Polycomplexes of Hyaluronic Acid and Borates in a Solid State and Solution: Synthesis, Characterization and Perspectives of Application in Boron Neutron Capture Therapy
Abstract
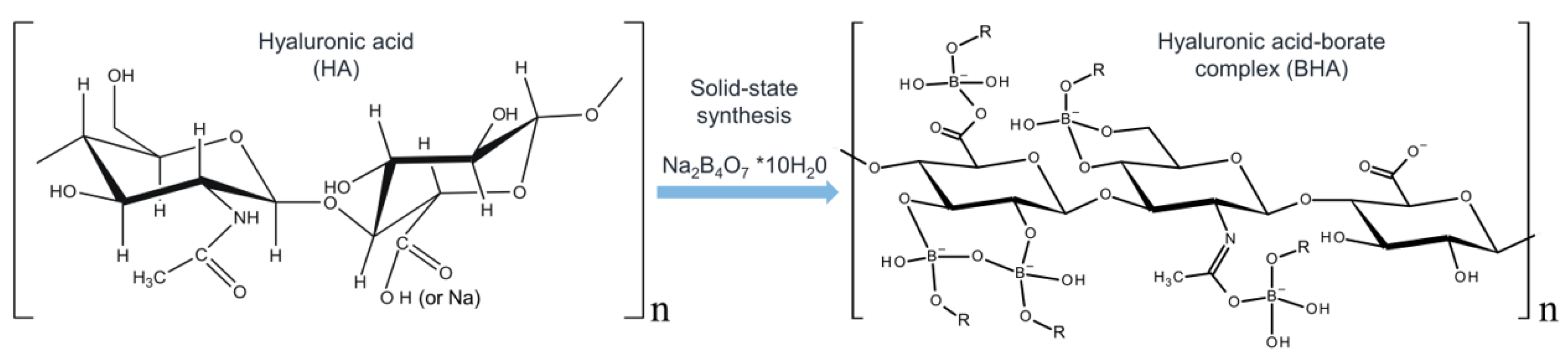
:1. Introduction
2. Materials and Methods
3. Results
3.1. Fourier Transform Infrared Spectroscopy
3.1.1. The Absorption Region of ОН–, NH– and СН– Valent Oscillations is 3650–2750 cm−1
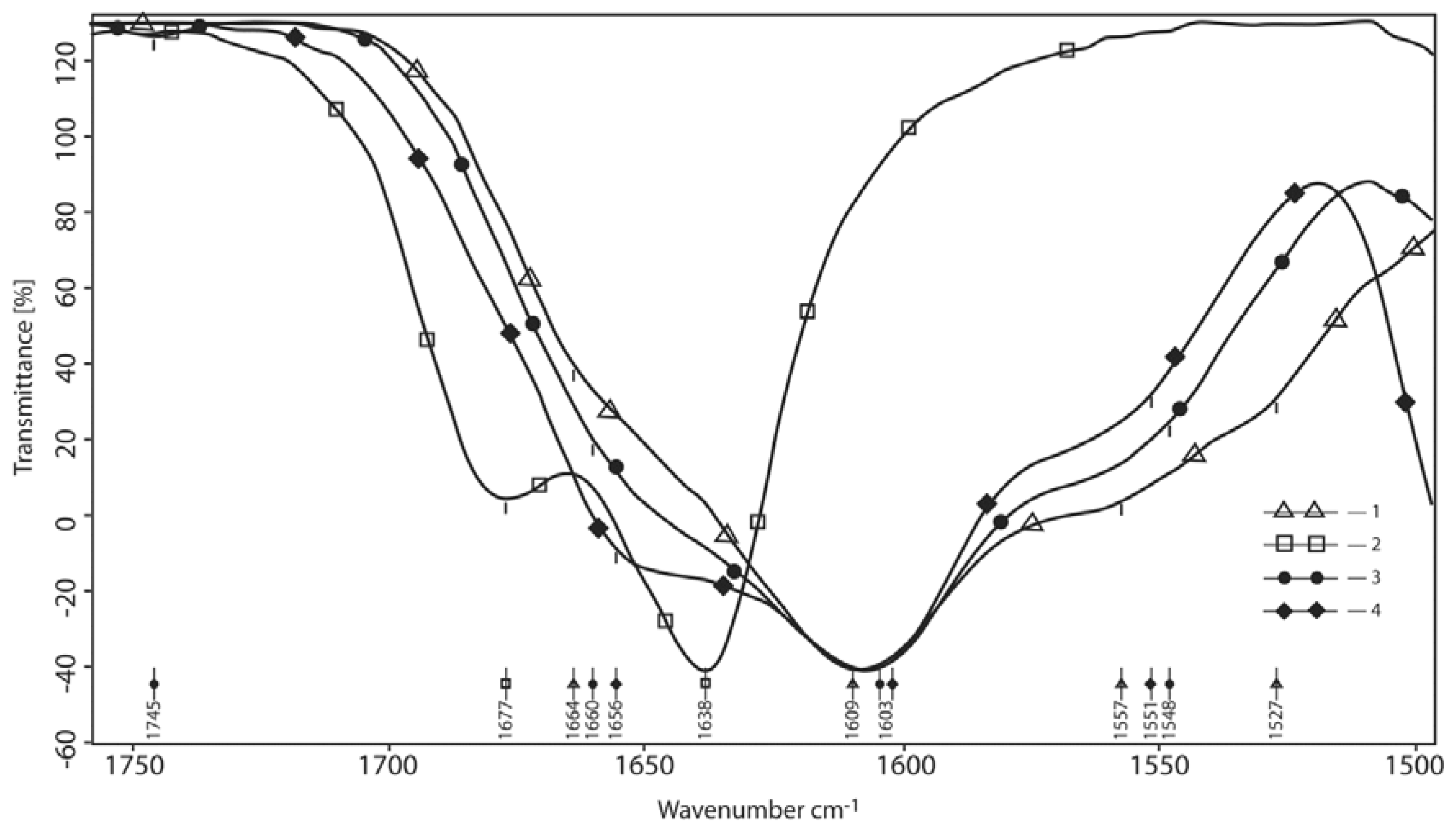
3.1.2. The Area of 1750–1200 cm−1 of the Carbonyls and Amide Bonds
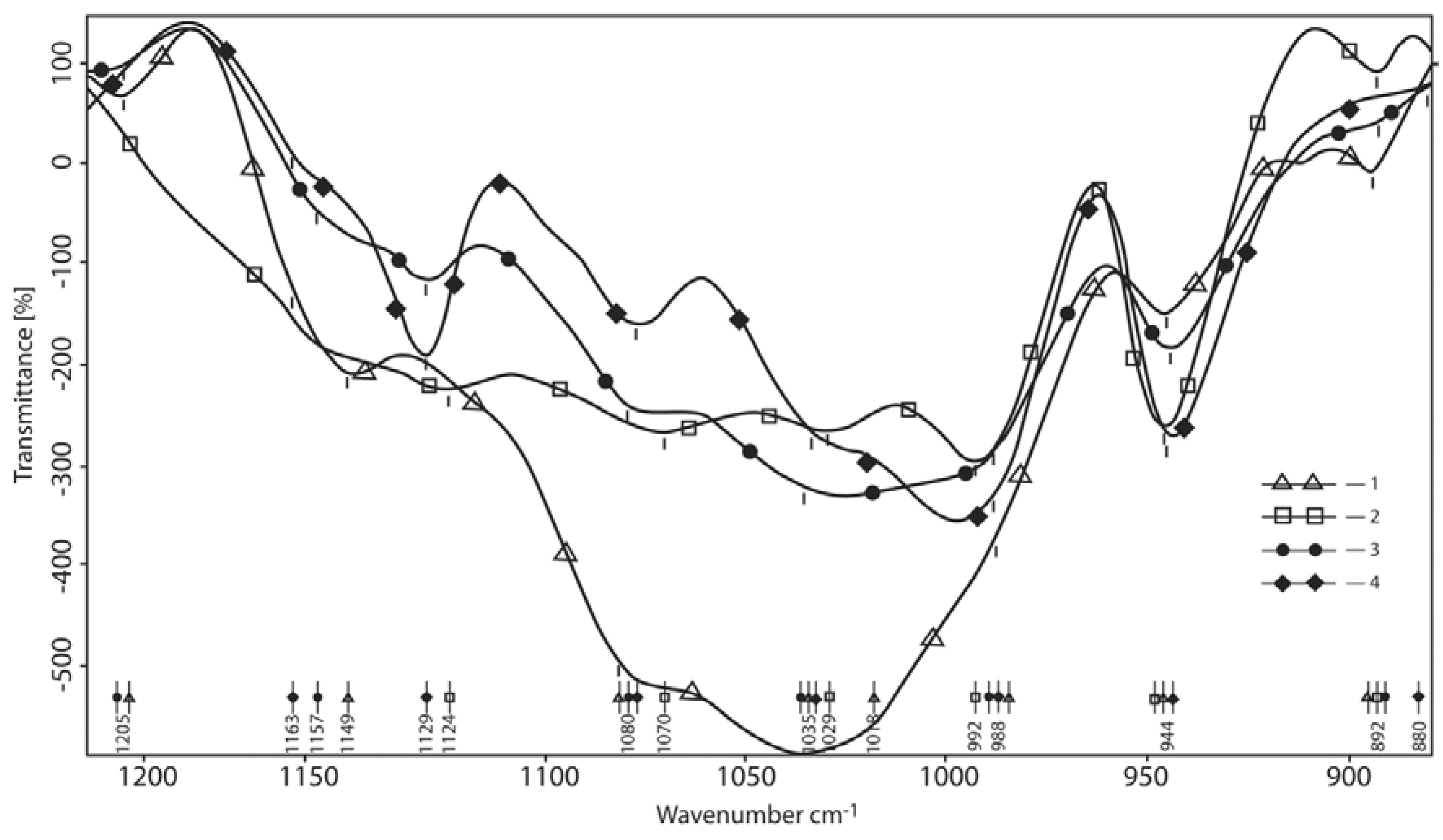
3.1.3. Oscillations Area of С–О– Containing Bonds
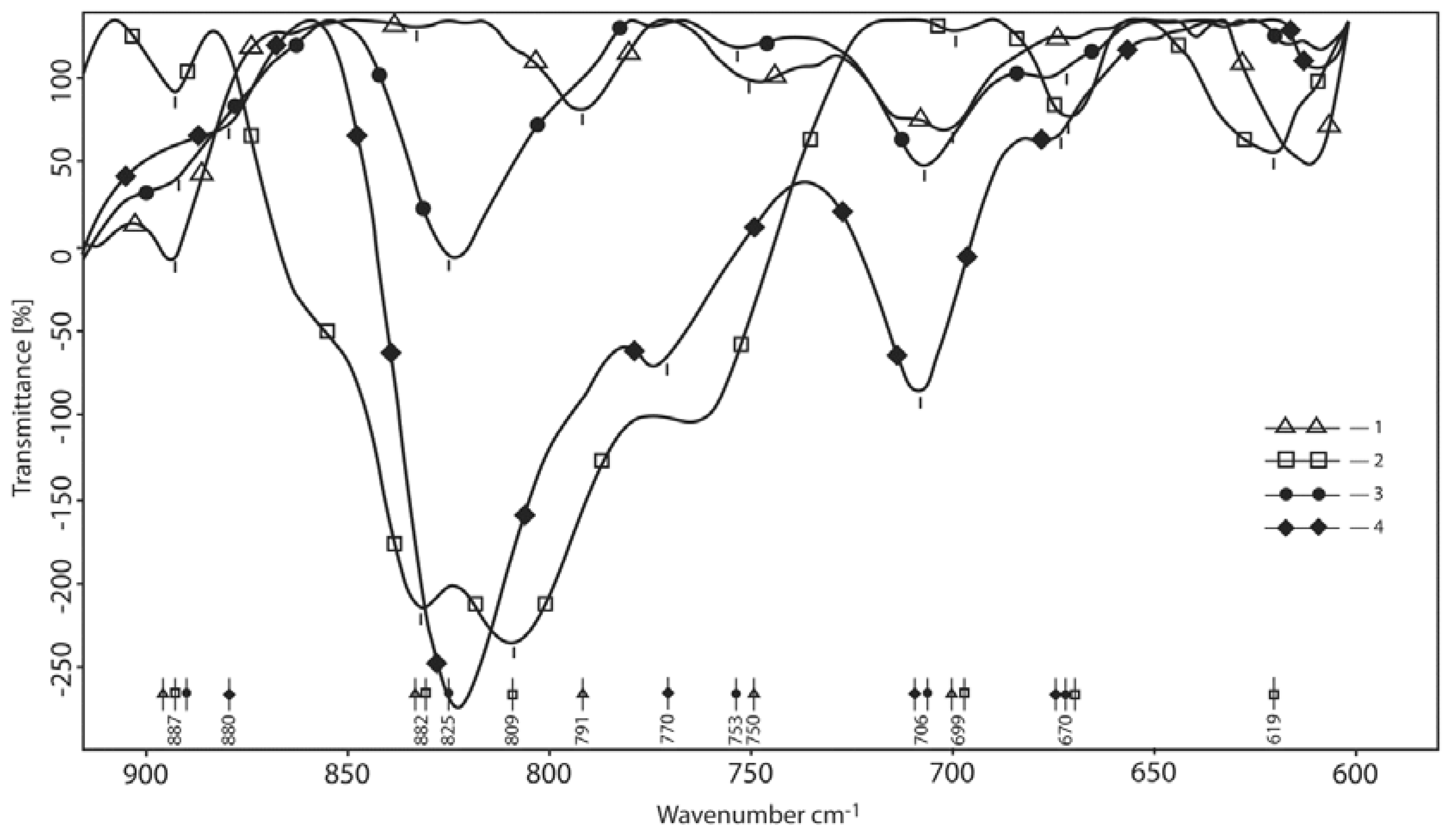
3.1.4. Area of the Deformational and Packaging Oscillations (Overall Normalization by CH)
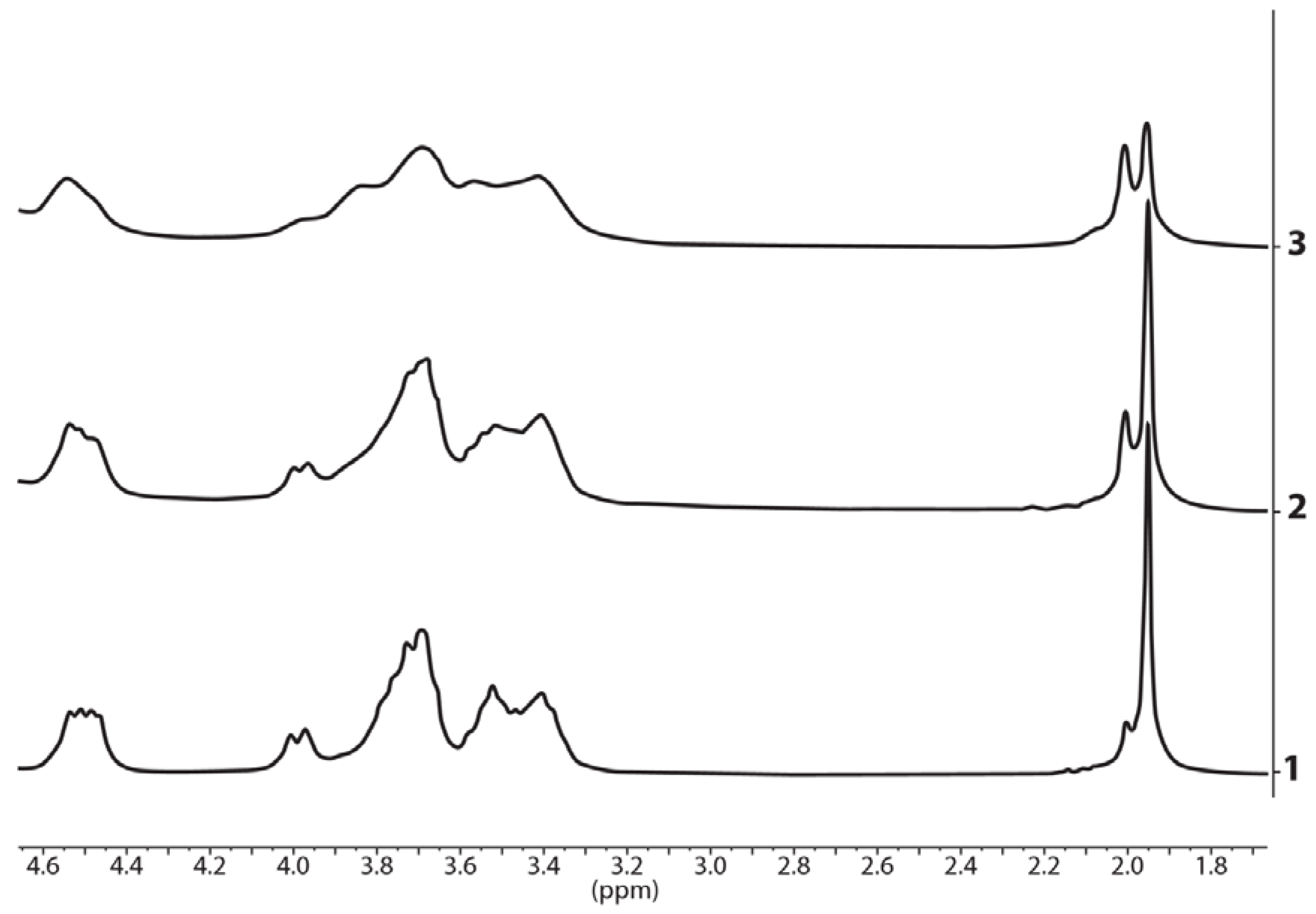
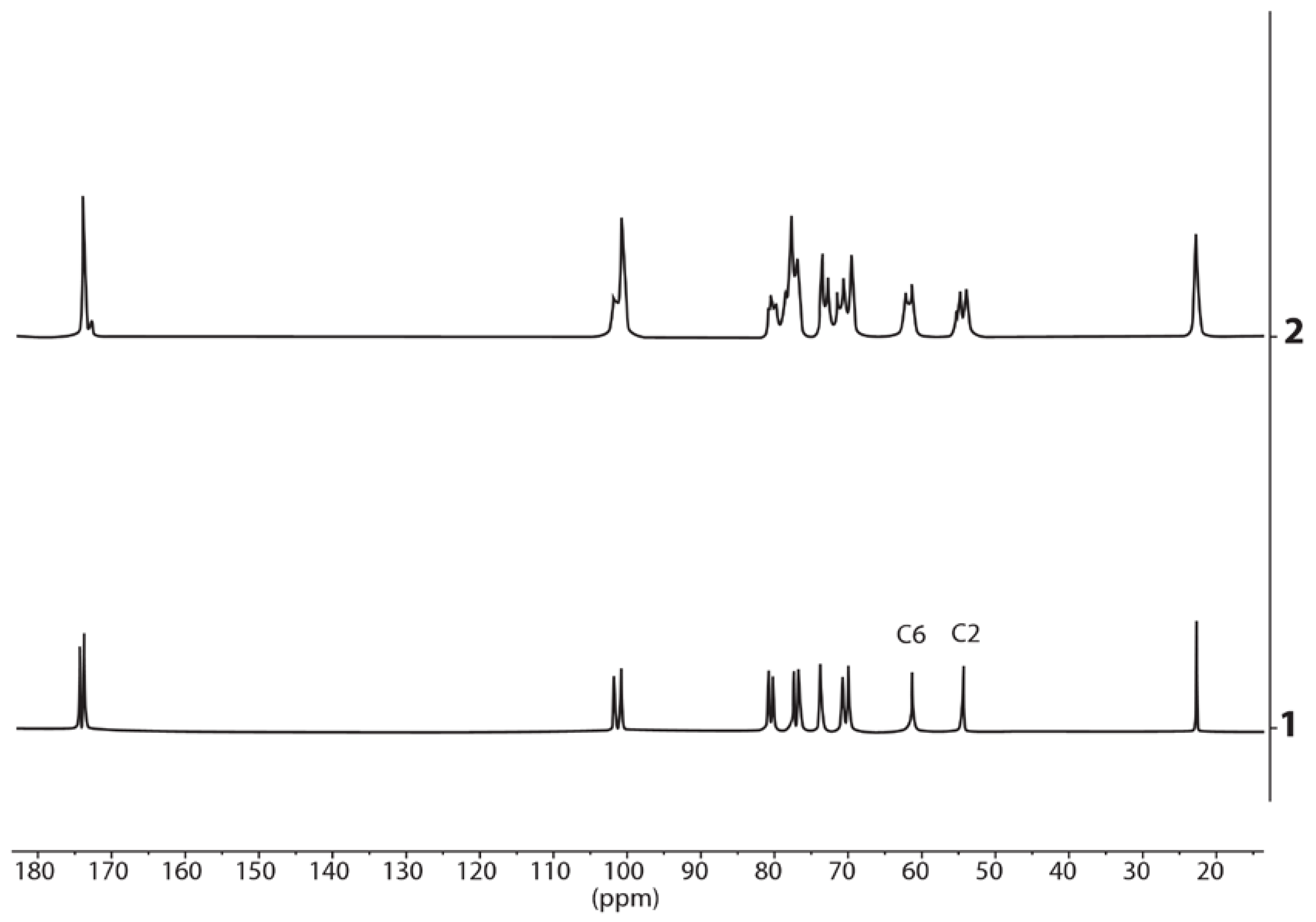
3.2. 1H and 13C NMR Spectra of the Polycomplexes
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grimes, R.N. Carboranes in Medicine. In Carboranes, 3rd ed.; Grimes, R.N., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 945–984. ISBN 9780128018941. [Google Scholar]
- Nakamura, H.; Ueno, M.; Lee, J.-D.; Ban, H.S.; Justus, E.; Fan, P.; Gabel, D. Synthesis of dodecaborate-conjugated cholesterols for efficient boron delivery in neutron capture therapy. Tetrahedron Lett. 2007, 48, 3151–3154. [Google Scholar] [CrossRef]
- Schaffran, T.; Lissel, F.; Samatanga, B.; Karlsson, G.; Burghardt, A.; Edwards, K.; Winterhalter, M.; Peschka-Süss, R.; Schubert, R.; Gabel, D. Dodecaborate cluster lipids with variable headgroups for boron neutron capture therapy: Synthesis, physical-chemical properties and toxicity. J. Organomet. Chem. 2009, 694, 1708–1712. [Google Scholar] [CrossRef]
- Crossley, E.L.; Ziolkowski, E.J.; Coderre, J.A.; Rendina, L.M. Boronated DNA-binding compounds as potential agents for boron neutron capture therapy. Mini Rev. Med. Chem. 2007, 7, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ching, H.Y.; Clarke, R.J.; Rendina, L.M. Supramolecular β-cyclodextrin adducts of boron-rich DNA metallointercalators containing dicarba-closo-dodecaborane(12). Inorg. Chem. 2013, 52, 10356–10367. [Google Scholar] [CrossRef] [PubMed]
- Yinghuai, Z.; Yan, K.C.; Maguire, J.A.; Hosmane, N.S. Resent developments in boron neutron capture therapy (BNCT) driven by nanotechnology. Curr. Chem. Biol. 2007, 1, 141–149. [Google Scholar] [CrossRef]
- Yinghuai, Z.; Maguire, J.A.; Hosmane, N.S. Resent developments in boron neutron capture therapy driven by nanotechnology, Boron for Living: Boron Neutron Capture Therapy. In Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 147–163. ISBN 9781439826621. [Google Scholar]
- Gao, Z.; Horiguchi, Y.; Nakai, K.; Matsumura, A.; Suzuki, M.; Ono, K.; Nagasaki, Y. Use of boron cluster-containing redox nanoparticles with ROS scavenging ability in boron neutron capture therapy to achieve high therapeutic efficiency and low adverse effects. Biomaterials 2016, 104, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Laurenţia, G.N.; Rodica, A.M. Boron neutron capture therapy: Delivery agents used in boron administration. Ther. Pharmacol. Clin. Toxicol. 2016, 20, 25–32. [Google Scholar]
- Barth, R.F.; Vicente, M.G.; Harling, O.K.; Kiger, W.S., 3rd; Riley, K.J.; Binns, P.J.; Wagner, F.M.; Suzuki, M.; Aihara, T.; Kato, I.; et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 2012, 7, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkov, V.P.; Zelenetskii, A.N.; Ivanov, P.L.; Mikhajlova, N.P.; Molin, A.A.; Seljanin, M.A.; Khabarov, V.N.; Chernyshenko, A.O. Method for preparing boron-containing hyaluronic acid. Russian Federation Patent 2445978, 2012. [Google Scholar]
- Haxaire, K.; Maréchal, Y.; Milas, M.; Rinaudo, M. Hydration of hyaluronan polysaccharide observed by IR spectrometry. II: Definition and quantitative analysis of elementary hydration spectra and water uptake. Biopolymers 2003, 72, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, Y.; Milas, M.; Rinaudo, M. Hydration of hyaluronan polysaccharide observed by IR spectrometry. III: Structure and mechanism of hydration. Biopolymers 2003, 72, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Vasi, A.M.; Popa, M.I.; Butnaru, M.; Dodi, G.; Verestiuc, L. Chemical functionalization of hyaluronic acid for drug delivery applications. Mater. Sci. Eng. C 2014, 38, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Alkrad, J.A.; Mrestani, Y.; Stroehl, D.; Wartewig, S.; Neubert, R. Characterization of enzymatically digested hyaluronic acid using NMR, Raman, IR, and UV-Vis spectroscopes. J. Pharm. Biomed. Anal. 2003, 31, 545–550. [Google Scholar] [CrossRef]
- Duarte, M.L.; Ferreira, M.C.; Marvão, M.R.; Rocha, J. An optimised method to determine the degree of acetylation of chitin and chitosan by FTIR spectroscopy. Int. J. Biol. Macromol. 2002, 31, 1–8. [Google Scholar] [CrossRef]
- Van den Berg, R.; Peters, J.A.; van Bekkum, H. The structure and (local) stability constants of borate esters of mono- and di-saccharides as studied by 11B and 13C NMR spectroscopy. Carbohydr. Res. 1994, 253, 1–12. [Google Scholar] [CrossRef]
- Sen, S. Temperature induced structural changes and transport mechanisms in borate, borosilicate and boroaluminate liquids: High-resolution and high-temperature NMR results. J. Non-Cryst. Solids 1999, 253, 84–94. [Google Scholar] [CrossRef]
- Dubrovskii, S.А.; Zelenetskii, A.N.; Uspenskii, S.A.; Khabarov, V.N. Effect of borax additives on the rheological properties of sodium hyaluronate aqueous solutions. Polym. Sci. Ser. A 2014, 56, 205–210. [Google Scholar] [CrossRef]
- Grafstein, D.; Dvorak, J. Neocarboranes, a new family of stable organoboranes isomeric with the carboranes. Inorg. Chem. 1963, 2, 1128–1133. [Google Scholar] [CrossRef]
- Snyder, H.R.; Reedy, A.J.; Lennarz, W.J. Synthesis of aromatic boronic acids. Aldehydo boronic acids and a boronic acid analog of tyrosine. J. Am. Chem. Soc. 1958, 80, 835–838. [Google Scholar] [CrossRef]
- Soloway, A.H.; Wright, R.L.; Messer, J.R. Evaluation of boron compounds for use in neutron-capture therapy of brain tumors. I. Animal investigations. J. Pharmacol. Exp. Therap. 1961, 134, 117–122. [Google Scholar]
- Ichihashi, M.; Nakanishi, T.; Mishima, Y. Specific killing effect of 10B1-para-boronophenylalanine in thermal neutron capture therapy of malignant melanoma: In vitro radiobiological evaluation. J. Investig. Dermatol. 1982, 78, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F. A critical assessment of boron neutron capture therapy: An overview. J. Neurooncol. 2003, 62, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; ISBN 978-0-470-51234-0. [Google Scholar]
- Lenz, R.W.; Heeschen, J.P. The application of nuclear magnetic resonance to structural studies of carbohydrates in aqueous solution. J. Polym. Sci. A 1961, 51, 247–261. [Google Scholar] [CrossRef]
- Smith, J.T.; Rassi, Z.E. Micellar electrokinetic capillary chromatography with in situ charged micelles IV. Influence of the nature of the alkylglycoside surfactant. J. Chromatogr. A 1994, 685, 131–143. [Google Scholar] [CrossRef]
- Hall, D.G. Boronic Acids. Preparation, Application in Organic Synthesis and Medicine; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; ISBN 978-3-527-32598-6. [Google Scholar]
- Choi, K.Y.; Saravanakumar, G.; Park, J.H.; Park, K. Hyaluronic acid-based nanocarriers for intracellular targeting: Interfacial interactions with proteins in cancer. Colloids Surf. B Biointerfaces 2012, 99, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, P.; Tammi, R.; Parkkinen, J.; Tammi, M.; Agren, U.; Johansson, R.; Hirvikoski, P.; Eskelinen, M.; Kosma, V.M. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am. J. Pathol. 2000, 156, 529–536. [Google Scholar] [CrossRef]
- Ropponen, K.; Tammi, M.; Parkkinen, J.; Eskelinen, M.; Tammi, R.; Lipponen, P.; Agren, U.; Alhava, E.; Kosma, V.M. Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res. 1998, 58, 342–347. [Google Scholar] [PubMed]
- Setala, L.P.; Tammi, M.I.; Tammi, R.H.; Eskelinen, M.J.; Lipponen, P.K.; Agren, U.M.; Parkkinen, J.; Alhava, E.M.; Kosma, V.M. Hyaluronan expression in gastric cancer cells is associated with local and nodal spread and reduced survival rate. Br. J. Cancer 1999, 79, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Selyanin, M.A.; Boykov, P.Y.; Khabarov, V.N.; Polyak, F. Hyaluronic Acid: Production, Properties, Application in Biology and Medicine; Translation, Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; ISBN 978-1-118-63379-3. [Google Scholar]
- De Maio, M.; Rzany, B. Injectable Fillers in Aesthetic Medicine; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-45125-6. [Google Scholar]
- Akopova, T.A.; Zelenetskii, A.N.; Ozerin, A.N. Solid state synthesis and modification of chitosan. In Focus on Chitosan Research; Ferguson, A.N., O’Neill, A.G., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 223–254. ISBN 978-1-62081-841-1. [Google Scholar]
- Demina, T.S.; Akopova, T.A.; Vladimirov, L.V.; Shchegolikhin, A.N.; Kechek’yan, A.S.; Perov, N.S.; Chernyshenko, A.O.; Zelenetskii, A.N. The study of the interaction between chitosan and 2,2-Bis(hydroxymethyl)propionic acid during solid-phase synthesis. Polym. Sci. Ser. B 2011, 53, 358–370. [Google Scholar] [CrossRef]
- Akopova, T.A.; Timashev, P.S.; Demina, T.S.; Bardakova, K.N.; Minaev, N.V.; Burdukovskii, V.F.; Cherkaev, G.V.; Vladimirov, L.V.; Istomin, A.V.; Svidchenko, E.A.; et al. Solid-state synthesis of unsaturated chitosan derivatives to design 3D structures through two-photon-induced polymerization. Mendeleev. Commun. 2015, 25, 280–282. [Google Scholar] [CrossRef]
- Schantea, C.E.; Zubera, G.; Herlinb, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelenetskii, A.N.; Uspenskii, S.; Zaboronok, A.; Cherkaev, G.; Shchegolihin, A.; Mathis, B.J.; Selyanin, M.; Yamamoto, T.; Matsumura, A. Polycomplexes of Hyaluronic Acid and Borates in a Solid State and Solution: Synthesis, Characterization and Perspectives of Application in Boron Neutron Capture Therapy. Polymers 2018, 10, 181. https://doi.org/10.3390/polym10020181
Zelenetskii AN, Uspenskii S, Zaboronok A, Cherkaev G, Shchegolihin A, Mathis BJ, Selyanin M, Yamamoto T, Matsumura A. Polycomplexes of Hyaluronic Acid and Borates in a Solid State and Solution: Synthesis, Characterization and Perspectives of Application in Boron Neutron Capture Therapy. Polymers. 2018; 10(2):181. https://doi.org/10.3390/polym10020181
Chicago/Turabian StyleZelenetskii, Alexander N., Sergey Uspenskii, Alexander Zaboronok, Georgij Cherkaev, Alexander Shchegolihin, Bryan J. Mathis, Mikhail Selyanin, Tetsuya Yamamoto, and Akira Matsumura. 2018. "Polycomplexes of Hyaluronic Acid and Borates in a Solid State and Solution: Synthesis, Characterization and Perspectives of Application in Boron Neutron Capture Therapy" Polymers 10, no. 2: 181. https://doi.org/10.3390/polym10020181






