Collagen-Coated Poly(lactide-co-glycolide)/Hydroxyapatite Scaffold Incorporated with DGEA Peptide for Synergistic Repair of Skull Defect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Coated Scaffolds
2.3. Characterization of Coated Scaffolds
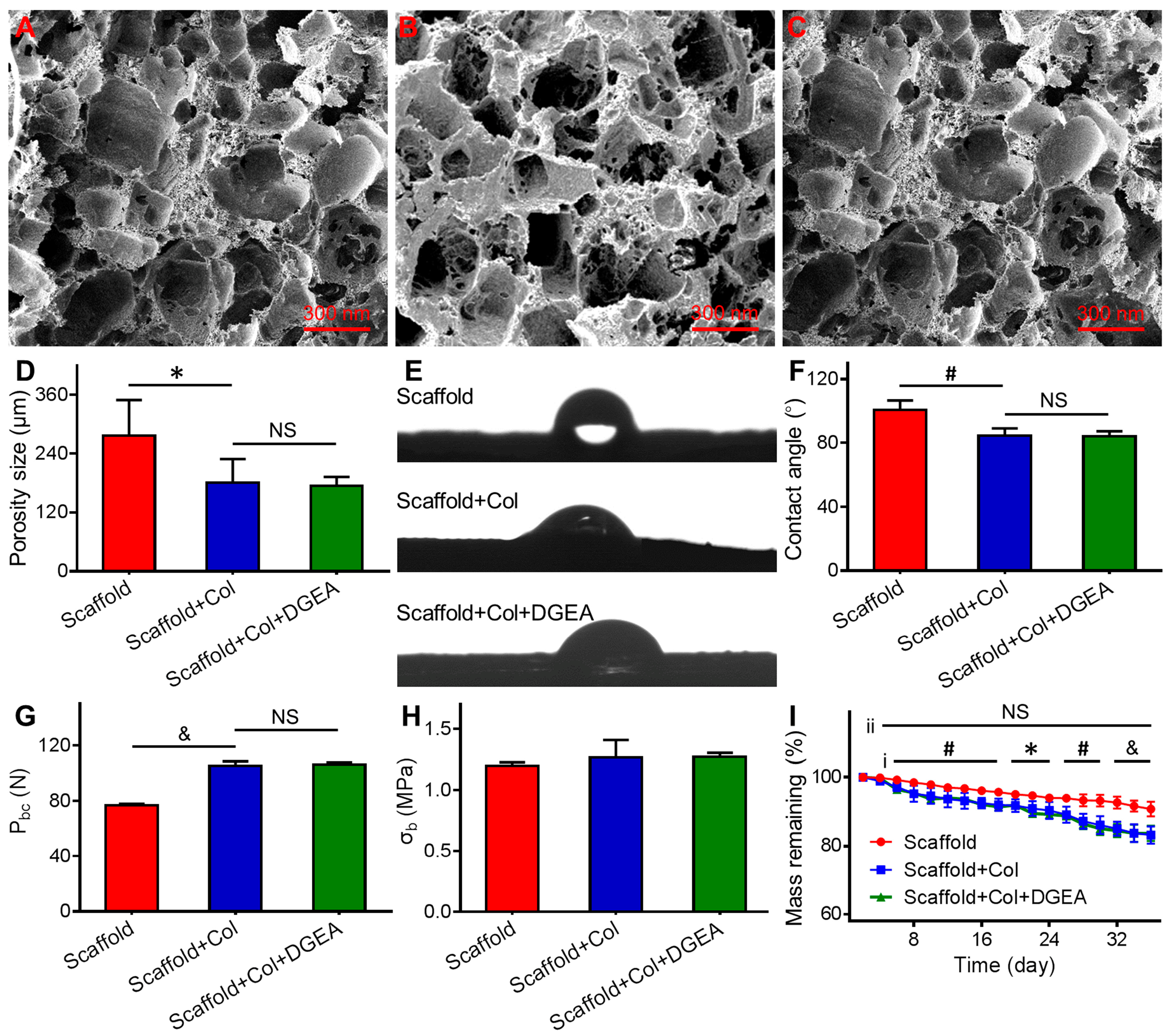
2.3.1. Morphological Observation
2.3.2. Mechanical-Property Testing
2.3.3. In Vitro Degradation Experiment
2.3.4. Hydrophilicity Detection
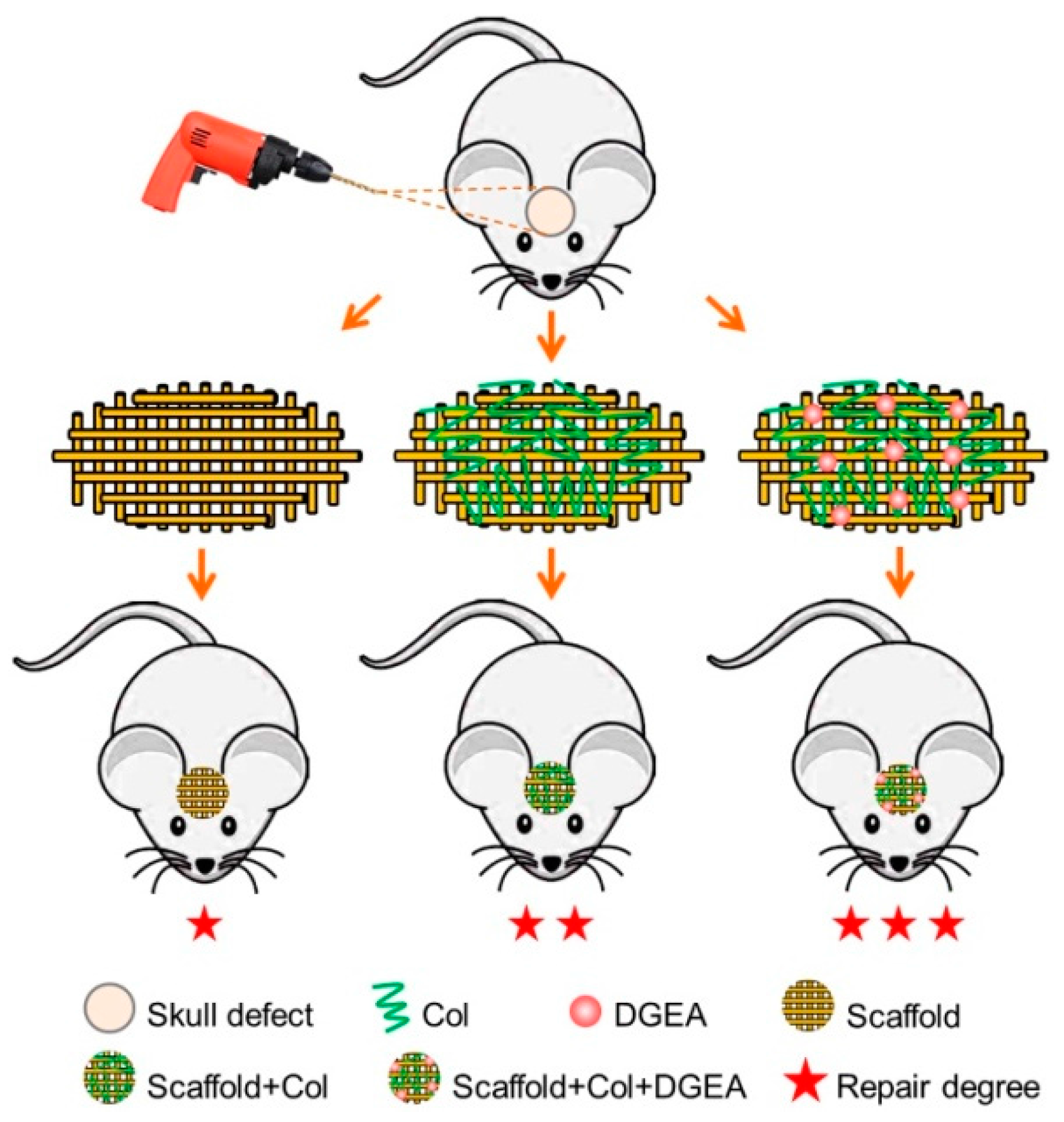
2.4. In Vivo Animal Experiments
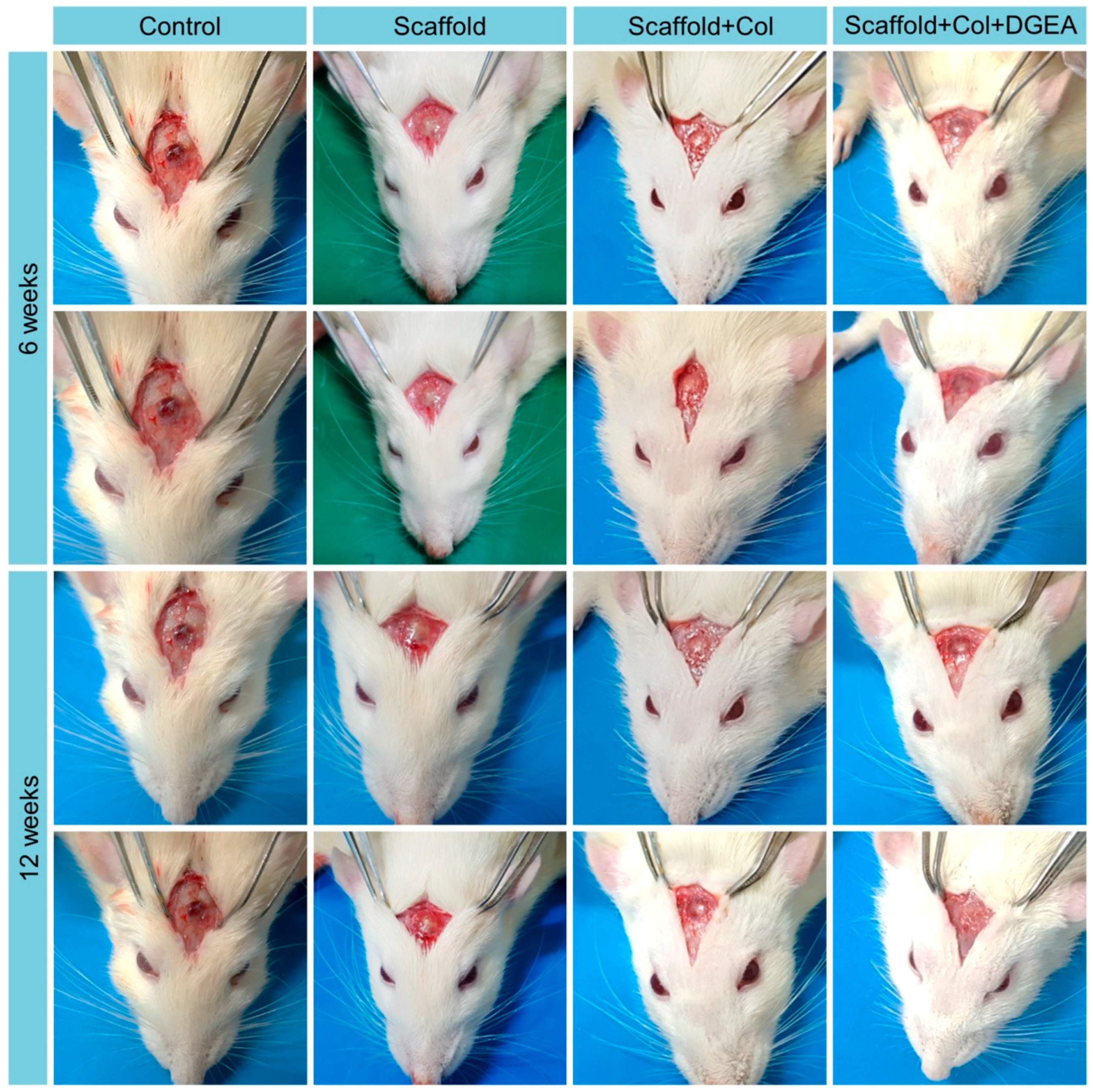
2.4.1. Establishment of Animal Models
2.4.2. Micro-CT Detection
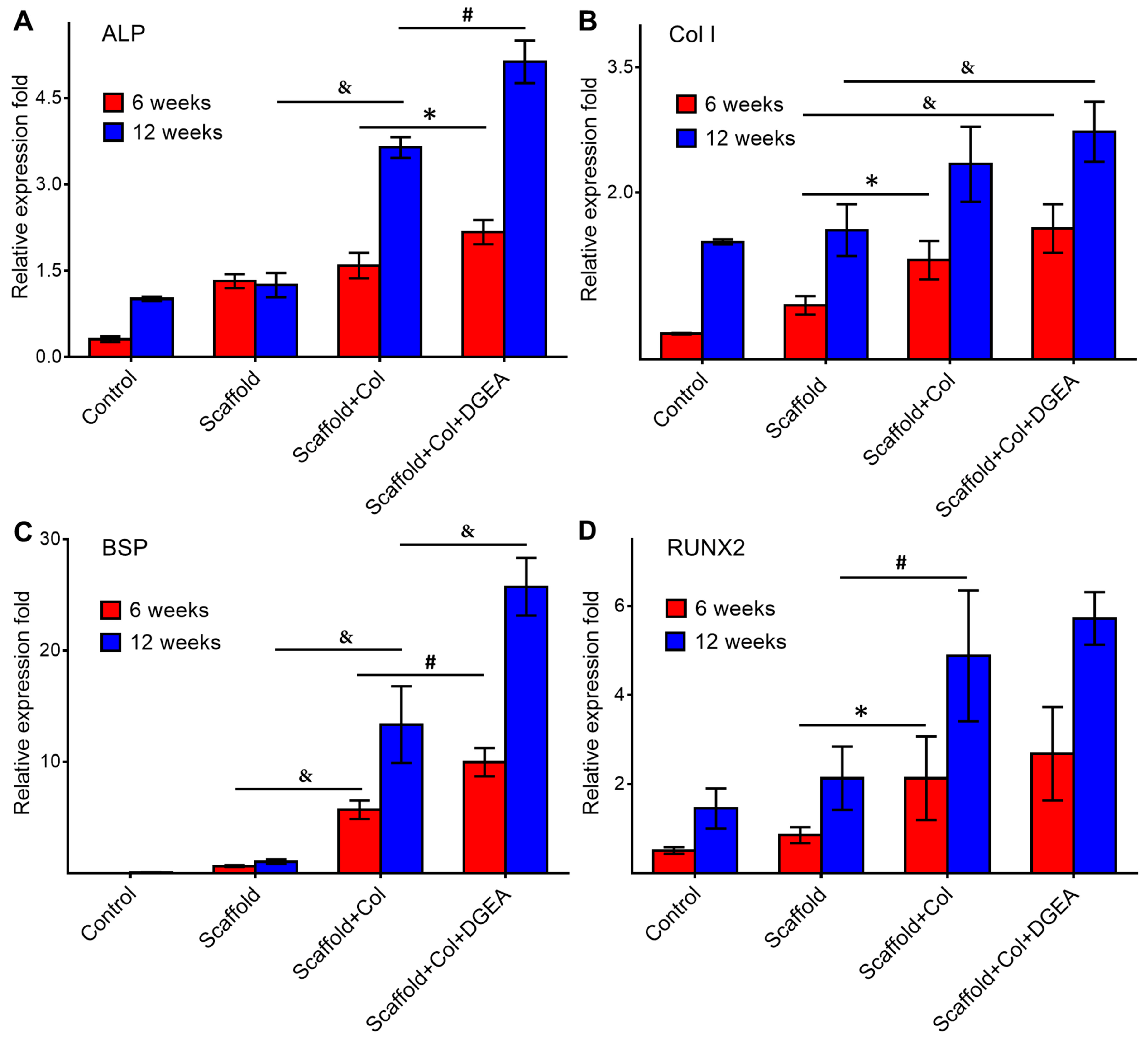
2.4.3. RNA Extraction and RT-PCR Assays
2.4.4. Immunofluorescence Staining
2.5. Statistical Analyses
3. Results and Discussion
3.1. Characterization of Coated Scaffolds
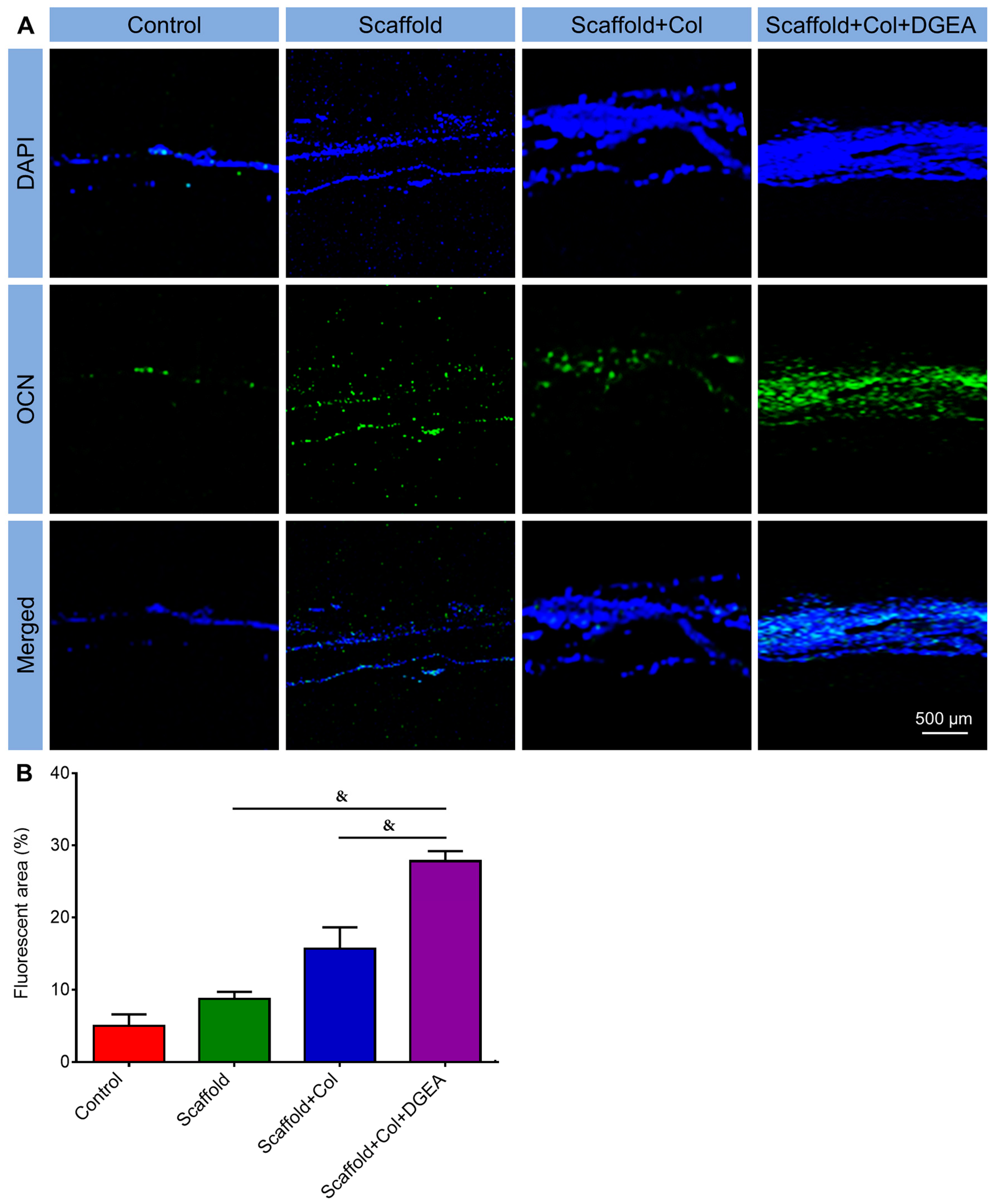
3.2. In Vivo Efficacy Verification
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kneser, U.; Schaefer, D.; Polykandriotis, E.; Horch, R. Tissue engineering of bone: The reconstructive surgeon’s point of view. J. Cell. Mol. Med. 2006, 10, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Cao, H.; Wang, X.; Chen, S.; Zhang, M.; Wang, N.; Yao, Z.; Dai, Y.; Xie, X.; Zhang, P.; et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, Y.; Zhang, J.; Xie, Q.; Wang, Z.; Yu, S.; Yuan, Y.; Liu, C. MBG-modified β-TCP scaffold promotes mesenchymal stem cells adhesion and osteogenic differentiation via a FAK/MAPK signalling pathway. ACS Appl. Mater. Interfaces 2017, 9, 30283–30296. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Giavaresi, G.; Parrilli, A.; Ferrari, A.; Aldini, N.N.; Morra, M.; Cassinelli, C.; Bollati, D.; Fini, M. Collagen type I coating stimulates bone regeneration and osteointegration of titanium implants in the osteopenic rat. Int. Orthop. 2015, 39, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Zhang, Z.Z.; Jiang, D.; Qi, Y.S.; Wang, H.J.; Zhang, J.Y.; Ding, J.X.; Yu, J.K. Thermogel-coated poly(ε-caprolactone) composite scaffold for enhanced cartilage tissue engineering. Polymers 2016, 8, 200. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.S.; Li, T.Y.; Ding, J.X.; Liu, J.G.; Li, B.S.; Chen, X.S. Gelatin tight-coated poly(lactide-co-glycolide) scaffold incorporating rhBMP-2 for bone tissue engineering. Materials 2015, 8, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, N.; Chase, H.; Brumfeld, V.; Shahar, R.; Weiner, S. The 3D structure of the collagen fibril network in human trabecular bone: Relation to trabecular organization. Bone 2015, 71, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Morra, M. Biochemical modification of titanium surfaces: Peptides and ECM proteins. Eur. Cells Mater. 2006, 12, 1–15. [Google Scholar] [CrossRef]
- Gentile, P.; Ferreira, A.M.; Callaghan, J.T.; Miller, C.A.; Atkinson, J.; Freeman, C.; Hatton, P.V. Multilayer nanoscale encapsulation of biofunctional peptides to enhance bone tissue regeneration in vivo. Adv. Healthc. Mater. 2017, 6, 1601182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Y.; Xu, W.P.; Hu, Y.; Ding, J.X. Poly(lactide-co-glycolide)/hydroxyapatite porous scaffold with microchannels for bone regeneration. Polymers 2016, 8, 218. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Ding, J.X.; Wu, J.; Zhuang, X.L.; Chen, X.S.; Wang, J.C.; Yin, J.B.; Li, Z.M. High-pressure compression-molded porous resorbable polymer/hydroxyapatite composite scaffold for cranial bone regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1471–1482. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R. Cartilage and bone extracellular matrix. Curr. Pharm. Des. 2009, 15, 1334–1348. [Google Scholar]
- Geissler, U.; Hempel, U.; Wolf, C.; Scharnweber, D.; Worch, H.; Wenzel, K.W. Collagen type I-coating of Ti6Al4V promotes adhesion of osteoblasts. J. Biomed. Mater. Res. Part A 2000, 51, 752–760. [Google Scholar] [CrossRef]
- Mushahary, D.; Wen, C.; Kumar, J.M.; Sravanthi, R.; Hodgson, P.; Pande, G.; Li, Y. Strontium content and collagen-I coating of magnesium–zirconia–strontium implants influence osteogenesis and bone resorption. Clin. Oral Implant. Res. 2016, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.M.; Shi, B.; Ding, J.X.; Zhuang, X.L.; Zhang, X.N.; Fu, C.F.; Chen, X.S. Prevention of postoperative tendon adhesion by biodegradable electrospun membrane of poly(lactide-co-glycolide). Chin. J. Polym. Sci. 2015, 33, 587–596. [Google Scholar] [CrossRef]
- Li, D.; Sun, H.; Ding, J.X.; Tang, Z.H.; Zhang, Y.; Xu, W.G.; Zhuang, X.L.; Chen, X.S. Polymeric topology and composition constrained polyether–polyester micelles for directional antitumor drug delivery. Acta Biomater. 2013, 9, 8875–8884. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.S.; Wang, N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: Biodegradation. J. Biomater. Sci. Polym. Ed. 2001, 12, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016, 15, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Gao, H.; Song, J.; Li, Y.; Zhang, L.; Cao, X.; Xu, M.; Cai, J. Tough and cell-compatible chitosan physical hydrogels for mouse bone mesenchymal stem cells in vitro. ACS Appl. Mater. Interfaces 2016, 8, 19739–19746. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Irie, N. Osteoclast–osteoblast communication. Arch. Biochem. Biophys. 2008, 473, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Schenk, R.K.; Buser, D. Guided bone regeneration at oral implant sites. Periodontology 1998, 17, 22–35. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, M.; Han, H.; Dong, S.; Zhang, Y.; Xu, W.; Zhu, B.; Wang, J.; Zhou, Y.; Ding, J. Collagen-Coated Poly(lactide-co-glycolide)/Hydroxyapatite Scaffold Incorporated with DGEA Peptide for Synergistic Repair of Skull Defect. Polymers 2018, 10, 109. https://doi.org/10.3390/polym10020109
Bi M, Han H, Dong S, Zhang Y, Xu W, Zhu B, Wang J, Zhou Y, Ding J. Collagen-Coated Poly(lactide-co-glycolide)/Hydroxyapatite Scaffold Incorporated with DGEA Peptide for Synergistic Repair of Skull Defect. Polymers. 2018; 10(2):109. https://doi.org/10.3390/polym10020109
Chicago/Turabian StyleBi, Ming, Hui Han, Shujun Dong, Ying Zhang, Weiguo Xu, Bitao Zhu, Jingyun Wang, Yanmin Zhou, and Jianxun Ding. 2018. "Collagen-Coated Poly(lactide-co-glycolide)/Hydroxyapatite Scaffold Incorporated with DGEA Peptide for Synergistic Repair of Skull Defect" Polymers 10, no. 2: 109. https://doi.org/10.3390/polym10020109





