme-Catalyzed Formation of Supramolecular Hydrogels as Promising Vaccine Adjuvants. Adv. Funct. Mater. 2016, 26, 1822–1829. [Google Scholar] [CrossRef]
- Wang, W.; Deng, L.; Xu, S.; Zhao, X.; Lv, N.; Zhang, G.; Gu, N.; Hu, R.; Zhang, J.; Liu, J.; et al. A reconstituted “two into one” thermosensitive hydrogel system assembled by drug-loaded amphiphilic copolymer nanoparticles for the local delivery of paclitaxel. J. Mater. Chem. 2013, 1, 552–563. [Google Scholar] [CrossRef]
- Cheng, T.; Liu, J.; Ren, J.; Huang, F.; Ou, H.; Ding, Y.; Zhang, Y.; Ma, R.; An, Y.; Liu, J.; et al. Green tea catechin-based complex micelles combined with doxorubicin to overcome cardiotoxicity and multidrug resistance. Theranostics 2016, 6, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wang, J.; Qu, A.; Shen, L.; Liu, J.; Liu, J.; Zhang, Z.; An, Y.; Shi, L. Maintenance of amyloid beta peptide homeostasis by artificial chaperones based on mixed-shell polymeric micelles. Angew. Chem. Int. Edit. 2014, 53, 8985–8990. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliver. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhao, W.; Liu, H.; Marquez, R.; Huang, Y.; Zhang, Y.; Li, J.; Xie, W.; Venkataramanan, R.; Xu, L.; et al. An improved D-alpha-tocopherol-based nanocarrier for targeted delivery of doxorubicin with reversal of multidrug resistance. J. Control. Release 2014, 196, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Jeong, G.-W.; Nah, J.-W. Preparation and anticancer effect of transferrin-modified pH-sensitive polymeric drug nanoparticle for targeted cancer therapy. J. Ind. Eng. Chem. 2017, 54, 298–303. [Google Scholar] [CrossRef]
- Gerweck, L.E. Tumor pH: Implications for treatment and novel drug design. Semin. Radiat. Oncol. 1998, 8, 176–182. [Google Scholar] [CrossRef]
- Shi, F.; Ding, J.; Xiao, C.; Zhuang, X.; He, C.; Chen, L.; Chen, X. Intracellular microenvironment responsive PEGylated polypeptide nanogels with ionizable cores for efficient doxorubicin loading and triggered release. J. Mater. Chem. 2012, 22, 14168–14179. [Google Scholar] [CrossRef]
- Reddy, G.R.; Bhojani, M.S.; McConville, P.; Moody, J.; Moffat, B.A.; Hall, D.E.; Kim, G.; Koo, Y.-E.; Woolliscroft, M.J.; Sugai, G.V.; et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin. Cancer Res. 2006, 12, 6677–6686. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-T.; Hong, C.-Y.; Pan, C.-Y. Fabrication of PDEAEMA-coated mesoporous silica nanoparticles and pH-responsive controlled release. J. Phys. Chem. 2010, 114, 12481–12486. [Google Scholar] [CrossRef]
- Jia, T.; Huang, S.; Yang, C.; Wang, M. Unimolecular micelles of amphiphilic cyclodextrin-core star-like copolymers with covalent pH-responsive linkage of anticancer prodrugs. Mol. Pharmaceut. 2017, 14, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Xu, C.-R.; Zhong, F.; Hong, C.-Y.; Pan, C.-Y. Fabrication of functional nano-objects through raft dispersion polymerization and influences of morphology on drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 18347–18359. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.E.; Wei, H.; Tan, N.; Boydston, A.J.; Pun, S.H. Sunflower polymers for folate-mediated drug delivery. Biomacromolecules 2016, 17, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhong, Y.; Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Acetal-linked paclitaxel prodrug micellar nanoparticles as a versatile and potent platform for cancer therapy. Biomacromolecules 2013, 14, 2772–2780. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Gu, J.; Qu, X.; Yang, Z. Preparation of multifunctional drug carrier for tumor-specific uptake and enhanced intracellular delivery through the conjugation of weak acid labile linker. Bioconjugate Chem. 2009, 20, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Matesic, L.; Locke, J.M.; Vine, K.L.; Ranson, M.; Bremner, J.B.; Skropeta, D. Synthesis and hydrolytic evaluation of acid-labile imine-linked cytotoxic isatin model systems. Bioorg. Med. Chem. 2011, 19, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Mueller, I.A.; Kratz, F.; Jung, M.; Warnecke, A. Schiff bases derived from p-aminobenzyl alcohol as trigger groups for pH-dependent prodrug activation. Tetrahedron Lett. 2010, 51, 4371–4374. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhou, X.; Jia, L.; Ma, C.; Song, R.; Deng, Y.; Hu, X.; Sun, W. Acetal-linked paclitaxel polymeric prodrug based on functionalized mPEG-PCL diblock polymer for ph-triggered drug delivery. Polymers 2017, 9, 698. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, F.; Ren, C.; Yang, L.; Liu, J.; Cheng, Z.; Chu, L.; Liu, J. A targeted chemo-photodynamic combination platform based on the dox prodrug nanoparticles for enhanced cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 13016–13028. [Google Scholar] [CrossRef] [PubMed]
- Du, J.-Z.; Du, X.-J.; Mao, C.-Q.; Wang, J. Tailor-made dual pH-sensitive polymer–doxorubicin nanoparticles for efficient anticancer drug delivery. J. Am. Chem. Soc. 2011, 133, 17560–17563. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Y.; Zhou, X.; Zhang, Z.; Zhang, L.; Wang, D.; Wang, X.; Sun, W. Design, Synthesis, and Characterization of Schiff Base Bond-Linked pH-Responsive Doxorubicin Prodrug Based on Functionalized mPEG-PCL for Targeted Cancer Therapy. Polymers 2018, 10, 1127. https://doi.org/10.3390/polym10101127
Zhai Y, Zhou X, Zhang Z, Zhang L, Wang D, Wang X, Sun W. Design, Synthesis, and Characterization of Schiff Base Bond-Linked pH-Responsive Doxorubicin Prodrug Based on Functionalized mPEG-PCL for Targeted Cancer Therapy. Polymers. 2018; 10(10):1127. https://doi.org/10.3390/polym10101127
Chicago/Turabian StyleZhai, Yinglei, Xing Zhou, Zhiqiang Zhang, Lei Zhang, Dianyu Wang, Xinhui Wang, and Wei Sun. 2018. "Design, Synthesis, and Characterization of Schiff Base Bond-Linked pH-Responsive Doxorubicin Prodrug Based on Functionalized mPEG-PCL for Targeted Cancer Therapy" Polymers 10, no. 10: 1127. https://doi.org/10.3390/polym10101127