Novel Multifunctional Luminescent Electrospun Fluorescent Nanofiber Chemosensor-Filters and Their Versatile Sensing of pH, Temperature, and Metal Ions
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
2.3. Synthesis of the Fluorescent Probe (RhBN2) and Fluorescent Monomer (RhBN2AM)
2.4. Synthesis of poly(NIPAAm-co-NMA-co-RhBN2AM)
2.5. Preparation of Electrospun Fibers and Drop-Cast Films
3. Results and Discussion
3.1. Characterization of RhBN2AM and poly(NIPAAm-co-NMA-co-RhBN2AM)
3.2. Morphologies of Electrospun Nanofibers
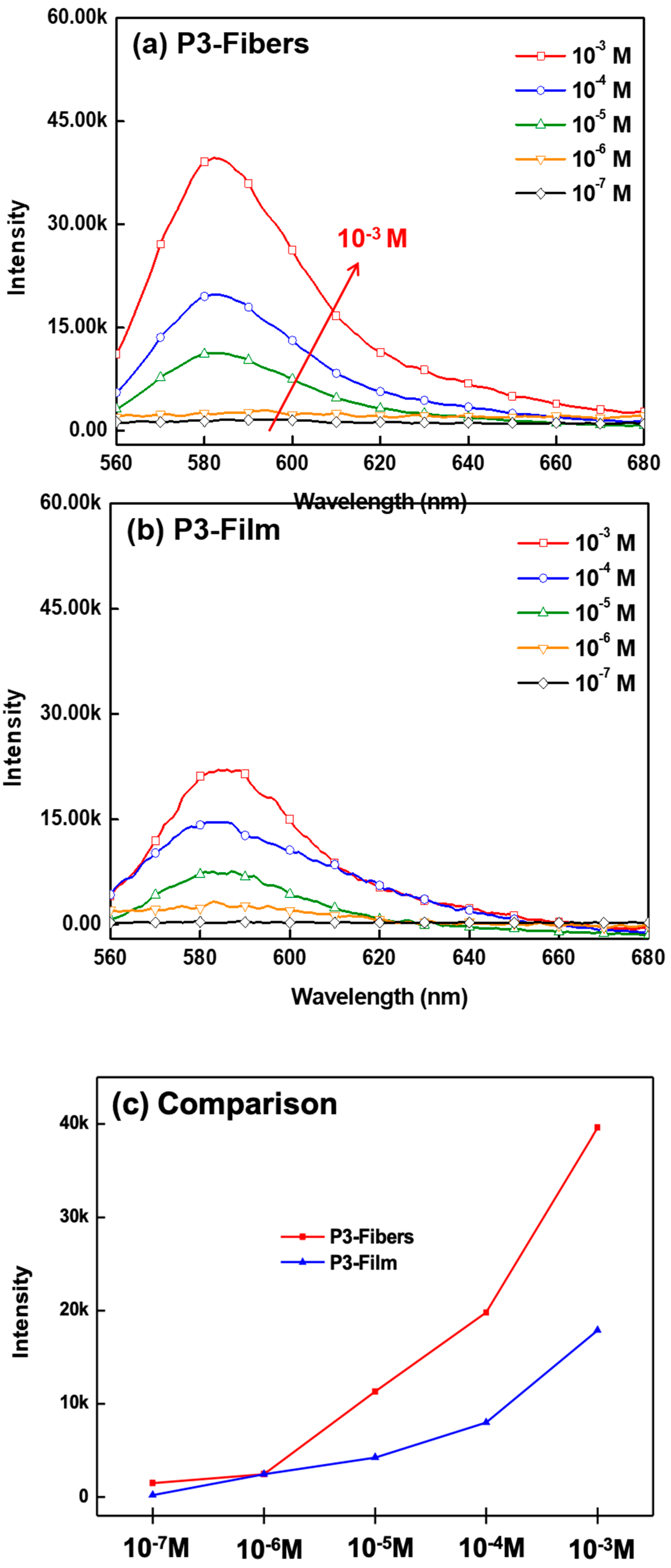
3.3. pH Sensing Property of ES Nanofibers
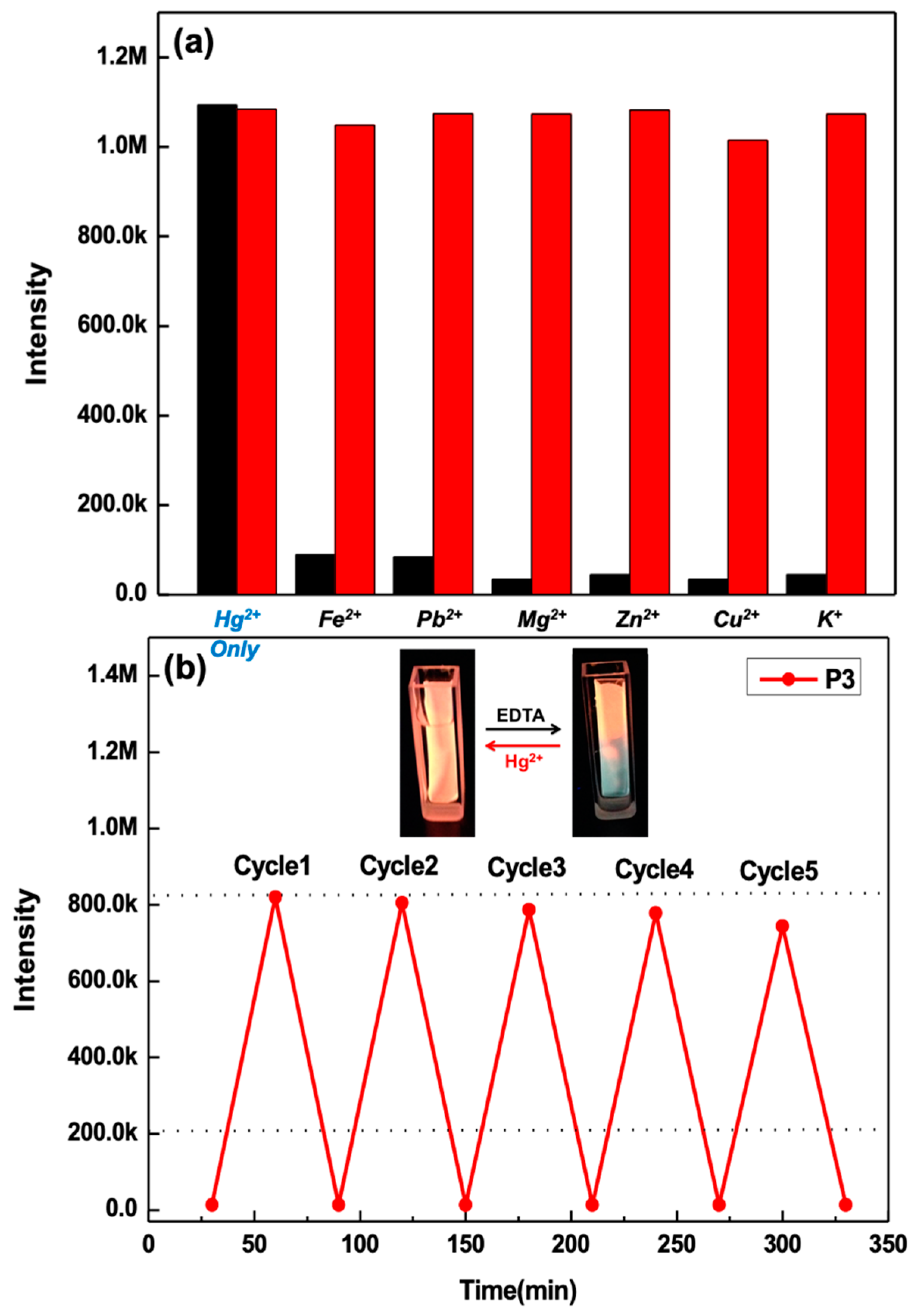
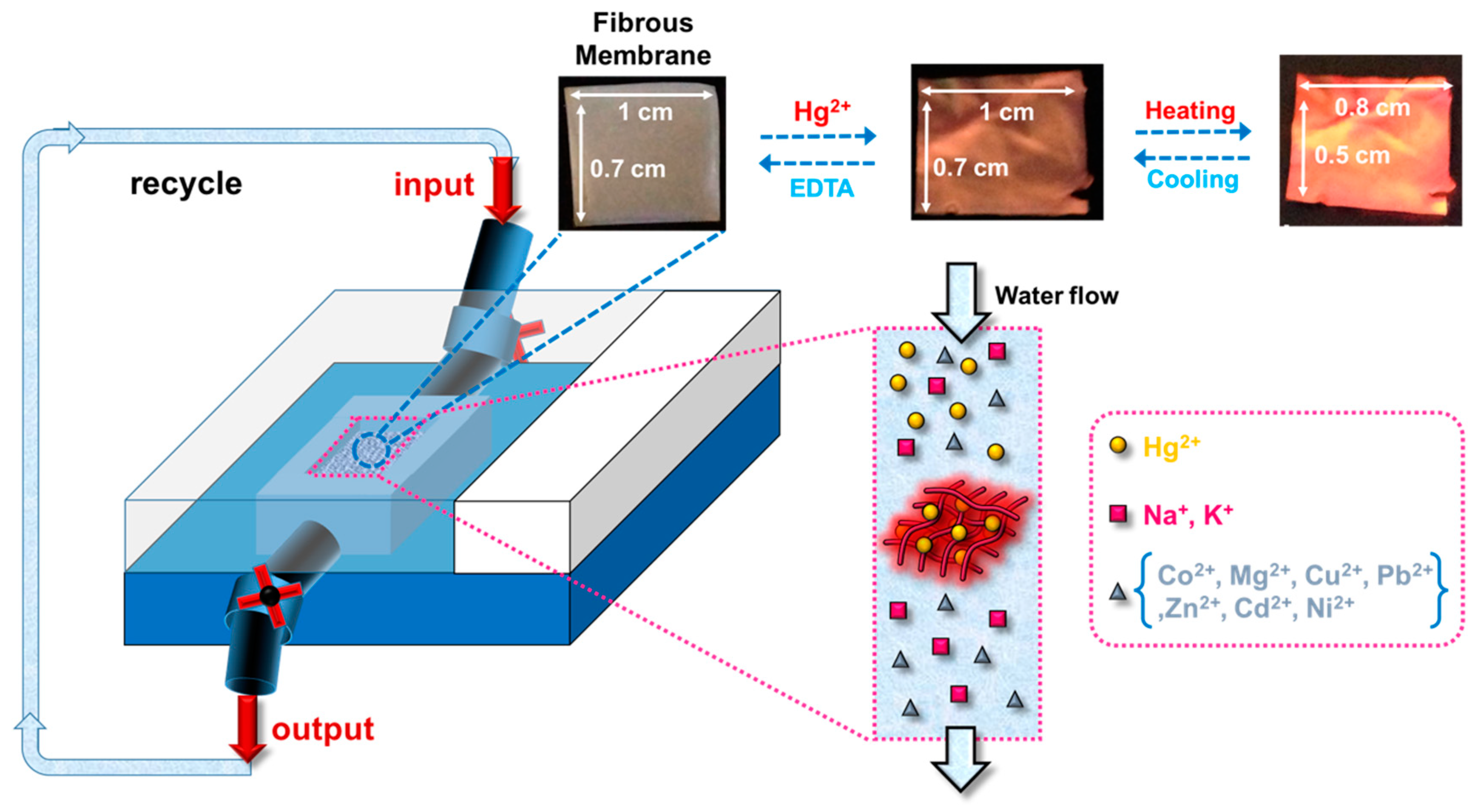
3.4. Hg2+ Sensing Property of ES Nanofibers
3.5. Thermo-Responsive Volume and Luminescence Variation of ES Nanofibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Day, J.J.; Reed, M.N.; Newland, M.C. Neuromotor Deficits and Mercury Concentrations in Rats Exposed to Methyl Mercury and Fish Oil. Neurotoxicol. Teratol. 2005, 27, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Harada, M. Minamata Disease: Methylmercury Poisoning in Japan Caused by Environmental Pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xu, X.; Boezen, H.M.; Huo, X. Children with Health Impairments by Heavy Metals in an E-Waste Recycling Area. Chemosphere 2016, 148, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.; Pal, A. Rhodamine-based Probes for Metal Ion-induced Chromo-/Fluorogenic Dual Signaling and Their Selectivity Towards Hg(II) ion. Org. Biomol. Chem. 2011, 9, 4467–4480. [Google Scholar] [CrossRef] [PubMed]
- Kaewtong, C.; Wanno, B.; Uppa, Y.; Morakot, N.; Pulpoka, B.; Tuntulani, T. Facile Synthesis of Rhodamine-based Highly Sensitive and Fast Responsive Colorimetric and Off-On Fluorescent Reversible Chemosensors for Hg2+: Preparation of a Fluorescent Thin Film Sensor. Dalton Trans. 2011, 40, 12578–12583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shiraishi, Y.; Hira, T. Cu(II)-Selective Green Fluorescence of a Rhodamine−Diacetic Acid Conjugate. Org. Lett. 2007, 9, 5039–5042. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, T.; Zhang, G.; Liu, S. Highly Selective Fluorescence Sensing of Mercury Ions over a Broad Concentration Range Based on Mixed Polymeric Micelles. Macromolecules 2012, 45, 3939–3947. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, X.; Wang, D.; Hu, X.; Liu, T.; Zhang, G.; Liu, S. Ultrasensitive Ratiometric Fluorescent pH and Temperature Probes Constructed from Dye-Labeled Thermoresponsive Double Hydrophilic Block Copolymers. J. Mater. Chem. 2011, 21, 19030–19038. [Google Scholar] [CrossRef]
- Lin, S.T.; Fuchise, K.; Chen, Y.; Sakai, R.; Satoh, T.; Kakuchi, T.; Chen, W.C. Synthesis, Thermomorphic Characteristics, and Fluorescent Properties of Poly[2,7-(9,9-dihexylfluorene)]-block-Poly(N-isopropylacrylamide)-block-Poly(N-hydroxyethylacrylamide) Rod-Coil-Coil Triblock Copolymers. Soft Matter 2009, 5, 3761–3770. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S. Responsive Polymers-Based Dual Fluorescent Chemosensors for Zn2+ Ions and Temperatures Working in Purely Aqueous Nedia. Anal. Chem. 2011, 83, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wu, S.; Zeng, F. Reusable Polymer Film Chemosensor for Ratiometric Fluorescence Sensing in Aqueous Media. Sens. Actuators B 2010, 145, 451–456. [Google Scholar] [CrossRef]
- Lv, F.; Feng, X.; Tang, H.; Liu, L.; Yang, Q.; Wang, S. Development of Film Sensors Based on Conjugated Polymers for Copper (II) Ion Detection. Adv. Funct. Mater. 2011, 21, 845–850. [Google Scholar] [CrossRef]
- Chen, J.Y.; Kuo, C.C.; Lai, C.S.; Chen, W.C.; Chen, H.L. Manipulation on the Morphology and Electrical Properties of Aligned Electrospun Nanofibers of Poly(3-hexylthiophene) for Field-Effect Transistor Applications. Macromolecules 2011, 44, 2883–2892. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Kuo, C.C.; Chen, B.Y.; Chiu, P.C.; Tsai, P.C. Multifunctional Polyacrylonitrile-ZnO/Ag Electrospun Nanofiber Membranes with Various ZnO Morphologies for Photocatalytic, UV-Shielding, and Antibacterial Applications. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 262–269. [Google Scholar] [CrossRef]
- Huang, Y.S.; Kuo, C.C.; Huang, C.C.; Jang, S.C.; Tsen, W.C.; Chuang, F.S.; Chen, B.Y.; Chen, J.J.; Chow, J.D.; Shu, Y.C. Novel Highly Aligned, Double-Layered, Hollow Fibrous Polycarbonate Membranes with a Perfectly Tightly Packed Pentagonal Pore Structure Fabricated Using the Electrospinning Process. RSC Adv. 2015, 5, 88857–88865. [Google Scholar] [CrossRef]
- Huang, Y.S.; Kuo, C.C.; Shu, Y.C.; Jang, S.C.; Tsen, W.C.; Chuang, F.S.; Chen, C.C. Highly Aligned and Single-Layered Hollow Fibrous Membranes Prepared from Polyurethane and Silica Blends Through a Two-Fluid Coaxial Electrospun Process. Macromol. Chem. Phys. 2014, 215, 879–887. [Google Scholar] [CrossRef]
- Kuo, C.C.; Lin, C.H.; Chen, W.C. Morphology and Photophysical Properties of Light-Emitting Electrospun Nanofibers Prepared from Poly(fluorene) Derivative/ PMMA Blends. Macromolecules 2007, 40, 6959–6966. [Google Scholar] [CrossRef]
- Kuo, C.C.; Tung, Y.C.; Chen, W.C. Morphology and pH Sensing Characteristics of New Luminescent Electrospun Fibers Prepared from Poly(phenylquinoline)-block-Polystyrene/Polystyrene Blends. Macromol. Rapid Commun. 2010, 31, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Tung, Y.C.; Lin, C.H.; Chen, W.C. Novel Luminescent Electrospun Fibers Prepared from Conjugated Rod–Coil Block Copolymer of Poly[2,7-(9,9-dihexylfluorene)]-block-Poly(methyl methacrylate). Macromol. Rapid Commun. 2008, 29, 1711–1715. [Google Scholar] [CrossRef]
- Kuo, C.C.; Wang, C.T.; Chen, W.C. Highly-Aligned Electrospun Luminescent Nanofibers Prepared from Polyfluorene/PMMA Blends: Fabrication, Morphology, Photophysical Properties and Sensory Applications. Macromol. Mater. Eng. 2008, 293, 999–1008. [Google Scholar] [CrossRef]
- Tzeng, P.; Kuo, C.C.; Lin, S.T.; Chiu, Y.C.; Chen, W.C. New Thermoresponsive Luminescent Electrospun Nanofibers Prepared from Poly[2,7-(9,9-dihexylfluorene)]-block-Poly(N-isopropylacrylamide)/PMMA Blends. Macromol. Chem. Phys. 2010, 211, 1408–1416. [Google Scholar] [CrossRef]
- Wang, C.T.; Kuo, C.C.; Chen, H.C.; Chen, W.C. Non-Woven and Aligned Electrospun Multicomponent Luminescent Polymer Nanofibers: Effects of Aggregated Morphology on the Photophysical Properties. Nanotechnology 2009, 20, 375604. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Yang, Y.Y.; Yu, D.G.; Du, Q.; Yang, X.L. Fast Dissolving Drug Delivery Membrane Based on The Ultra-Thin Shell of Electrospun Core-Shell Nanofibers. Eur. J. Pharm. Sci. 2018, 122, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shao, W.; Luo, M.; Bian, J.; Yu, D.G. Electrospun Blank Nanocoating for Improved Sustained Release Profiles from Medicated Gliadin Nanofibers. Nanomaterials 2018, 8, 184. [Google Scholar]
- Yu, D.G.; Li, J.J.; Williams, G.R.; Zhao, M. Electrospun Amorphous Solid Dispersions of Poorly Water-Soluble Drugs: A Review. J. Control. Release 2018, 292, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Yu, D.G.; Zhu, M.J.; Zhao, M.; Williams, G.R. Tunable Zero-Order Drug Delivery Systems Created by Modified Triaxial Electrospinning. Chem. Eng. J. 2019, 356, 886–894. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Yu, D.G.; Wang, G.; Williams, G.R.; Zhang, Z. Tunable Drug Release from Nanofibers Coated with Blank Cellulose Acetate Layers Fabricated Using Tri-Axial Electrospinning. Carbohydr. Polym. 2019, 203, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Kuo, C.C.; Cho, C.J.; Liang, F.C.; Jeng, R.J. Novel Fluorescent Chemosensory Filter Membranes Composed of Electrospun Nanofibers with Ultra-Selective and Reversible pH and Hg2+ Sensing Characteristics. Dyes Pigm. 2017, 143, 129–142. [Google Scholar] [CrossRef]
- Chen, B.Y.; Kuo, C.C.; Huang, Y.S.; Lu, S.T.; Liang, F.C.; Jiang, D.H. Novel Highly Selective and Reversible Chemosensors Based on Dual-Ratiometric Fluorescent Electrospun Nanofibers with pH− and Fe3+-Modulated Multicolor Fluorescence Emission. ACS Appl. Mater. Interfaces 2015, 7, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.N.; Kuo, C.C.; Chiu, Y.C.; Chen, W.C. Ultra Metal Ions and pH Sensing Characteristics of Thermoresponsive Luminescent Electrospun Nanofibers Prepared from Poly(HPBO-co-NIPAAm-co-SA). RSC Adv. 2014, 4, 45345–45353. [Google Scholar] [CrossRef]
- Cho, C.J.; Lu, S.T.; Kuo, C.C.; Liang, F.C.; Chen, B.Y.; Chu, C.C. Pyrene or Rhodamine Derivative–Modified Surfaces of Electrospun Nanofibrous Chemosensors for Colorimetric and Fluorescent Determination of Cu2+, Hg2+, and pH. React. Funct. Polym. 2016, 108, 137–147. [Google Scholar] [CrossRef]
- Liang, F.C.; Kuo, C.C.; Chen, B.Y.; Cho, C.J.; Hung, C.C.; Chen, W.C.; Borsali, R. RGB-Switchable Porous Electrospun Nanofiber Chemoprobe-Filter Prepared from Multifunctional Copolymers for Versatile Sensing of pH and Heavy Metals. ACS Appl. Mater. Interfaces 2017, 9, 16381–16396. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.C.; Chen, Y.; Kuo, C.C.; Tung, S.H.; Kakuchi, T.; Chen, W.C. Synthesis, Morphology, and Sensory Applications of Multifunctional Rod-Coil-Coil Triblock Copolymers and Their Electrospun Nanofibers. ACS Appl. Mater. Interfaces 2012, 4, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.C.; Kuo, C.C.; Hsu, J.C.; Chen, W.C. Thermoresponsive Luminescent Electrospun Fibers Prepared from Poly(DMAEMA-co-SA-co-StFl) Multifunctional Random Copolymers. ACS Appl. Mater. Interfaces 2010, 2, 3340–3347. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.C.; Luo, Y.L.; Kuo, C.C.; Chen, B.Y.; Cho, C.J.; Lin, F.J.; Yu, Y.Y.; Borsali, R. Novel Magnet and Thermoresponsive Chemosensory Electrospinning Fluorescent Nanofibers and Their Sensing Capability for Metal Ions. Polymers 2017, 9, 136. [Google Scholar] [CrossRef]
- Chen, L.N.; Chiu, Y.C.; Hung, J.J.; Kuo, C.C.; Chen, W.C. Multifunctional Electrospun Nanofibers Prepared from Poly((N-isopropylacrylamide)-co-(N-hydroxymethylacrylamide)) and Their Blends with 1,2-diaminoanthraquinone for NO Gas Detection. Macromol. Chem. Phys. 2014, 215, 286–294. [Google Scholar] [CrossRef]
- Hung, C.C.; Kuo, C.C.; Weng, N.K.; Wu, W.C.; Chen, B.Y.; Cho, C.J.; Hsu, I.J.; Chiu, Y.C.; Chen, W.C. Novel Highly Sensitive and Reversible Electrospun Nanofibrous Chemosensor-Filters Composed of Poly(HEMA-co-MNA) and bpy-F-bpy with Metal-Ion-Modulated Multicolor Fluorescence Emission. Polym. J. 2016, 48, 439–449. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ebara, M.; Aoyagi, T. Temperature-Responsive Electrospun Nanofibers for ‘On-Off’ Switchable Release of Dextran. Sci. Technol. Adv. Mater. 2012, 13, 064203. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.J.; Chiu, W.Y. Thermo-Responsive Nanofibers Prepared from Poly(N-isopropylacrylamide-co-N-methylol acrylamide). Polymer 2012, 53, 2829–2838. [Google Scholar] [CrossRef]
- Chuang, W.J.; Chiu, W.Y.; Tai, H.J. Thermally Crosslinkable Poly(N-isopropylacrylamide) Copolymers: Synthesis and Characterization of Temperature-Responsive Hydrogel. Mater. Chem. Phys. 2012, 134, 1208–1213. [Google Scholar] [CrossRef]







| Polymers | Feeding Molar Ratio NIPAAm:NMA:RhB | Experimental Ratio a NIPAAm:NMA:RhB | Mn b | Mw/Mn b | Tdc (°C) | LCST d (°C) |
|---|---|---|---|---|---|---|
| P1 | 10:1:0.1 | 90.2:9.4:0.4 | 33,000 | 1.85 | 343 | 30.0 |
| P2 | 10:3:0.1 | 80.5:19.2:0.3 | 35,300 | 1.92 | 351 | 35.0 |
| P3 | 10:5:0.1 | 71.1:28.4:0.5 | 35,500 | 1.88 | 352 | 45.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.-Y.; Lung, Y.-C.; Kuo, C.-C.; Liang, F.-C.; Tsai, T.-L.; Jiang, D.-H.; Satoh, T.; Jeng, R.-J. Novel Multifunctional Luminescent Electrospun Fluorescent Nanofiber Chemosensor-Filters and Their Versatile Sensing of pH, Temperature, and Metal Ions. Polymers 2018, 10, 1259. https://doi.org/10.3390/polym10111259
Chen B-Y, Lung Y-C, Kuo C-C, Liang F-C, Tsai T-L, Jiang D-H, Satoh T, Jeng R-J. Novel Multifunctional Luminescent Electrospun Fluorescent Nanofiber Chemosensor-Filters and Their Versatile Sensing of pH, Temperature, and Metal Ions. Polymers. 2018; 10(11):1259. https://doi.org/10.3390/polym10111259
Chicago/Turabian StyleChen, Bo-Yu, Yen-Chen Lung, Chi-Ching Kuo, Fang-Cheng Liang, Tien-Liang Tsai, Dai-Hua Jiang, Toshifumi Satoh, and Ru-Jong Jeng. 2018. "Novel Multifunctional Luminescent Electrospun Fluorescent Nanofiber Chemosensor-Filters and Their Versatile Sensing of pH, Temperature, and Metal Ions" Polymers 10, no. 11: 1259. https://doi.org/10.3390/polym10111259
APA StyleChen, B.-Y., Lung, Y.-C., Kuo, C.-C., Liang, F.-C., Tsai, T.-L., Jiang, D.-H., Satoh, T., & Jeng, R.-J. (2018). Novel Multifunctional Luminescent Electrospun Fluorescent Nanofiber Chemosensor-Filters and Their Versatile Sensing of pH, Temperature, and Metal Ions. Polymers, 10(11), 1259. https://doi.org/10.3390/polym10111259







