Interface Modulation of Core-Shell Structured BaTiO3@polyaniline for Novel Dielectric Materials from Its Nanocomposite with Polyarylene Ether Nitrile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PEN
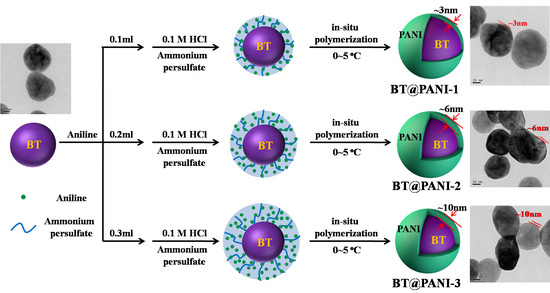
2.3. In-Situ Preparation of Surface Functionalized BaTiO3 Nanoparticles
2.4. Preparation of PEN/BT@PANI Nanocomposite Films
2.5. Characterization
3. Results and Discussion
3.1. Characterization of BT@PANI Nanoparticles
3.2. Morphology of the Nanocomposite Films
3.3. Thermal Properties of the Nanocomposite Films
3.4. Mechanical Properties of the Nanocomposite Films
3.5. Dielectric Properties of the Nanocomposite Films
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Wang, Q. Ferroelectric Polymers and Their Energy-Related Applications. Macromol. Chem. Phys. 2016, 217, 1228–1244. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y. Recent Progress in Supercapacitors: From Materials Design to System Construction. Adv. Mater. 2013, 25, 5336–5342. [Google Scholar] [CrossRef] [PubMed]
- Chu, B. A Dielectric Polymer with High Electric Energy Density and Fast Discharge Speed. Science (80- ) 2006, 313, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Khanchaitit, P.; Han, K.; Gadinski, M.R.; Li, Q.; Wang, Q. Ferroelectric polymer networks with high energy density and improved discharged efficiency for dielectric energy storage. Nat. Commun. 2013, 4, 2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Seok, S.I.; Chu, B.; Doğan, F.; Zhang, Q.; Wang, Q. Nanocomposites of Ferroelectric Polymers with TiO2 Nanoparticles Exhibiting Significantly Enhanced Electrical Energy Density. Adv. Mater. 2009, 21, 217–221. [Google Scholar] [CrossRef]
- Dang, Z.-M.; Yuan, J.-K.; Yao, S.-H.; Liao, R.-J. Flexible Nanodielectric Materials with High Permittivity for Power Energy Storage. Adv. Mater. 2013, 25, 6334–6365. [Google Scholar] [CrossRef]
- Arbatti, M.; Shan, X.; Cheng, Z.-Y.; Cheng, Z.-Y. Ceramic–Polymer Composites with High Dielectric Constant. Adv. Mater. 2007, 19, 1369–1372. [Google Scholar] [CrossRef]
- Wei, R.; Wang, J.; Zhang, H.; Han, W.; Liu, X. Crosslinked Polyarylene Ether Nitrile Interpenetrating with Zinc Ion Bridged Graphene Sheet and Carbon Nanotube Network. Polymers 2017, 9, 342. [Google Scholar] [CrossRef]
- Liu, X.; Long, S.; Luo, D.; Chen, W.; Cao, G. Preparation and properties of polyarylene ether nitrites/multi-walled carbon nanotubes composites. Mater. Lett. 2008, 62, 19–22. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Gu, J.; Liu, H.; Liu, C.; Luo, C.; Kong, J.; Shao, Q.; Wang, N.; Guo, Z.; Liu, X. Ultralight, highly compressible and fire-retardant graphene aerogel with self-adjustable electromagnetic wave absorption. Carbon 2018, 139, 1126–1135. [Google Scholar] [CrossRef]
- Dang, Z.-M.; Lin, Y.-H.; Nan, C.-W. Novel Ferroelectric Polymer Composites with High Dielectric Constants. Adv. Mater. 2003, 15, 1625–1629. [Google Scholar] [CrossRef]
- Pu, Z.; Tong, L.; Long, Y.; Yang, W.; Huang, X.; Liu, X. Composites of Core/Shell-Structured Copper-Phthalocyanine-Decorated TiO2 Particles Embedded in Poly(Arylene Ether Nitrile) Matrix with Enhanced Dielectric Properties. J. Electron. Mater. 2014, 43, 2597–2606. [Google Scholar] [CrossRef]
- Yao, Z.; Cao, M.; Zhang, S.; Lanagan, M.T.; Liu, H.; Song, Z.; Hao, H.; Yu, Z. Homogeneous/Inhomogeneous-Structured Dielectrics and their Energy-Storage Performances. Adv. Mater. 2017, 29, 1601727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Wong, C. Recent advances in high-k nanocomposite materials for embedded capacitor applications. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 1322–1328. [Google Scholar]
- Kim, P.; Doss, N.M.; Tillotson, J.P.; Hotchkiss, P.J.; Pan, M.-J.; Marder, S.R.; Li, J.; Calame, J.P.; Perry, J.W. High Energy Density Nanocomposites Based on Surface-Modified BaTiO3 and a Ferroelectric Polymer. ACS Nano 2009, 3, 2581–2592. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhong, J.; Yang, J.; Ma, Z.; Liu, X. Flexible polyarylene ether nitrile/BaTiO3 nanocomposites with high energy density for film capacitor applications. J. Electron. Mater. 2011, 40, 141–148. [Google Scholar] [CrossRef]
- Zheng, L.; Liang, G.; Gu, A.; Yuan, L.; Guan, Q. Unique pure barium titanate foams with three-dimensional interconnecting pore channels and their high-k cyanate ester resin composites at very low barium titanate loading. J. Mater. Chem. C 2016, 4, 10654–10663. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, J.; Yuan, Q.; Niu, Y.; Bai, Y.; Wang, H. Significantly Enhanced Breakdown Strength and Energy Density in Sandwich-Structured Barium Titanate/Poly(vinylidene fluoride) Nanocomposites. Adv. Mater. 2015, 27, 6658–6663. [Google Scholar] [CrossRef]
- Wei, R.; Yang, R.; Xiong, Z.; Xiao, Q.; Li, K.; Liu, X. Enhanced dielectric properties of polyarylene ether nitriles filled with core–shell structured PbZrO3 around BaTiO3 nanoparticles. J. Electron. Mater. 2018, 47, 6177–6184. [Google Scholar] [CrossRef]
- Pan, Z.; Yao, L.; Zhai, J.; Shen, B.; Liu, S.; Wang, H. Excellent energy density of polymer nanocomposites containing BaTiO3@Al2O3 nanofibers induced by moderate interfacial area. J. Mater. Chem. A 2016, 4, 13259–13264. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, X.; Bi, K.; Zhang, J.; Huang, Y.; Wu, L.; Zhao, P.; Xu, K.; Lei, M.; Li, L. Significantly enhanced energy storage performance promoted by ultimate sized ferroelectric BaTiO3 fillers in nanocomposite films. Nano Energy 2017, 31, 49–56. [Google Scholar] [CrossRef]
- You, Y.; Wei, R.; Yang, R.; Yang, W.; Hua, X.; Liu, X. Crystallization behaviors of polyarylene ether nitrile filled in multi-walled carbon nanotubes. RSC Adv. 2016, 6, 70877–70883. [Google Scholar] [CrossRef]
- Tang, H.; Pu, Z.; Huang, X.; Wei, J.; Liu, X.; Lin, Z. Novel blue-emitting carboxyl-functionalized poly(arylene ether nitrile)s with excellent thermal and mechanical properties. Polym. Chem. 2014, 5, 3673–3679. [Google Scholar] [CrossRef]
- You, Y.; Han, W.; Tu, L.; Wang, Y.; Wei, R.; Liu, X. Double-layer core/shell-structured nanoparticles in polyarylene ether nitrile-based nanocomposites as flexible dielectric materials. RSC Adv. 2017, 7, 29306–29311. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Du, Y.; Xu, P.; Qiang, R.; Wang, Y.; Ding, D.; Xue, J.; Ma, J.; Zhao, H.; Han, X. Constructing Uniform Core–Shell PPy@PANI Composites with Tunable Shell Thickness toward Enhancement in Microwave Absorption. ACS Appl. Mater. Interfaces 2015, 7, 20090–20099. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Hao, Y.; Zhang, J.; Lei, M.; Bi, K. Particle size effect of BaTiO3 nanofillers on the energy storage performance of polymer nanocomposites. Nanoscale, 2017, 9, 16386–16395. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Du, X.; Mao, H.; Tang, X.; Wei, R.; Liu, X. Synergistic enhancement of mechanical, crystalline and dielectric properties of polyarylene ether nitrile-based nanocomposites by unidirectional hot stretching-quenching. Polymer Int. 2017, 66, 1151–1158. [Google Scholar] [CrossRef]
- Zhang, B.; Du, Y.; Zhang, P.; Zhao, H.; Kang, L.; Han, X.; Xu, P. Microwave absorption enhancement of Fe3O4/polyaniline core/shell hybrid microspheres with controlled shell thickness. J. Appl. Polym. Sci. 2013, 130, 1909–1916. [Google Scholar] [CrossRef]
- Tang, H.; Wang, P.; Zheng, P.; Liu, X. Core-shell structured BaTiO3@polymer hybrid nanofiller for poly(arylene ether nitrile) nanocomposites with enhanced dielectric properties and high thermal stability. Compos. Sci. Technol. 2016, 123, 134–142. [Google Scholar] [CrossRef]
- Yu, K.; Wang, H.; Zhou, Y.; Bai, Y.; Niu, Y. Enhanced dielectric properties of BaTiO3/poly(vinylidene fluoride) nanocomposites for energy storage applications. J. Appl. Phys. 2013, 113, 34105. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P. Core-Shell Structured High- k Polymer Nanocomposites for Energy Storage and Dielectric Applications. Adv. Mater. 2014, 27, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Pu, Z.; Feng, M.; Tong, L.; Liu, X. BaTiO3@MWCNTs core/shell nanotubes embedded PEN nanocomposite films with high thermal stability and highpermittivity. Mater. Lett. 2013, 96, 139–142. [Google Scholar] [CrossRef]
- Niu, Y.; Bai, Y.; Yu, K.; Wang, Y.; Xiang, F.; Wang, H. Effect of the Modifier Structure on the Performance of Barium Titanate/Poly(vinylidene fluoride) Nanocomposites for Energy Storage Applications. ACS Appl. Mater. Interfaces 2015, 7, 24168–24176. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Huang, X.; Huang, Y.; Yang, K.; Jiang, P. Core@Double-Shell Structured BaTiO3–Polymer Nanocomposites with High Dielectric Constant and Low Dielectric Loss for Energy Storage Application. J. Phys. Chem. C 2013, 117, 22525–22537. [Google Scholar] [CrossRef]
- Wei, R.; Li, K.; Ma, J.; Zhang, H.; Liu, X. Improving dielectric properties of polyarylene ether nitrile with conducting polyaniline. J. Mater. Sci. Mater. Electron. 2016, 27, 9565–9571. [Google Scholar] [CrossRef]
- Yang, R.; Wei, R.; Tong, L.; Jia, K.; Liu, X.; Li, K. Crosslinked polyarylene ether nitrile film as flexible dielectric materials with ultrahigh thermal stability. Sci. Rep. 2016, 6, 36434. [Google Scholar] [CrossRef] [PubMed] [Green Version]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Y.; Wang, Y.; Tu, L.; Tong, L.; Wei, R.; Liu, X. Interface Modulation of Core-Shell Structured BaTiO3@polyaniline for Novel Dielectric Materials from Its Nanocomposite with Polyarylene Ether Nitrile. Polymers 2018, 10, 1378. https://doi.org/10.3390/polym10121378
You Y, Wang Y, Tu L, Tong L, Wei R, Liu X. Interface Modulation of Core-Shell Structured BaTiO3@polyaniline for Novel Dielectric Materials from Its Nanocomposite with Polyarylene Ether Nitrile. Polymers. 2018; 10(12):1378. https://doi.org/10.3390/polym10121378
Chicago/Turabian StyleYou, Yong, Yajie Wang, Ling Tu, Lifen Tong, Renbo Wei, and Xiaobo Liu. 2018. "Interface Modulation of Core-Shell Structured BaTiO3@polyaniline for Novel Dielectric Materials from Its Nanocomposite with Polyarylene Ether Nitrile" Polymers 10, no. 12: 1378. https://doi.org/10.3390/polym10121378