1. Introduction
Increasing concern for the environment and volatile petroleum prices has led to growth of bio-based and biodegradable materials as alternatives to petroleum derived plastics [
1]. Plastics from bio-derived sources, or bioplastics, can be processed from a variety of feedstock including raw and refined plant sources and methane gas from biological degradation processes [
2]. One class of bioplastics, poly(hydroxyalkanoates) (PHAs), such as poly(hydroxybutyrate) (PHB) and its industrially-produced copolymer poly(hydroxybutyrate-
co-hydroxyvalerate) (PHBV), is synthesized by microorganisms as a storage polymer and can be harvested to produce a usable plastic [
3]. PHBV has mechanical properties most similar to polypropylene, however thermal processing of PHBV is challenging due the proximity of the thermal decomposition temperature to the melting temperature [
4,
5]. Poly(lactic acid) (PLA) is another important bioplastic, that can be produced through renewable resources, and has mechanical properties most similar to that of polystyrene [
5]. Alone, both bioplastics are brittle, with relatively poor impact strength and low thermal degradation temperatures. The toughness and processability of these bioplastics can be improved through multiphase blends of PHBV and PLA, resulting in attractive material properties not obtainable in the neat biopolymers [
2,
6].
One growing application space for bioplastics is additive manufacturing, and an emergent area of this space is conductive filament. Conductive filament is used in the rapid prototyping and production of electrically conductive components on a variety of 3D printers [
7,
8]. This production method enables various applications from low cost sensors to conductive traces, branching into electromagnetic and radio frequency shielding [
9,
10]. Typically, conductivity in thermoplastics is achieved through the addition of a conductive filler, such as silver nanoparticles, carbon black nanofillers, or graphene [
10,
11]. In addition to electrical conductivity, these value-added composites can reduce cost and weight, add color, provide anti-static potential at low volume percent, and enhance the mechanical and thermal properties over that of the neat polymer [
12]. Of these fillers, the majority of conductive filaments are produced with carbon black (CB), a commercially available petroleum-derived filler.
Though CB offers many positive benefits to polymer blends, when designing biobased composites having a renewable source for and considering the fate of filler materials is also important. Biochar (BioC) carries many of the same benefits as CB, but comes from renewable plant-derived sources and, as a widely applied agricultural amendment, is compatible with the bioplastic’s ability to biodegrade. This bio-sourced form of carbon is produced in a similar way to charcoal. Thermal decomposition of biomass in the absence of oxygen results in roughly 35% syngas, 30% bio-oil, and 35% BioC [
13]. The electrical conductivity of lignin sourced BioC has been shown to improve with pyrolysis at high treatment temperatures (over 800
C) [
14,
15,
16].
The bulk of the work exploring conductive nanocomposites, including with polymer blends, consists of polymers mixed with CB, graphene, carbon nanotubes (CNTs), or other graphite fillers [
7,
8,
17,
18,
19]. The conductivity of PLA-based composites has been successfully modified with CNTs [
20,
21], graphene [
22,
23,
24,
25], and CB [
26,
27]. Conductive filament made from PLA and conductive fillers (CB, graphene) is commercially available [
28,
29]. Fewer conductivity studies have been done with PHB and PHBV-based carbon nanocomposites; this work has been primarily with CNTs and graphene or graphene oxide, with conductivities in the range of ∼0.1 S/m to 30 S/m with loadings above the percolation threshold [
30,
31,
32].
Studies of conductive polymer blends have shown that nanofillers lower the percolation threshold of the blend over that of nanofiller incorporated into a single polymer, due to the ability to reside in either the minor or major phase, or the interfacial region [
19]. This partitioning of the nanofiller produces higher conductivity at lower weight percent filler than in their non-blended counterparts. Phase-separation has been used to control nanofiller localization in blended PLA composites [
20,
21,
22,
33]. Differences in nanofiller aspect ratio contribute to the phase-localization behavior in addition to phase separation in these composite blends [
27,
34,
35]. The studies of BioC involve particulate in a single polymer, conductivity of non-incorporated monolithic BioC, or large (microns to millimeters) fillers in a polymer blend [
12,
14,
36,
37].
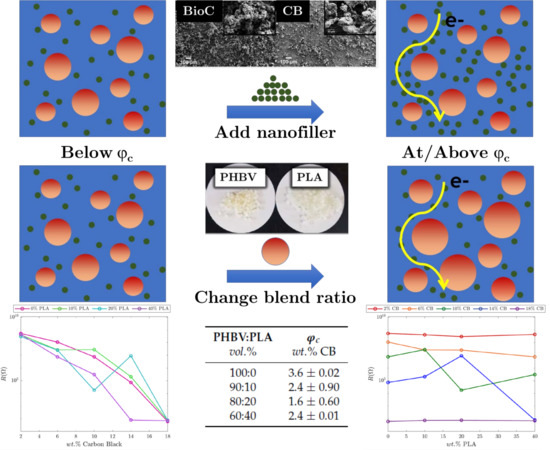
This study provides a novel investigation into the localization of CB and BioC nanofiller in biopolymer blends, and the resultant impact on mechanical and electrical properties of nanocomposites of interest for 3D printing. The focus is to investigate: (i) Nanofiller localization and resultant morphology in a blended biopolymer system, PHBV:PLA, (ii) electrical and mechanical percolation of the nanofilled composites, and (iii) the impact of the two nanofillers on processing and melt rheology. To elucidate how nanofiller localization would be expected to occur in the blends, we predict the interfacial energies of the blends using contact angle measurements and calculations of surface tension and verify these predictions using field emission scanning electron microscopy (FE-SEM). We then use impedance spectroscopy, Raman spectroscopy, and dynamic mechanical analysis (DMA) to evaluate the electrical and mechanical properties of the nanocomposites and calculate the percolation threshold in blended and non-blended systems. We use melt rheology during compounding and differential scanning calorimetry (DSC) to evaluate the impact of nanofiller addition during processing and how these interactions impact blend microstructure, thermal stability, and processability.
2. Theory
Polymer blend phase separation and incorporation of nanofillers can be described through the use of the Owens-Wendt theory [
38]. The thermodynamics of phase behavior of polymers in a blend is governed by the surface tension of the polymers. The surface tension is comprised of polar and dispersive components, and is typically measured using the contact angle between the polymer surface and liquids with known polar and dispersive values. The Owens-Wendt theory combines the Goods Equation (
1) with the Young’s Equation (
2) to create the linear form (
3) [
38]:
Substituting into the linear form (
) gives:
where
is the overall surface tension of the wetting liquid,
is the overall surface energy of the solid, the polar and dispersive components are represented by
,
and
,
respectively,
represents the interfacial tension between the solid and the liquid, and
is the contact angle between the liquid and the solid. A solid’s unknown polar and dispersive components (
,
) are calculated using contact angles with liquids of a known polar and dispersive component (
,
). This calculation is done by plotting contact angle data (
: Equations (
4) and (
6)) and using a line of best fit to determine a slope and
y-intercept [
39]. This form of the Harmonic Mean Method requires a minimum of two liquids for which surface tension data is well known to develop a best fit line.
In general, binary polymer blends exhibit either a blended morphology representative of miscibility, or they may exhibit a sea-island structure representative of an immiscible blend. For binary polymer composites, there are typically three potential locations of the nanofiller: It may exist in the major phase, the minor phase, or in the interfacial region between the two. The interfacial tension between the blended polymers and the nanoparticulates was calculated using the Harmonic Mean Equation (
8), and used to predict the morphology of the system [
39].
To determine miscibility of the polymer blend, the spreading coefficient
was calculated for phase
i on phase
j (
9). A positive
indicates that polymer
i will spread and is miscible on
j, while a negative number indicates immiscibility between
i on
j [
39].
The localization of the nanofiller in the blend can be predicted by determining the wetting coefficient of the polymer on the particulate,
. This coefficient is given in Equation (
10), where
represents the interfacial tension of the nanofiller on polymer
i [
39].
By this definition, if is greater than 1 the particulate will localize in the j phase of the polymer blend. In turn, if is less than −1 it will localize in the i phase and finally if is between 1 and −1 the particulate will localize in the interfacial region between the blends.
The contact angle between the polymers during molten flow can be characterized by Equation (
11) and used to predict the shape of the minor phase in the major [
39].
3. Materials and Methods
3.1. Materials
Commercially available PHBV (ENMAT Y 1000p, >98% purity) in pellet form was provided by Tianan (Nigbo City, China). PLA (2003D), also in pellet form, was supplied by Nature Works (Minnetonka, MN, USA). Powdered CB (Vulcan XCMAX22) with a density of 0.19 g/cm was provided by Cabot Chemical Corporation (Boston, MA, USA). Kraft lignin, with a density of 1.3 g/cm at 25 C, was purchased from Sigma-Aldrich, St. Louis, MO, USA and used to produce the BioC. All materials were stored in a desiccator prior to composite fabrication.
3.2. BioC Production
BioC was produced through slow pyrolysis of ball milled kraft lignin. Powdered kraft lignin (20 g) was milled with zirconia media for 24 h at 60 rpm. The media were removed and the milled lignin was stored at 105 C to remove moisture. Prior to pyrolysis in a tube furnace, nitrogen gas was purged through the tube at 0.95 CCM for 15 min to establish an oxygen free environment. After the initial purging, nitrogen flow was reduced to 0.55 CCM and heating began at 10 C/min to 750 C. After one hour at 750 C the temperature was ramped to 950 C at a rate of at 10 C/min and held for an hour. The sample was then allowed to cool to room temperature while still under nitrogen flow. Post pyrolysis, samples were stored at 105 C.
3.3. Composite Fabrication
Composites were prepared by melt compounding in a Thermo Fisher Scientific HAAKE Minilab II dual screw extruder at 50 rpm and 190
C for 5 min. During this time, rheology data were collected and viscosity was calculated from Minilab outputs as shown in
Appendix B. After mixing, blends were extruded into a Thermo Fisher Scientific Minijet Pro injection molder. Initial injection pressure was 600 bar for 10 s followed by 450 bar for 60 s. Injection temperatures were 190
C in the gun and 60
C in the mold, as established through prior optimization. All composites were injected into a DMA sample mold (Thermo Fisher Scientific, Waltham, MA USA, Part # 557-2295) with dimensions of 60 × 10 × 1 mm
.
Table 1 gives the blend ratios used for the PHBV:PLA blends and blends with nanofillers. CB nanofilled blends were made with all PHBV:PLA blend ratios, while samples of BioC were produced as feasible due to viscosity challenges during processing. Nanofiller was measured as a weight fraction of the total polymer blend.
3.4. Characterization
Interfacial parameters, polymer blend morphology, moduli, thermo-mechanical, and electrical properties were evaluated by video contact analysis, FE-SEM, DMA, DSC, Raman spectroscopy, and 4-point probe impedance spectroscopy measurements.
3.4.1. Contact Angle Analysis
To better understand the interactions of the polymer blends during mixing, polar and dispersive components of polymer surface tensions were calculated by measuring contact angles with deionized water and diiodomethane (MI). Contact angles were measured using a video contact angle system with drop sizes of 2.45 ± 0.5
L and a minimum of 5 measurements. Angles were divided into their dispersive and polar components using the Owens-Wendt relationship as described in
Section 2 (Equations (
1)–(
3)).
Table 2 shows the known dispersive and polar components of water and MI used as the contact liquid. Matlab code was developed to analyze the surface tensions and predict interfacial interactions, phase separation, and nanofiller localization in the polymer blends and nanofilled composites [
40].
It is particularly difficult to consistently measure contact angles for nanofillers in order to determine surface energies. Instead, alternate methods such as absorption and heat of immersion are standard techniques and have been explored in previous studies which provided the literature values for CB used in this study [
41,
42].
3.4.2. Dynamic Mechanical Analysis
Dynamic mechanical measurements were conducted on a TA instruments Q800 DMA. A multi frequency-strain experiment in the 3-pt bending configuration was run at a frequency of 1 Hz, amplitude of 20 m, and a force track of 125%. The temperature was equilibrated at −40 C for five minutes and increased to 150 C at a constant rate of 5 C/min. During the temperature ramp, storage modulus, loss modulus, and tan data were collected.
3.4.3. Differential Scanning Calorimetry
A TA Instruments Discovery DSC (Serial Number DSCI-0220) was used to assess the impact of the nanofillers on the polymers and blends. The nitrogen flow rate was 50 mL/min, as optimized in previous work [
43]. Samples were encapsulated in aluminum pans with a target sample weight of 5 mg ± 2 mg, and heated from −20
C to 180
C in the first heating cycle at a rate of 10
C/min. After equilibrating to 190
C, they were held at 190
C for 2 min prior to cooling at 10
C/min to −20
C. The samples were then heated at 10
C/min to 195
C in the second and final heating cycle. The glass transition temperature (
) was taken to be the midpoint of the heat capacity change, the melting temperature (
) was measured as the minimum of the endothermic peak upon heating, the cold crystallization temperature (
) was measured as the maximum of the exothermic peak (when present) upon heating, and the crystallization temperature (
) was taken as the maximum temperature of the exothermic peak upon cooling (in between the first and second heating cycles).
The percent crystallinity of the PHBV and PLA in the matrix,
, was determined using a modification to the standard equation for single phase composites [
44]:
where
and
are the enthalpies of melting and cold crystallization measured upon heating,
is the weight fraction for the PHBV or PLA, and
) is the reference value for 100% crystalline polymer: 146 J/g or 12.5 kJ/mole [
45,
46] for PHB and 93.7 J/g for PLA [
47]. As a reference, a typical value for the crystallinity of annealed PHB samples measured by Barham was 86% et al. [
45]. To convert between volume and weight percent, densities of 1.24 g/cm
for PHBV and 1.25 g/cm
for PLA were used. Sample density was measured using a Mettler Toledo XS205DU Excellence series analytical balance with the Mettler Toledo Density determination kit for Excellence XP/XS analytical balances. The measurement is a buoyancy technique based on the Archimedes’ principle.
To evaluate the effect of BioC on each of the individual biopolymers, DSC was used to evaluate neat PHBV and PLA compared with each of the polymers with BioC. BioC was added on top of each of the biopolymers prior to the first heating cycle. The melting endotherms of the neat polymers were compared to the endotherms of the first and second heating cycles in the BioC nanofilled biopolymers.
3.4.4. Scanning Electron Microscopy
To obtain a cross section of the nanofilled blends, samples were cryo-fractured using liquid nitrogen. Examination of fracture surfaces though FE-SEM was conducted on a Supra 55VP System 2512 at 1 kV with an SE2 detector. Samples were uncoated. Nanofiller and matrix microstructure were characterized and the particle-matrix interfaces were imaged along with assessing potential localization of the nanofillers.
3.4.5. Impedance Spectroscopy
The polymers’ resistivity, impedance, capacitance, and phase angle were measured using a Hioki 3522-50 LCR HiTester in the 4 point configuration at room temperature. Each data point is the average of 3 measurements taken from 0.1 Hz to 10
Hz. The percolation threshold for the composites was calculated using a Sigmoidal–Boltzmann function [
48]:
where
is the measured resistivity,
is the lower limit for resistivity,
is the upper limit for resistivity,
is the percentage of CB in the blend,
is the percolation threshold, and
is the slope in proximity to the percolation threshold. The data for resistivity from impedance spectroscopy at 10
Hz were fitted using the curve fitting toolbox in Matlab R2018b.
3.4.6. Raman Spectroscopy
A fully integrated high resolution Raman microscope for confocal Raman analysis, Horiba LabRam HR Evolution NIR, was used to evaluate CB, BioC, and nanofilled composites for graphitic content. The confocal microscope was used for optical images of the composite samples at 20× LWD and 50× LWD. During Raman spectral acquisition, Raman spectra were acquired at 50× LWD and 100×, the stigmatic spectrometer was used with a grating of 1800 gr/mm, and the 532 nm 100 mW laser at 1%. To reduce the impact of heating in the samples, the acquisition time was 3 s, and the spectra were accumulated for 3 acquisitions. Raman spectra were recorded between 1000 and 1800 cm
, which corresponds to the spectral region that provides data on the microstructure of carbons giving a measure of the graphite band at 1530–1610 cm
(G) and the disorder-induced band at 1320–1370 cm
(D) [
23,
49,
50]. The cftool in Matlab was used to fit gaussian exponentials to the CB and BioC peaks observed in this region to determine the peak locations for D and G peaks and the intensity ratio,
[
51].
5. Conclusions
Conductive nanofiller localization was tailored through phase separation in the PHBV:PLA blends. The partitioning of the nanofiller for the phase-separated morphology of the blends was calculated from surface energies derived from contact angle analysis. FE-SEM was used to verify the location of the nanofillers; CB localized within the PHBV phase as predicted by our calculations. The lignin-derived BioC had a more complex reaction with the biopolymers, as evidenced by the change in blend morphology as observed via FE-SEM, a rapid reduction in melt upon addition of BioC, and DSC results that show a significant reduction in the PHBV melting peak and the absence of a melting peak for the PLA phase. These material changes upon the addition of BioC are likely due to a reaction occurring between the biopolymers and BioC, potentially due to residual species from the Kraft lignin process. The rapid reduction in melt viscosity and source of lignin-BioC/polymer interaction will be critical to implementing BioC as a potential alternative to CB.
The impedance of CB in PHBV:PLA blends was measured for weight percentages of 0, 2, 6, 10, 14, and 18 wt.% CB and PHBV:PLA blends of 100:0, 90:10, 80:20, and 60:40. The phase-separated blends also modified the percolation threshold, which varied between 3.6% and 1.6% CB with the maximum value for 100% PHBV and the minimum for the 80:20 blend. When comparing CB composites with BioC composites with 2% nanofiller (approximately at the percolation threshold), the 2% BioC had a resistance of 3.43 × 10 as compared to 2.99 × 10 for the CB, indicating that BioC could perform comparably to CB as a conductive nanofiller if the processing challenges can be overcome. Results at higher BioC loading would be required to establish if this comparable behavior is present for more conductive samples. Both nanofillers exhibited graphitic content individually and incorporated into PHBV:PLA blends, as determined via Raman spectroscopy, which is necessary for a conductive network of nanoparticles. These results further support the use of nano sized lignin-derived BioC as an electrically conductive nanofiller in biocomposites.