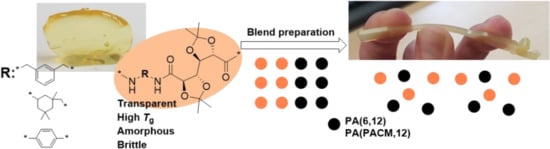
Towards High-performance Materials Based on Carbohydrate-Derived Polyamide Blends
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Methods
A Typical Procedure for Blend Preparation
2.3. Characterization
3. Results and Discussion
3.1. Preparation of Blends
3.2. Thermal and Morphological Characterization of the Blends
3.2.1. Thermal Properties of PA(6,12) Blends
3.2.2. Thermal Properties of PA(PACM,12) Trogamid CX Blends
3.2.3. Morphological Study of the Blends by SEM
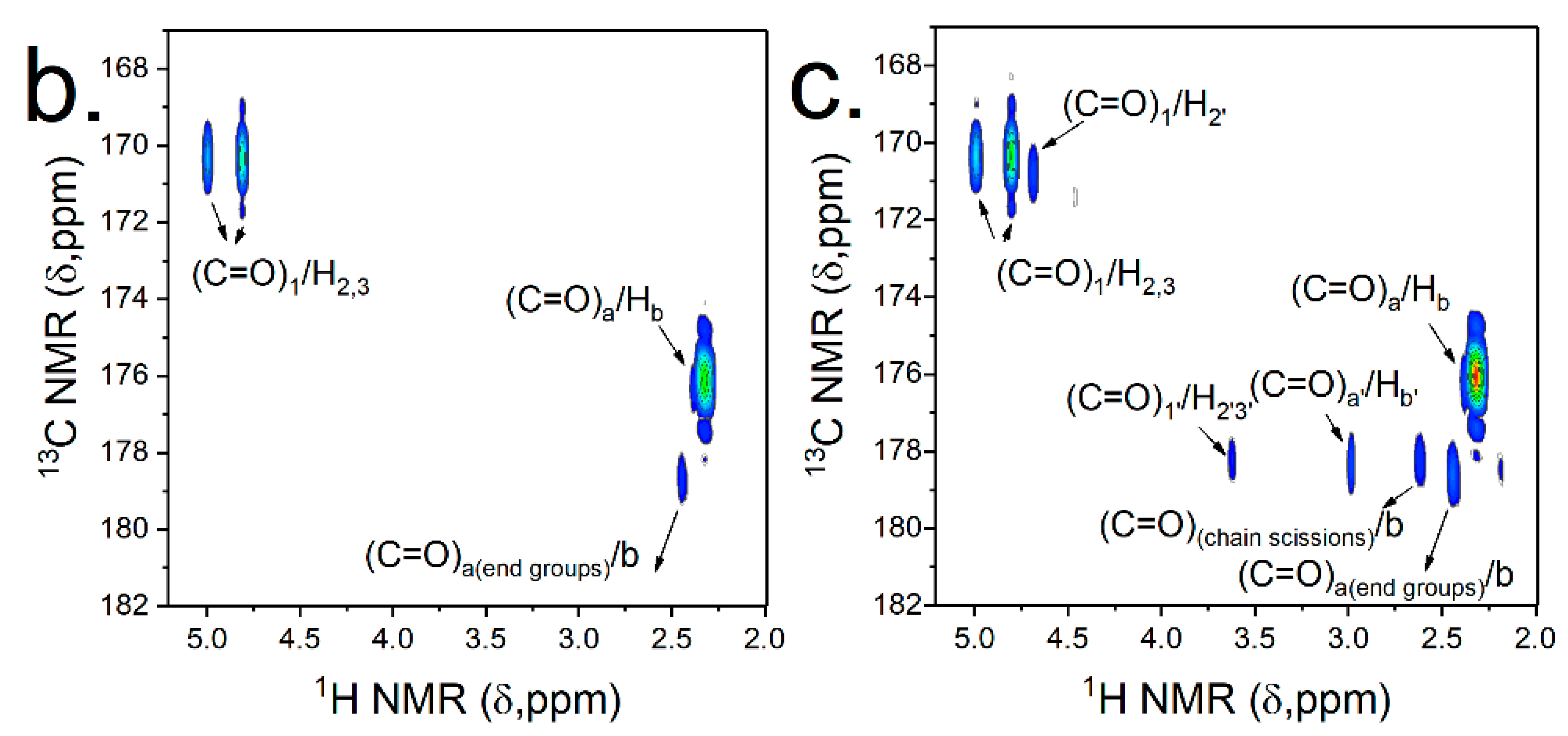
3.2.4. Structural Transformations Induced by the Thermal Processing of PA(PPDA,GalXMe) with PA(PACM,12)
3.3. Material Properties of Blends
Mechanical Performance of Blends
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability
Conflicts of Interest
References
- Black, W.A.P.; Dewar, E.T.; Rutherford, D. Carbohydrate Derived Polyamides. U.S. Patent 3,225,012, 21 December 1965. [Google Scholar]
- Winnacker, M.; Rieger, B. Biobased Polyamides: Recent Advances in Basic and Applied Research. Macromol. Rapid Commun. 2016, 37, 1391–1413. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Galbis, J.A.; de Gracia Garcia-Martin, M.; de Paz, M.V.; Galbis, E. Synthetic Polymers from Sugar-Based Monomers. Chem. Rev. 2016, 116, 1600–1636. [Google Scholar] [CrossRef] [PubMed]
- Kiely, D.E.; Vishwanathan, A.; Jarman, B.P.; Manley-Harris, M. Synthesis of Poly(galactaramides) from Alkylene- and Substituted Alkylenediammonium Galactarates. J. Carbohydr. Chem. 2009, 28, 348–368. [Google Scholar] [CrossRef]
- Chnari, E.; Lari, H.B.; Tian, L.; Uhrich, K.E.; Moghe, P.V. Nanoscale anionic macromolecules for selective retention of low-density lipoproteins. Biomaterials 2005, 26, 3749–3758. [Google Scholar] [CrossRef] [PubMed]
- Srirangan, K.; Akawib, L.; Moo-Younga, M.; Choua, C.P. Towards sustainable production of clean energy carriers from biomass resources. Appl. Energy 2012, 100, 172–186. [Google Scholar] [CrossRef]
- Kshirsagar, C.M.; Anand, R. An overview of biodiesel extraction from the third generation biomass feedstock: Prospects and challenges. Appl. Mech. Mater. 2014, 592–594, 1881–1885. [Google Scholar] [CrossRef]
- Wróblewska, A.A.; Bernaerts, K.V.; De Wildeman, S.M.A. Rigid, bio-based polyamides from galactaric acid derivatives with elevated glass transition temperatures and their characterization. Polymer 2017, 124, 252–262. [Google Scholar] [CrossRef]
- Butler, K.; Lawrance, D.R. Linear Polyamides. GB Patent 750,822, 20 June 1956. [Google Scholar]
- Spinella, S.; Cai, J.; Samuel, C.; Zhu, J.; McCallum, S.A.; Habibi, Y.; Raquez, J.M.; Dubois, P.; Gross, R.A. Polylactide/Poly(omega-hydroxytetradecanoic acid) Reactive Blending: A Green Renewable Approach to Improving Polylactide Properties. Biomacromolecules 2015, 16, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, K.; Kiekens, P. Biopolymers: Overview of several properties and consequences on their applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Urayama, H.; Kanamori, T.; Fukushima, K.; Kimura, Y. Controlled crystal nucleation in the melt-crystallization of poly(l-lactide) and poly(l-lactide)/poly(d-lactide) stereocomplex. Polymer 2003, 44, 5635–5641. [Google Scholar] [CrossRef]
- Lavilla, C.; Alla, A.; Martínez de Ilarduya, A.; Benito, E.; García-Martín, M.G.; Galbis, J.A.; Muñoz-Guerra, S. Biodegradable aromatic copolyesters made from bicyclic acetalized galactaric acid. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3393–3406. [Google Scholar] [CrossRef]
- Lavilla, C.; Alla, A.; Martínez de Ilarduya, A.; Benito, E.; García-Martín, M.G.; Galbis, J.A.; Muñoz-Guerra, S. Carbohydrate-based copolyesters made from bicyclic acetalized galactaric acid. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1591–1604. [Google Scholar] [CrossRef]
- Gubbels, E.; Lavilla, C.; de Ilarduya, A.M.; Noordover, B.A.J.; Koning, C.E.; Muñoz-Guerra, S. Partially renewable copolyesters prepared from acetalized D-glucitol by solid-state modification of poly(butylene terephthalate). J. Polym. Sci. Part A Polym. Chem. 2014, 52, 164–177. [Google Scholar] [CrossRef]
- Lavilla, C.; Gubbels, E.; Martínez de Ilarduya, A.; Noordover, B.A.J.; Koning, C.E.; Muñoz-Guerra, S. Solid-State Modification of PBT with Cyclic Acetalized Galactitol and D-Mannitol: Influence of Composition and Chemical Microstructure on Thermal Properties. Macromolecules 2013, 46, 4335–4345. [Google Scholar] [CrossRef]
- Mehtio, T.; Nurmi, L.; Ramo, V.; Mikkonen, H.; Harlin, A. Synthesis and characterization of copolyanhydrides of carbohydrate-based galactaric acid and adipic acid. Carbohydr. Res. 2015, 402, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, S.; Nayak, S.K.; Mohanty, S. Analysis and evaluation of biobased polyester of PTT/PBAT blend: Thermal, dynamic mechanical, interfacial bonding, and morphological properties. Polym. Adv. Technol. 2016, 27, 938–945. [Google Scholar] [CrossRef]
- Enriquez, E.; Mohanty, A.K.; Misra, M. Biobased polymer blends of poly(trimethylene terephthalate) and high density polyethylene. Mater. Des. 2016, 90, 984–990. [Google Scholar] [CrossRef]
- Cicala, G.; Latteri, A.; Saccullo, G.; Recca, G.; Sciortino, L.; Lebioda, S.; Saake, B. Investigation on Structure and Thermomechanical Processing of Biobased Polymer Blends. J. Polym. Environ. 2016, 25, 750–758. [Google Scholar] [CrossRef]
- Reulier, M.; Avérous, L. Elaboration, morphology and properties of renewable thermoplastics blends, based on polyamide and polyurethane synthesized from dimer fatty acids. Eur. Polym. J. 2015, 67, 418–427. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Huang, Z.-G.; Diao, X.-Q.; Weng, Y.-X.; Wang, Y.-Z. Characterization of the effect of REC on the compatibility of PHBH and PLA. Polym. Test. 2015, 42, 17–25. [Google Scholar] [CrossRef]
- Spinella, S.; Samuel, C.; Raquez, J.-M.; McCallum, S.A.; Gross, R.; Dubois, P. Green and Efficient Synthesis of Dispersible Cellulose Nanocrystals in Biobased Polyesters for Engineering Applications. Sustain. Chem. Eng. 2016, 4, 2517–2527. [Google Scholar] [CrossRef]
- Bendaoud, A.; Kehrbusch, R.; Baranov, A.; Duchemin, B.; Maigret, J.E.; Falourd, X.; Staiger, M.P.; Cathala, B.; Lourdin, D.; Leroy, E. Nanostructured cellulose-xyloglucan blends via ionic liquid/water processing. Carbohydr. Polym. 2017, 168, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.S.; Janke, A.; Gohs, U.; Fery, A.; Heinrich, G. Some nanomechanical properties and degree of branching of electron beam modified polyamide 6. Eur. Polym. J. 2017, 88, 221–230. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Janke, A.; Jehnichen, D.; Gohs, U.; Heinrich, G. Influence of electron-induced reactive processing on structure, morphology and nano-mechanical properties of polyamide 6/fluoroelastomer blends. Polymer 2018, 142, 394–402. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Bhowmick, A.K. Dynamic vulcanization of novel nanostructured polyamide 6/fluoroelastomer thermoplastic elastomeric blends with special reference to morphology, physical properties and degree of vulcanization. Polymer 2015, 57, 105–116. [Google Scholar] [CrossRef]
- Lavilla, C.; Alla, A.; de Ilarduya, A.M.; Benito, E.; Garcia-Martin, M.G.; Galbis, J.A.; Munoz-Guerra, S. Carbohydrate-based polyesters made from bicyclic acetalized galactaric acid. Biomacromolecules 2011, 12, 2642–2652. [Google Scholar] [CrossRef] [PubMed]
- Lavilla, C.; Alla, A.; Martínez de Ilarduya, A.; Muñoz-Guerra, S. High Tg Bio-Based Aliphatic Polyesters from Bicyclic d-Mannitol. Biomacromolecules 2013, 14, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Lavilla, C.; Muñoz-Guerra, S. Sugar-based aromatic copolyesters: A comparative study regarding isosorbide and diacetalized alditols as sustainable comonomers. Green Chem. 2013, 15, 144–151. [Google Scholar] [CrossRef]
- Lavilla, C.; Muñoz-Guerra, S. Biodegradation and hydrolytic degradation of poly(butylene terephthalate) copolyesters containing cyclic sugar units. Polym. Degrad. Stab. 2012, 97, 1762–1771. [Google Scholar] [CrossRef]
- Muñoz-Guerra, S. Carbohydrate-based polyamides and polyesters: An overview illustrated with two selected examples. High Perform. Polym. 2012, 24, 9–23. [Google Scholar] [CrossRef]
- Muñoz-Guerra, S.; Lavilla, C.; Japu, C.; Martínez de Ilarduya, A. Renewable terephthalate polyesters from carbohydrate-based bicyclic monomers. Green Chem. 2014, 16, 1716–1739. [Google Scholar] [CrossRef]
- Wróblewska, A.A.; Noordijk, J.; Das, N.; Gerards, C.; Wildeman, S.M.A.; Bernaerts, K.V. Structure–Property Relations in New Cyclic Galactaric Acid Derived Monomers and Polymers Therefrom: Possibilities and Challenges. Macromol. Rapid Commun. 2018, 34, 1800077. [Google Scholar] [CrossRef] [PubMed]
- Wroblewska, A.; Stevens, S.; Garsten, W.; Wildeman, S.M.A.; Bernaerts, K.V. A solvent-free method for the copolymerization of labile sugar-derived building blocks into polyamides. Sustain. Chem. Eng. 2018, 6, 13504–13517. [Google Scholar] [CrossRef] [PubMed]
- Koning, C.; Van Duin, M.; Pagnoulle, C.; Jerome, R. Strategies for compatibilization of polymer blends. Prog. Polym. Sci. 1998, 23, 707–757. [Google Scholar] [CrossRef] [Green Version]
- Grohens, Y.; Thomas, S.; Jyotishkumar, P. Characterization of Polymer Blends: Miscibility, Morphology and Interfaces; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Joy, J.; Wilson, R.; Mathew, P.L.; Thomas, S. Natural polymer blends and their composites: Micro- and nanostructured polymer systems. In Micro- and Nanostructured Polymer Systems: From Synthesis to Applications; Thomas, S., Shanks, R.A., Joy, J., Eds.; Apple Academic Press: Oakville, ON, Canada, 2016. [Google Scholar]
- Imre, B.; García, L.; Puglia, D.; Vilaplana, F. Reactive compatibilization of plant polysaccharides and biobased polymers: Review on current strategies, expectations and reality. CarbohydR. Polym. 2019, 209, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Walha, F.; Lamnawar, K.; Maazouz, A.; Jaziri, M. Biosourced blends based on poly (lactic acid) and polyamide 11: Structure–properties relationships and enhancement of film blowing processability. Adv. Polym. Technol. 2018, 37, 2061–2074. [Google Scholar] [CrossRef]
- Gug, J.; Soule, J.; Tan, B.; Sobkowicz, M.J. Effects of chain-extending stabilizer on bioplastic poly(lactic acid)/polyamide blends compatibilized by reactive extrusion. Polym. Degrad. Stab. 2018, 153, 118–129. [Google Scholar] [CrossRef]
- Gug, J.; Sobkowicz, M.J. Improvement of the mechanical behavior of bioplastic poly(lactic acid)/polyamide blends by reactive compatibilization. J. Appl. Polym. Sci. 2016, 133, 43350–43362. [Google Scholar] [CrossRef]
- Yang, S.; Madbouly, S.A.; Schrader, J.A.; Grewell, D.; Kessler, M.R.; Graves, W.R. Processing and characterization of bio-based poly (hydroxyalkanoate)/poly(amide) blends: Improved flexibility and impact resistance of PHA-based plastics. J. Appl. Polym. Sci. 2015, 132, 42209–42219. [Google Scholar] [CrossRef]
- Isayev, A.I. Encyclopedia of Polymer Blends; Wiley-VCH: Weinheim, Germany, 2011; Volume 2. [Google Scholar]
- Morgan, P.W.; Kwolek, S.L. Polyamides from Phenylenediamines and Aliphatic Diacids. Macromolecules 1974, 8, 104–111. [Google Scholar] [CrossRef]
- Gies, A.P.; Ellison, S.T.; Stow, S.M.; Hercules, D.M. Matrix-assisted laser desorption/ionization-time-of-flight/time-of-flight collision-induced dissociation study of poly(p-phenylenediamine terephthalamide) fragmentation reactions. Anal. Chim. Acta 2014, 808, 124–143. [Google Scholar] [CrossRef] [PubMed]
- Van Krevelen, D.W.; Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure: Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewska, A.A.; Leoné, N.; De Wildeman, S.M.A.; Bernaerts, K.V. Towards High-performance Materials Based on Carbohydrate-Derived Polyamide Blends. Polymers 2019, 11, 413. https://doi.org/10.3390/polym11030413
Wróblewska AA, Leoné N, De Wildeman SMA, Bernaerts KV. Towards High-performance Materials Based on Carbohydrate-Derived Polyamide Blends. Polymers. 2019; 11(3):413. https://doi.org/10.3390/polym11030413
Chicago/Turabian StyleWróblewska, Aleksandra A., Nils Leoné, Stefaan M. A. De Wildeman, and Katrien V. Bernaerts. 2019. "Towards High-performance Materials Based on Carbohydrate-Derived Polyamide Blends" Polymers 11, no. 3: 413. https://doi.org/10.3390/polym11030413