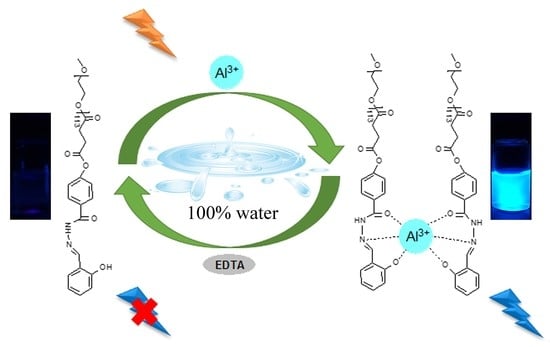
A Highly Selective Turn-on and Reversible Fluorescent Chemosensor for Al3+ Detection Based on Novel Salicylidene Schiff Base-Terminated PEG in Pure Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Salicylidene Schiff Base Derivative BAB
2.4. Synthesis of Chemosensor PEGBAB
2.5. Preparation of Test Solutions for Spectroscopic Measurements
2.6. Application as Test Strips
3. Results and Discussion
3.1. Synthesis of PEGBAB
3.2. UV-Vis and Fluorescence Studies
3.3. Competition Experiment
3.4. Effect of pH and Temperature
3.5. Reversibility Study
3.6. Construction of Logic Gate
3.7. Application as Test Strips
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bhoomika, K.; Pyngrope, S.; Dubey, R.S. Effect of aluminum on protein oxidation, non-protein thiols and protease activity in seedlings of rice cultivars differing in aluminum tolerance. J. Plant Physiol. 2014, 171, 497–508. [Google Scholar] [CrossRef]
- Boonkitpatarakul, K.; Wang, J.; Niamnont, N.; Liu, B.; Mcdonald, L.; Pang, Y.; Sukwattanasinitt, M. Novel turn-on fluorescent sensors with mega stoke shifts for dual detection of Al and Zn. ACS Sens. 2016, 1, 144–150. [Google Scholar] [CrossRef]
- Parkinson, I.S.; Ward, M.K.; Kerr, D.N. Dialysis encephalopathy, bone disease and anaemia: The aluminum intoxication syndrome during regular haemodialysis. J. Clin. Pathol. 1981, 34, 1285–1294. [Google Scholar] [CrossRef]
- Nayak, P. Aluminum: Impacts and disease. Environ. Res. 2002, 89, 101–115. [Google Scholar] [CrossRef]
- Exley, C. The coordination chemistry of aluminium in neurodegenerative disease. Coord. Chem. Rev. 2012, 256, 2142–2146. [Google Scholar] [CrossRef]
- Altschuler, E. Aluminum-containing antacids as a cause of idiopathic Parkinson’s disease. Med. Hypotheses 1999, 53, 22–23. [Google Scholar] [CrossRef]
- Krejpcio, Z.; Wojciak, R.W. The influence of Al3+ ions on pepsin and trypsin activity in vitro. Pol. J. Environ. Stud. 2002, 11, 251–254. [Google Scholar]
- Frankowski, M.; Zioła-Frankowska, A.; Kurzyca, I.; Novotný, K.; Vaculovič, T.; Kanický, V.; Siepak, M.; Siepak, J. Determination of aluminium in groundwater samples by GF-AAS, ICP-AES, ICP-MS and modelling of inorganic aluminium complexes. Environ. Monit. Assess. 2011, 182, 71–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolfson, A.D.; Gracey, G.M. Matrix effects in the determination of aluminium in dialysis fluids by graphite furnace atomic absorption spectrometry. Analyst 1987, 112, 1387–1389. [Google Scholar] [CrossRef]
- Frankowski, M.; Frankowska, A.Z.; Siepak, J. New method for speciation analysis of aluminium fluoride complexes by HPLC-FAAS hyphenated technique. Talanta 2010, 80, 2120–2126. [Google Scholar] [CrossRef]
- Sanz-Medel, A.; Cabezuelo, A.B.S.; Milačič, R.; Polak, T.B. The chemical speciation of aluminium in human serum. Coord. Chem. Rev. 2002, 228, 373–383. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, S.; Liu, Y.; He, S.; Zhao, L.; Zeng, X. Highly selective and sensitive fluorescent turn-on chemosensor for Al3+ based on a novel photoinduced electron transfer approach. Org. Lett. 2011, 13, 5274–5277. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.N.; Gupta, V.K.; Chatterjee, S. A sensitive voltammetric sensor for determination of synthetic corticosteroid triamcinolone, abused for doping. Biosens. Bioelectron. 2005, 24, 3562–3568. [Google Scholar] [CrossRef]
- Quang, D.T.; Kim, J.S. Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem. Rev. 2010, 110, 6280–6301. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, W.; Guo, Z. Metal coordination in photoluminescent sensing. Chem. Soc. Rev. 2013, 42, 1568–1600. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Chhatwal, M.; Kumar, A.; Singh, V.; Gupta, R.D.; Awasthi, S.K. Addressing of multiple-metal ions on a single platform. Coord. Chem. Rev. 2015, 292, 30–55. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Natale, C.D. Porphyrinoids for chemical sensor applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Yu, C.; Chen, L.; Zhang, J.; Li, J.; Liu, P.; Wang, W.; Yan, B. “Off-On” based fluorescent chemosensor for Cu2+ in aqueous media and living cells. Talanta 2011, 85, 1627–1633. [Google Scholar] [CrossRef]
- Wang, R.; Yu, F.; Liu, P.; Chen, L. A turn-on fluorescent probe based on hydroxylamine oxidation for detecting ferric ion selectively in living cells. Chem. Commun. 2012, 48, 5310–5312. [Google Scholar] [CrossRef] [Green Version]
- Nandre, J.P.; Patil, S.R.; Sahoo, S.K.; Pradeep, C.P.; Churakov, A.; Yu, F.; Chen, L.; Redshaw, C.; Patil, A.A.; Patil, U.D. A chemosensor for micro-to nano-molar detection of Ag+ and Hg2+ ions in pure aqueous media and its applications in cell imaging. Dalton Trans. 2017, 46, 14201–14209. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, M.; Chen, Q.; Yu, F.; Jiang, G.; Chen, L. Associated detection of superoxide anion and mercury (II) under chronic mercury exposure in cells and mice models via a three-channel fluorescent probe. Anal. Chem. 2018, 90, 9769–9778. [Google Scholar] [CrossRef] [PubMed]
- Soroka, K.; Vithanage, R.S.; Phillips, D.A.; Walker, B.; Dasgupta, P.K. Fluorescence properties of metal complexes of 8-hydroxyquinoline-5-sulfonic acid and chromatographic applications. Anal. Chem. 1987, 59, 629–636. [Google Scholar] [CrossRef]
- Sahana, S.; Bose, S.; Mukhopadhyay, S.K.; Bharadwaj, P.K. A highly selective and sensitive turn-on fluorescence chemosensor based on a rhodamine-adenine conjugate for Al3+ in aqueous medium: Bioimaging and DFT studies. J. Lumin. 2016, 169, 334–341. [Google Scholar] [CrossRef]
- Yao, D.; Huang, X.; Guo, F.; Xie, P. A new fluorescent enhancement chemosensor for Al3+ and Fe3+ based on naphthyridine and benzothiazole groups. Sens. Actuators B 2018, 256, 276–281. [Google Scholar] [CrossRef]
- Hariharan, P.S.; Anthony, S.P. Selective fluorescence sensing of Mg2+ ions by Schiff base chemosensor: Effect of diamine structural rigidity and solvent. RSC Adv. 2014, 4, 41565–41571. [Google Scholar] [CrossRef]
- Kim, S.; Noh, J.Y.; Kim, K.Y.; Kim, J.H.; Kang, H.K.; Nam, S.W.; Kim, S.H.; Park, S.; Kim, C.; Kim, J. Salicylimine-based fluorescent chemosensor for aluminum ions and application to bioimaging. Inorg. Chem. 2012, 51, 3597–3602. [Google Scholar] [CrossRef]
- Saluja, P.; Bhardwaj, V.K.; Pandiyan, T.; Kaur, S.; Kaur, N.; Singh, N. Imine-linked chemosensors for the detection of Zn2+ in biological samples. RSC Adv. 2014, 4, 9784–9790. [Google Scholar] [CrossRef]
- Wang, S.; Men, G.; Zhao, L.; Hou, Q.; Jiang, S. Binaphthyl-derived salicylidene Schiff base for dual-channel sensing of Cu, Zn cations and integrated molecular logic gates. Sens. Actuators B 2010, 145, 826–831. [Google Scholar] [CrossRef]
- Purkaita, R.; Patraa, C.; Mahapatrab, A.D.; Chattopadhyayb, D.; Sinhaa, C. A visible light excitable chromone appended hydrazide chemosensor for sequential sensing of Al3+ and F- in aqueous medium and in Vero cells. Sens. Actuators B 2018, 257, 545–552. [Google Scholar] [CrossRef]
- Jeong, J.W.; Rao, B.A.; Son, Y.A. Rhodamine-chloronicotinaldehyde-based “OFF-ON” chemosensor for the colorimetric and fluorescent determination of Al3+ ions. Sens. Actuators B 2015, 208, 75–84. [Google Scholar] [CrossRef]
- Balamurugan, G.; Velmathi, S.; Thirumalaivasan, N.; Wu, S.P. New phenazine based AIE probes for selective detection of aluminium(III) ions in presence of other trivalent metal ions in living cells. Analyst 2017, 142, 4721–4726. [Google Scholar] [CrossRef]
- Sarkar, D.; Ghosh, P.; Gharami, S.; Mondal, T.K.; Murmu, N. A novel coumarin based molecular switch for the sequential detection of Al3+ and F-: Application in lung cancer live cell imaging and construction of logic gate. Sens. Actuators B 2017, 242, 338–346. [Google Scholar] [CrossRef]
- Wang, G.Q.; Qin, J.C.; Li, C.R.; Yang, Z.Y. A highly selective fluorescent probe for Al3+ based on quinoline derivative. Spectrochim. Acta A 2015, 150, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, Y.; Xu, N.Z.; Zhang, M.Q.; Wu, G.Z.; Yao, C. A colorimetric and ratiometric fluorescent chemosensor based on furan-pyrene for selective and sensitive sensing Al3+. Chin. Chem. Lett. 2016, 27, 1673–1678. [Google Scholar] [CrossRef]
- Erdemir, S.; Kocyigit, O. Dual recognition of Zn2+ and Al3+ ions by a novel probe containing two fluorophore through different signaling mechanisms. Sens. Actuators B 2018, 273, 56–61. [Google Scholar] [CrossRef]
- Jiang, X.M.; Mi, W.H.; Zhu, W.; Yao, H.; Zhang, Y.M.; We, T.B. A biacylhydrazone-based chemosensor for fluorescence ‘turn-on’ detection of Al3+ with high selectivity and sensitivity. Supramol. Chem. 2019, 31, 80–88. [Google Scholar] [CrossRef]
- Li, Y.; Niu, Q.; Wei, T.; Li, T. Novel thiophene-based colorimetric and fluorescent turn-on sensor for highly sensitive and selective simultaneous detection of Al3+ and Zn2+ in water and food samples and its application in bioimaging. Anal. Chim. Acta 2019, 1049, 196–212. [Google Scholar] [CrossRef]
- Shoora, S.K.; Jain, A.K.; Gupta, V.K. A simple Schiff base based novel opticalprobe for aluminium(III) ions. Sens. Actuators B 2015, 216, 86–104. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, A.K.; Shoora, S.K. New on-off optical probe based on Schiffbase responding to Al3+ions: Logic gate application. Sens. Actuators B 2015, 219, 218–231. [Google Scholar] [CrossRef]
- Kim, H.N.; Guo, Z.; Zhu, W.; Yoon, J.; Tian, H. Recent progress on polymer-based fluorescent and colorimetric chemosensors. Chem. Soc. Rev. 2011, 40, 79–93. [Google Scholar] [CrossRef]
- Carvalho, W.S.P.; Wei, M.; Ikpo, N.; Gao, Y.; Serpe, M.J. Polymer-based technologies for sensing applications. Anal. Chem. 2018, 90, 459–479. [Google Scholar] [CrossRef]
- Wu, B.; Xu, L.; Wang, S.; Wang, Y.; Zhang, W. A PEGylated colorimetric and turn-on fluorescent sensor based on BODIPY for Hg(II) detection in water. Polym. Chem. 2015, 6, 4279–4289. [Google Scholar] [CrossRef]
- Kong, F.; Lin, M.; Qiu, T. Multi-functional ratiometric fluorescent chemosensors of poly(N-isopropylacrylamide) containing rhodamine 6G and 1,8-naphthalimide moieties. Polymer 2018, 151, 117–124. [Google Scholar] [CrossRef]
- Li, G.; Tao, F.; Wang, H.; Li, Y.; Wang, L. A novel reversible colorimetric chemosensor for rapid naked-eye detection of Cu2+ in pure aqueous solution. Sens. Actuators B 2015, 211, 325–331. [Google Scholar] [CrossRef]
- Li, G.; Tao, F.; Liu, Q.; Wang, L.; Wei, Z.; Zhu, F.; Chen, W.; Sun, H.; Zhou, Y. A highly selective and reversible water-soluble polymer based-colorimetric chemosensor for rapid detection of Cu2+ in pure aqueous solution. N. J. Chem. 2016, 40, 4513–4518. [Google Scholar] [CrossRef]
- Li, G.; Bai, L.; Tao, F.; Deng, A.; Wang, L. A dual chemosensor for Cu2+ and Hg2+ based on a rhodamine-terminated water-soluble polymer in 100% aqueous solution. Analyst 2018, 143, 5395–5403. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Li, G.; Li, L.; Gao, M.; Li, H.; Tao, F.; Deng, A.; Wang, S.; Wang, L. Schiff base functionalized PEG as a high efficient fluorescent chemosensor for Al3+ detection in 100% aqueous solution. React. Funct. Polym. 2019, 139, 1–8. [Google Scholar] [CrossRef]
- Bai, L.; Tao, F.; Li, L.; Deng, A.; Yan, C.; Li, G.; Wang, L. A simple turn-on fluorescent chemosensor based on Schiff base-terminated water-soluble polymer for selective detection of Al3+ in 100% aqueous solution. Spectrochim. Acta A 2019, 214, 436–444. [Google Scholar] [CrossRef]
- Hong, S.W.; Ahn, C.H.; Huh, J.; Jo, W.H. Synthesis of a PEGylated polymeric pH sensor and its pH sensitivity by fluorescence resonance energy transfer. Macromolecules 2006, 39, 7694–7700. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef] [Green Version]
- Hossain, S.M.; Singh, K.; Lakma, A.; Pradhan, R.N.; Singh, A.K. A schiff base ligand of coumarin derivative as an ICT-Based fluorescence chemosensor for Al3+. Sens. Actuators B 2017, 239, 1109–1117. [Google Scholar] [CrossRef]
- Maity, D.; Govindaraju, T. Naphthaldehyde-urea/thiourea conjugates as turn on fluorescent probes for Al3+ based on restricted C=N isomerization. Eur. J. Inorg. Chem. 2011, 36, 5479–5485. [Google Scholar] [CrossRef]
- Tang, W.; Xiang, Y.; Tong, A. Salicylaldehyde azines as fluorophores of aggregation-induced emission enhancement characteristics. J. Org. Chem. 2009, 74, 2163–2166. [Google Scholar] [CrossRef]
- Han, T.; Feng, X.; Tong, B.; Shi, J.; Chen, L.; Zhi, J.; Dong, Y. A novel “turn-on” fluorescent chemosensor for the selective detection of Al3+ based on aggregation-induced emission. Chem. Commun. 2012, 48, 416–418. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, H.; Jin, L.; Wang, W.; Zhang, Z.; Chen, Y. A novel turn on and reversible sensor for Al3+ and its applications in bioimaging. J. Lumin. 2018, 203, 113–120. [Google Scholar] [CrossRef]
- Xu, Y.; Mao, S.; Peng, H.; Wang, F.; Zhang, H.; Aderinto, S.O.; Wu, H. A fluorescent sensor for selective recognition of Al3+ based on naphthalimide Schiff-base in aqueous media. J. Lumin. 2017, 192, 56–63. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, H.; Sheng, L.; Chen, S.; Huang, D.; Liu, J. A highly selective colorimetric and fluorescent chemosensor for Al (III) based-on simple naphthol in aqueous solution. Spectrochim. Acta A. 2016, 157, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.Y.; Li, C.Y.; Li, Y.F.; Fu, Y.J.; Nie, S.X.; Tan, H.Y. A near-infrared chemosensor for determination of trivalent aluminum ions in living cells and tissues. Dyes Pigm. 2017, 136, 817–824. [Google Scholar] [CrossRef]
- Das, B.; Dey, S.; Maiti, G.P.; Bhattacharjee, A.; Dhara, A.; Jana, A. Hydrazinopyrimidine derived novel Al3+ chemosensor: Molecular logic gate and biological applications. N. J. Chem. 2018, 42, 9424–9435. [Google Scholar] [CrossRef]
- Sen, B.; Sheet, S.K.; Thounaojam, R.; Jamatia, R.; Pal, A.K.; Aguan, K.; Khatua, S. A coumarin based Schiff base probe for selective fluorescence detection of Al3+ and its application in live cell imaging. Spectrochim. Acta A 2017, 173, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, A.; Faizi, M.S.H.; Kumar, S.; Singh, M.K.; Sahu, S.K.; Kishor, S.; John, R.P. A selective ‘turn-on’fluorescent chemosensor for detection of Al3+ in aqueous medium: Experimental and theoretical studies. Sens. Actuators B 2018, 260, 888–899. [Google Scholar] [CrossRef]
- De Silva, A.P.; Gunaratne, H.Q.N.; McCoy, C.P. A molecular photoionic AND gate based on fluorescent signaling. Nature 1993, 364, 42–44. [Google Scholar] [CrossRef]
- Szaciłowski, K. Digital information processing in molecular systems. Chem. Rev. 2008, 108, 3481–3548. [Google Scholar] [CrossRef] [PubMed]
- Andréasson, J.; Pischel, U. Molecules with a sense of logic: A progress report. Chem. Soc. Rev. 2015, 44, 1053–1069. [Google Scholar] [CrossRef]
- Erbas-Cakmak, S.; Kolemen, S.; Sedgwick, A.C.; Gunnlaugsson, T.; James, T.D.; Yoon, J.; Akkaya, E.U. Molecular logic gates: The past, present and future. Chem. Soc. Rev. 2018, 47, 2228–2248. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, X.; Lu, H.; Wang, H.; Qin, Z. Highly selective and reversible chemosensor for Pd2+ detected by fluorescence, colorimetry, and test paper. ACS Appl. Mater. Interfaces 2015, 7, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Bui, M.P.N.; Abbas, A. Paper-based chemical and biological sensors: Engineering aspects. Biosens. Bioelectron. 2016, 77, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Ponram, M.; Balijapalli, U.; Sambath, B.; Iyer, S.K.; Venkatachalapathy, B.; Cingarama, R.; Sundaramurthy, K.N. Development of paper-based chemosensor for the detection of mercury ions using mono-and tetra-sulfur bearing phenanthridines. New J. Chem. 2018, 42, 8530–8536. [Google Scholar] [CrossRef]










| Chemosensor | Association Constant (M−1) | Detection Limit (M) | Testing Media | Refs. |
|---|---|---|---|---|
 | 8.5 × 105 | 1.05 × 10−8 | DMSO/H2O (1:5, v/v) | [35] |
 | 1.30 × 105 | 9.67 × 10−9 | 100% H2O | [51] |
 | 3.21 × 106 | 6.7 × 10−6 | DMF/water (9:1, v/v) | [54] |
 | 7.62 × 106 | 3.7 × 10−7 | EtOH/H2O (1:1, v/v) | [58] |
 | 5.49 × 104 | 1.78 × 10−7 | MeOH/H2O (1:1, v/v) | [59] |
 | 4.8 × 105 | 8.87 × 10−7 | CH3CN/H2O (1:1, v/v) | [60] |
 | 9.87 × 104 | 3.0 × 10−8 | EtOH/H2O (1:99, v/v) | [61] |
 | 1.9 × 104 | 2.78 × 10−6 | EtOH | [62] |
 | (1.06 ± 0.2) × 104 | 1.34 × 10−6 | MeOH/H2O (9:1, v/v) | [63] |
 | 2.1 × 104 | 4.32 × 10−6 | MeOH/H2O (9:1, v/v) | [64] |
 | 1.28 × 105 (UV-Vis) 1.18 × 105 (fluorescence) | 4.05 × 10−9 | 100% H2O | This work |
| Input 1 (Al3+) | Input 2 (EDTA) | Output (λem = 447 nm) |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 0 | 1 |
| 0 | 1 | 0 |
| 1 | 1 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.; Xu, Y.; Li, G.; Tian, S.; Li, L.; Tao, F.; Deng, A.; Wang, S.; Wang, L. A Highly Selective Turn-on and Reversible Fluorescent Chemosensor for Al3+ Detection Based on Novel Salicylidene Schiff Base-Terminated PEG in Pure Aqueous Solution. Polymers 2019, 11, 573. https://doi.org/10.3390/polym11040573
Bai L, Xu Y, Li G, Tian S, Li L, Tao F, Deng A, Wang S, Wang L. A Highly Selective Turn-on and Reversible Fluorescent Chemosensor for Al3+ Detection Based on Novel Salicylidene Schiff Base-Terminated PEG in Pure Aqueous Solution. Polymers. 2019; 11(4):573. https://doi.org/10.3390/polym11040573
Chicago/Turabian StyleBai, Liping, Yuhang Xu, Guang Li, Shuhui Tian, Leixuan Li, Farong Tao, Aixia Deng, Shuangshuang Wang, and Liping Wang. 2019. "A Highly Selective Turn-on and Reversible Fluorescent Chemosensor for Al3+ Detection Based on Novel Salicylidene Schiff Base-Terminated PEG in Pure Aqueous Solution" Polymers 11, no. 4: 573. https://doi.org/10.3390/polym11040573