Polymeric Nanocomposites Membranes with High Permittivity Based on PVA-ZnO Nanoparticles for Potential Applications in Flexible Electronics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Preparation of PVA/ZnO Membranes
2.2. Characterization
3. Results and Discussion
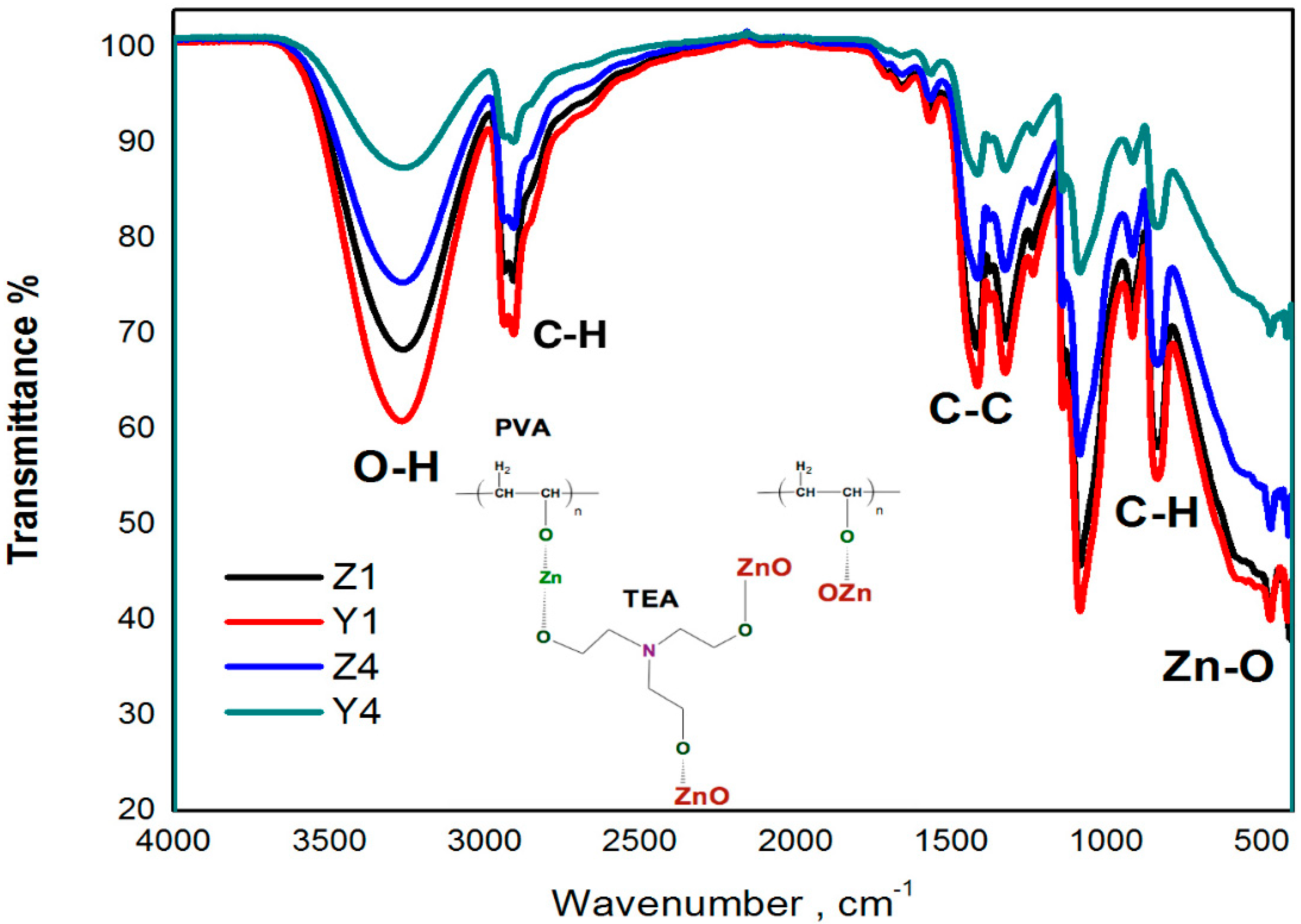
3.1. FTIR Analysis
3.2. Absorbance Characteristics and Optical Band Gap
3.3. Surface Morphology
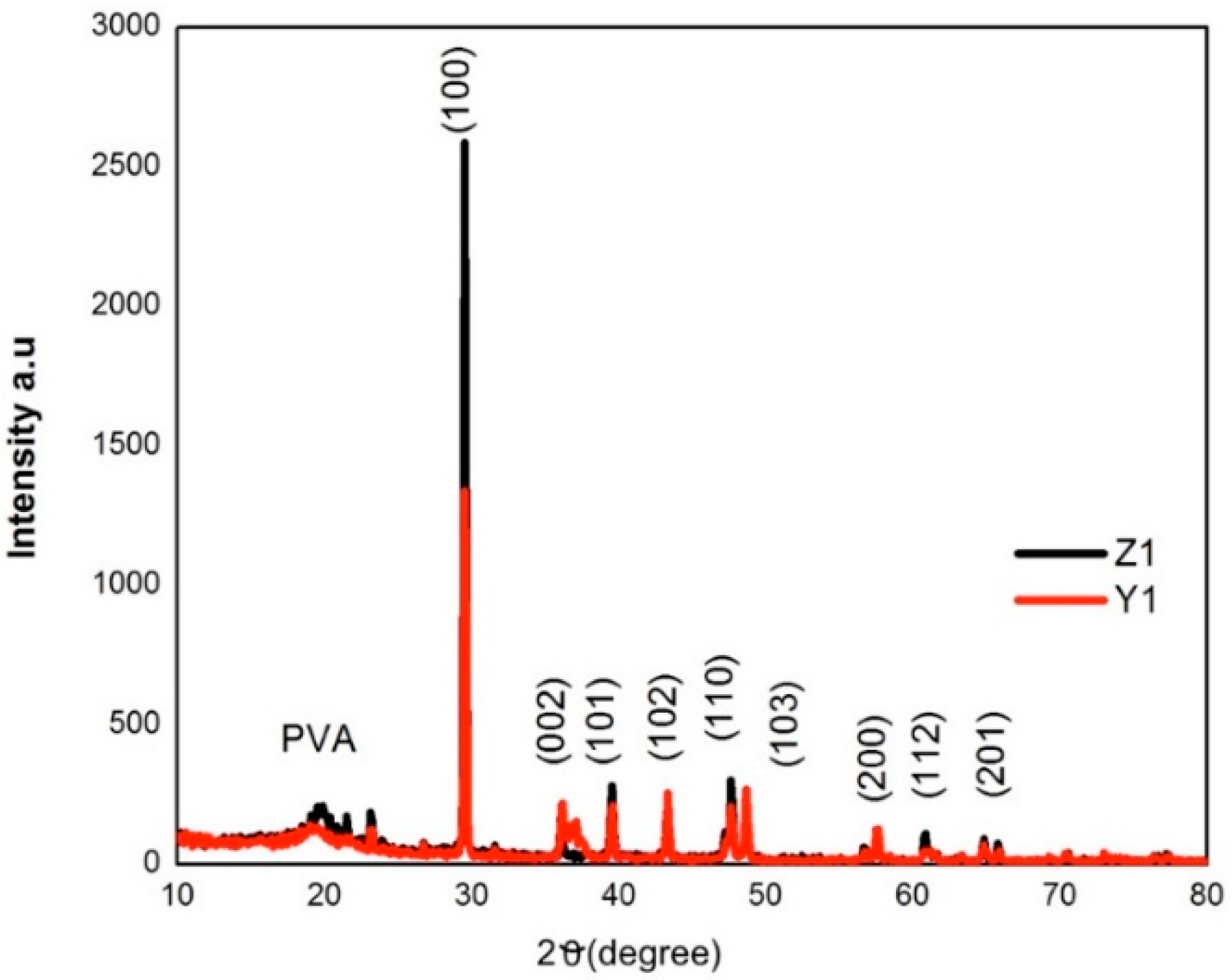
3.4. Structural Analysis
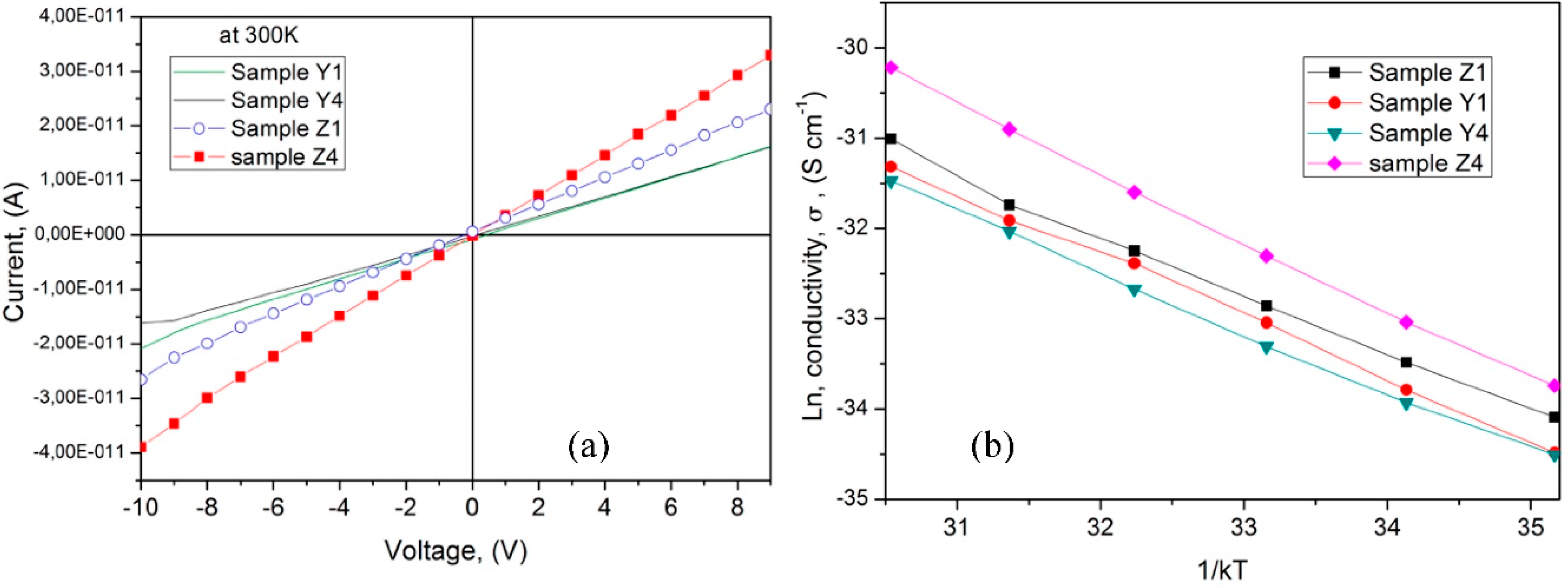
3.5. Electrical Properties
3.6. Dielectric Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, M.; Prasad, N.; Sivalingam, M.M.; Sastikumar, D.; Karthikeyan, B. Optical, phonon properties of ZnO–PVA, ZnO–GO–PVA nanocomposite free standing polymer films for UV sensing. J. Mater. Sci. Mater. Electron. 2018, 29, 365–373. [Google Scholar] [CrossRef]
- Mathen, J.J.; Madhavan, J.; Thomas, A.; Edakkara, A.J.; Sebastian, J.; Joseph, G.P. Transparent ZnO–PVA binary composite for UV-A photo detector: Optical, electrical and thermal properties followed by laser induced fluorescence. J. Mater. Sci. Mater. Electron. 2017, 28, 7190–7203. [Google Scholar] [CrossRef]
- Wang, Z.; Han, N.M.; Wu, Y.; Liu, X.; Shen, X.; Zheng, Q.; Kim, J.K. Ultrahigh dielectric constant and low loss of highly-aligned graphene aerogel/poly(vinyl alcohol) composites with insulating barriers. Carbon N. Y. 2017, 123, 385–394. [Google Scholar] [CrossRef]
- Pawar, S.P.; Biswas, S.; Kar, G.P.; Bose, S. High frequency millimetre wave absorbers derived from polymeric nanocomposites. Polymer 2016, 84, 398–419. [Google Scholar] [CrossRef]
- Peng, C.; Feng, Y.; Hu, J. Enhancing High-Frequency Dielectric Properties of Beta-SiC Filled Nanocomposites from Synergy between Percolation and Polarization. Materials 2018, 11, 1699. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.-M.; Yuan, J.-K.; Yao, S.-H.; Liao, R.-J. Flexible Nanodielectric Materials with High Permittivity for Power Energy Storage. Adv. Mater. 2013, 25, 6334–6365. [Google Scholar] [CrossRef]
- MacHado, W.S.; Hummelgen, I.A. Low-voltage poly(3-Hexylthiophene)/Poly(Vinyl Alcohol) field-effect transistor and inverter. IEEE Trans. Electron. Devices 2012, 59, 1529–1533. [Google Scholar] [CrossRef]
- Van Etten, E.A.; Ximenes, E.S.; Tarasconi, L.T.; Garcia, I.T.S.; Forte, M.M.C.; Boudinov, H. Insulating characteristics of polyvinyl alcohol for integrated electronics. Thin Solid Films 2014, 568, 111–116. [Google Scholar] [CrossRef]
- Choudhary, S.; Sengwa, R.J. ZnO nanoparticles dispersed PVA–PVP blend matrix based high performance flexible nanodielectrics for multifunctional microelectronic devices. Curr. Appl. Phys. 2018, 18, 1041–1058. [Google Scholar] [CrossRef]
- Mahendia, S.; Tomar, A.K.; Kumar, S. Electrical conductivity and dielectric spectroscopic studies of PVA-Ag nanocomposite films. J. Alloys Compd. 2010, 508, 406–411. [Google Scholar] [CrossRef]
- Son, D.I.; Park, D.H.; Choi, W.K.; Cho, S.H.; Kim, W.T.; Kim, T.W. Carrier transport in flexible organic bistable devices of ZnO nanoparticles embedded in an insulating poly(methyl methacrylate) polymer layer. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.S.; Afzalzadeh, R.; Abadyan, M. ZnO Nanoparticles as Ethanol Gas Sensors and the Effective Parameters on Their Performance. J. Mater. Sci. Technol. 2013, 29, 393–400. [Google Scholar] [CrossRef]
- Hmar, J.J.L. Flexible resistive switching bistable memory devices using ZnO nanoparticles embedded in polyvinyl alcohol (PVA) matrix and poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS). RSC Adv. 2018, 8, 20423–20433. [Google Scholar] [CrossRef]
- Wu, W.; Huang, X.; Li, S.; Jiang, P.; Toshikatsu, T. Novel three-dimensional zinc oxide superstructures for high dielectric constant polymer composites capable of withstanding high electric field. J. Phys. Chem. C 2012, 116, 24887–24895. [Google Scholar] [CrossRef]
- Devi, P.I.; Ramachandran, K. Dielectric studies on hybridised PVDF-ZNO nanocomposites. J. Exp. Nanosci. 2011, 6, 281–293. [Google Scholar] [CrossRef]
- Sugumaran, S.; Bellan, C.S.; Muthu, D.; Raja, S.; Bheeman, D.; Rajamani, R. Novel hybrid PVA–InZnO transparent thin films and sandwich capacitor structure by dip coating method: Preparation and characterizations. RSC Adv. 2015, 5, 10599–10610. [Google Scholar] [CrossRef]
- Hdidar, M.; Chouikhi, S.; Fattoum, A.; Arous, M.; Kallel, A. Influence of TiO2 rutile doping on the thermal and dielectric properties of nanocomposite films based PVA. J. Alloys Compd. 2018, 750, 375–383. [Google Scholar] [CrossRef]
- Komarov, S.A.; Komarov, A.S.; Barber, D.G.; Lemes, M.J.L.; Rysgaard, S. Open-Ended Coaxial Probe Technique for Dielectric Spectroscopy of Artificially Grown Sea Ice. IEEE Trans. Geosci. Remote Sens. 2016, 54, 4941–4951. [Google Scholar] [CrossRef]
- Roy, A.S.; Gupta, S.; Sindhu, S.; Parveen, A.; Ramamurthy, P.C. Dielectric properties of novel PVA/ZnO hybrid nanocomposite films. Compos. Part B Eng. 2013, 47, 314–319. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Pandiyarajan, T.; Mangalaraja, R.V. Enhanced blue light emission in transparent ZnO:PVA nanocomposite free standing polymer films. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2016, 152, 485–490. [Google Scholar] [CrossRef]
- Rashmi, S.H.; Raizada, A.; Madhu, G.M.; Kittur, A.A.; Suresh, R.; Sudhina, H.K. Influence of zinc oxide nanoparticles on structural and electrical properties of polyvinyl alcohol films. Plast. Rubber Compos. 2015, 44, 33–39. [Google Scholar] [CrossRef]
- Schroeder, R.; Majewski, L.A.; Grell, M. High-performance organic transistors using solution-processed nanoparticle-filled high-k polymer gate insulators. Adv. Mater. 2005, 17, 1535–1539. [Google Scholar] [CrossRef]
- Bouropoulos, N.; Psarras, G.C.; Moustakas, N.; Chrissanthopoulos, A.; Baskoutas, S. Optical and dielectric properties of ZnO-PVA nanocomposites. Phys. Status Solidi Appl. Mater. Sci. 2008, 205, 2033–2037. [Google Scholar] [CrossRef]
- Guirguis, O.W.; Moselhey, M.T.H. Optical study of poly(vinyl alcohol)/hydroxypropyl methylcellulose blends. J. Mater. Sci. 2011, 46, 5775–5789. [Google Scholar] [CrossRef]
- El-Khodary, A. Vibrational, thermal, optical and magnetic investigations of PVA films filled with FeCl3 and CoCl2. Phys. B Condens. Matter 2009, 404, 1287–1294. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Baek, J.B. Nanocomposites derived from polymers and inorganic nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef]
- Dazhi Sun, H.-J.S. Multifunctional polymer/ZnO nanocomposites: Controlled dispersion and physical properties. In Multifunctionality of Polymer Composites; Friedrich Klaus, U.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 858–874. ISBN 9780323264341. [Google Scholar]
- Xiong, H.M.; Zhao, X.; Chen, J.S. New polymer-inorganic nanocomposites: PEO-ZnO and PEO-ZnO-LiClO4films. J. Phys. Chem. B 2001, 105, 10169–10174. [Google Scholar] [CrossRef]
- Nakhaei, O.; Shahtahmassebi, N.; Rezaeeroknabadi, M.; Bagheri Mohagheghi, M.M. Synthesis, characterization and study of optical properties of polyvinyl alcohol/CaF2 nanocomposite films. Sci. Iran. 2012, 19, 1979–1983. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; Elashmawi, I.S.; El-khodary, A.; Yassin, A. Structural, optical, thermal and electrical studies on PVA/PVP blends filled with lithium bromide. Curr. Appl. Phys. 2010, 10, 607–613. [Google Scholar] [CrossRef]
- Yeow, Y.K.; Abbas, Z.; Khalid, K.; Rahman, M.Z.A. Improved dielectric model for polyvinyl alcohol-water hydrogel at microwave frequencies. Am. J. Appl. Sci. 2010, 7, 270–276. [Google Scholar] [CrossRef]
- Rajeswari, N.; Selvasekarapandian, S.; Karthikeyan, S.; Prabu, M.; Hirankumar, G.; Nithya, H.; Sanjeeviraja, C. Conductivity and dielectric properties of polyvinyl alcohol- polyvinylpyrrolidone poly blend film using non-aqueous medium. J. Non. Cryst. Solids 2011, 357, 3751–3756. [Google Scholar] [CrossRef]
- Abd El-kader, F.H.; Gaafer, S.A.; Mahmoud, K.H.; Mohamed, S.I.; Abd El-kader, M.F.H. Electrical Conduction in (Polyvinyl Alcohol/Glycogen) Blend Films. Polym. Compos. 2008, 30, 214–220. [Google Scholar] [CrossRef]
- Alakanandana, A.; Subrahmanyam, A.R.; Siva Kumar, J. Structural and Electrical Conductivity studies of pure PVA and PVA doped with Succinic acid polymer electrolyte system. Mater. Today Proc. 2016, 3, 3680–3688. [Google Scholar] [CrossRef]
- Latif, I.E.; AL-Abodi, E.H.; Badri, D.; Al Khafagi, J. Preparation, Characterization and Electrical Study of (Carboxymethylated Polyvinyl Alcohol/ZnO) Nanocomposites. Am. J. Polym. Sci. 2013, 2, 135–140. [Google Scholar] [CrossRef]
- Chandrakala, H.N.; Ramaraj, B.; Shivakumaraiah; Madhu, G.M.; Siddaramaiah. The influence of zinc oxide-cerium oxide nanoparticles on the structural characteristics and electrical properties of polyvinyl alcohol films. J. Mater. Sci. 2012, 47, 8076–8084. [Google Scholar] [CrossRef]
- Levinson, L.M.; Philipp, H.R. Low-temperature ac properties of metal-oxide varistors. J. Appl. Phys. 1978, 49, 6142–6146. [Google Scholar] [CrossRef]
- Wang, G.; Deng, Y.; Xiang, Y.; Guo, L. Fabrication of radial ZnO nanowire clusters and radial ZnO/PVDF composites with enhanced dielectric properties. Adv. Funct. Mater. 2008, 18, 2584–2592. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Varghese, M.; Jayakrishnan, P.; Periyat, P. Silver-Doped Zinc Oxide as a Nanofiller for Development of Poly(vinyl alcohol)/Poly(vinyl pyrrolidone) Blend Nanocomposites. Adv. Polym. Technol. 2018, 37, 137–143. [Google Scholar] [CrossRef]
- Makled, M.H.; Sheha, E.; Shanap, T.S.; El-Mansy, M.K. Electrical conduction and dielectric relaxation in p-type PVA/CuI polymer composite. J. Adv. Res. 2013, 4, 531–538. [Google Scholar] [CrossRef]
- Roscow, J.I.; Bowen, C.R.; Almond, D.P. Breakdown in the Case for Materials with Giant Permittivity? ACS Energy Lett. 2017, 2, 2264–2269. [Google Scholar] [CrossRef]
- Nan, C.-W.; Shen, Y.; Ma, J. Physical Properties of Composites Near Percolation. Annu. Rev. Mater. Res. 2010, 40, 131–151. [Google Scholar] [CrossRef]













| Labeled Sample | SDS | ZnCl2 | C6H8O7 | Solution |
|---|---|---|---|---|
| Z1 | 5 mL 288.3 (g/mol) 0.00005 mol | 1 mL 136.28 (g/mol) 0.0002 mol | 1 mL 192.15 (g/mol) 0.00002 (mol) | NH4 |
| Y1 | 5 mL 288.3 (g/mol) 0.00005 mol | 1 mL 136.28 (g/mol) 0.0002 mol | 1 mL 192.15 (g/mol) 0.00002 (mol) | KOH |
| Z4 | 5 mL 288.3 (g/mol) 0.00005 (mol) | 2.5 mL 136.28 (g/mol) 0.0005 (mol) | 1.2 mL 192.15 (g/mol) 0.000025 (mol) | NH4 |
| Y4 | 5 mL 288.3 (g/mol) 0.00005 (mol) | 2.5 mL 136.28 (g/mol) 0.00005 (mol) | 1.2 mL 192.15 (g/mol) 0.000025 (mol) | KOH |
| Sample | Activation Energy, Ea, (eV) | Conductivity, (300 K), (S/cm) | Band Gap, Eg, (eV) |
|---|---|---|---|
| Y1 | 0.72 | 1.18 × 10−12 | 5.8 |
| Z1 | 0.62 | 1.20 × 10−12 | 5.6 |
| Y4 | 0.68 | 1.71 × 10−12 | 5.5 |
| Z4 | 0.78 | 2.44 × 10−12 | 5.83 |
| Material | Deposition Method and Temperature | Permittivity (ε), Frequency | Reference |
|---|---|---|---|
| PVA/ZnO membranes | Solution casting/50 °C | 17.8 at 100 Hz | [2] |
| PVDF/xGnPs | Solution mixing process | 2.080 at 103 Hz (4.1 vol%) | [3] |
| PVDF/ZnO composites | combination of solution blend, sequential precipitation, and hot-press processes/60 °C | 102 at 500 HZ | [14] |
| PVA/ZnO nanocomposites | Solution casting/40 °C | 502 at 102 Hz 102 at 106 Hz | [19] |
| PVDF/ZnO nanowires clusters | Microemulsion/80 °C | 113 at 102 Hz | [38] |
| (PVA)/(PVP)/silver-doped zinc oxide (Ag-doped ZnO) | solution blended process and calcined at 500 °C | 804 at 103 Hz | [39] |
| p-type PVA/CuI | Solution casting at 24 °C | 103 at 102 Hz | [40] |
| PVA/ZnO | Solution casting method at 80 °C | ≈104 at 500 Hz | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosio, R.; Carrillo, A.; Mota, M.L.; De la Torre, K.; Torrealba, R.; Moreno, M.; Vazquez, H.; Flores, J.; Vivaldo, I. Polymeric Nanocomposites Membranes with High Permittivity Based on PVA-ZnO Nanoparticles for Potential Applications in Flexible Electronics. Polymers 2018, 10, 1370. https://doi.org/10.3390/polym10121370
Ambrosio R, Carrillo A, Mota ML, De la Torre K, Torrealba R, Moreno M, Vazquez H, Flores J, Vivaldo I. Polymeric Nanocomposites Membranes with High Permittivity Based on PVA-ZnO Nanoparticles for Potential Applications in Flexible Electronics. Polymers. 2018; 10(12):1370. https://doi.org/10.3390/polym10121370
Chicago/Turabian StyleAmbrosio, Roberto, Amanda Carrillo, Maria L. Mota, Karla De la Torre, Richard Torrealba, Mario Moreno, Hector Vazquez, Javier Flores, and Israel Vivaldo. 2018. "Polymeric Nanocomposites Membranes with High Permittivity Based on PVA-ZnO Nanoparticles for Potential Applications in Flexible Electronics" Polymers 10, no. 12: 1370. https://doi.org/10.3390/polym10121370
APA StyleAmbrosio, R., Carrillo, A., Mota, M. L., De la Torre, K., Torrealba, R., Moreno, M., Vazquez, H., Flores, J., & Vivaldo, I. (2018). Polymeric Nanocomposites Membranes with High Permittivity Based on PVA-ZnO Nanoparticles for Potential Applications in Flexible Electronics. Polymers, 10(12), 1370. https://doi.org/10.3390/polym10121370










