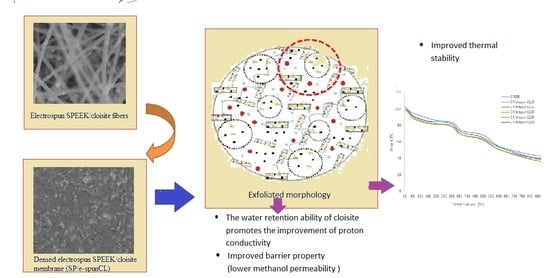
Thermal Stability and Water Content Study of Void-Free Electrospun SPEEK/Cloisite Membrane for Direct Methanol Fuel Cell Application
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Sulfonated Poly (Ether Ether Ketone) (SPEEK)
2.3. Preparation of Electrospinning Dope Solution
2.4. Electrospinning of Nanofibers
2.5. Preparation of Void-Free SP/e-spun Cloisite Membrane
2.6. Thermogravimetric Analysis (TGA)
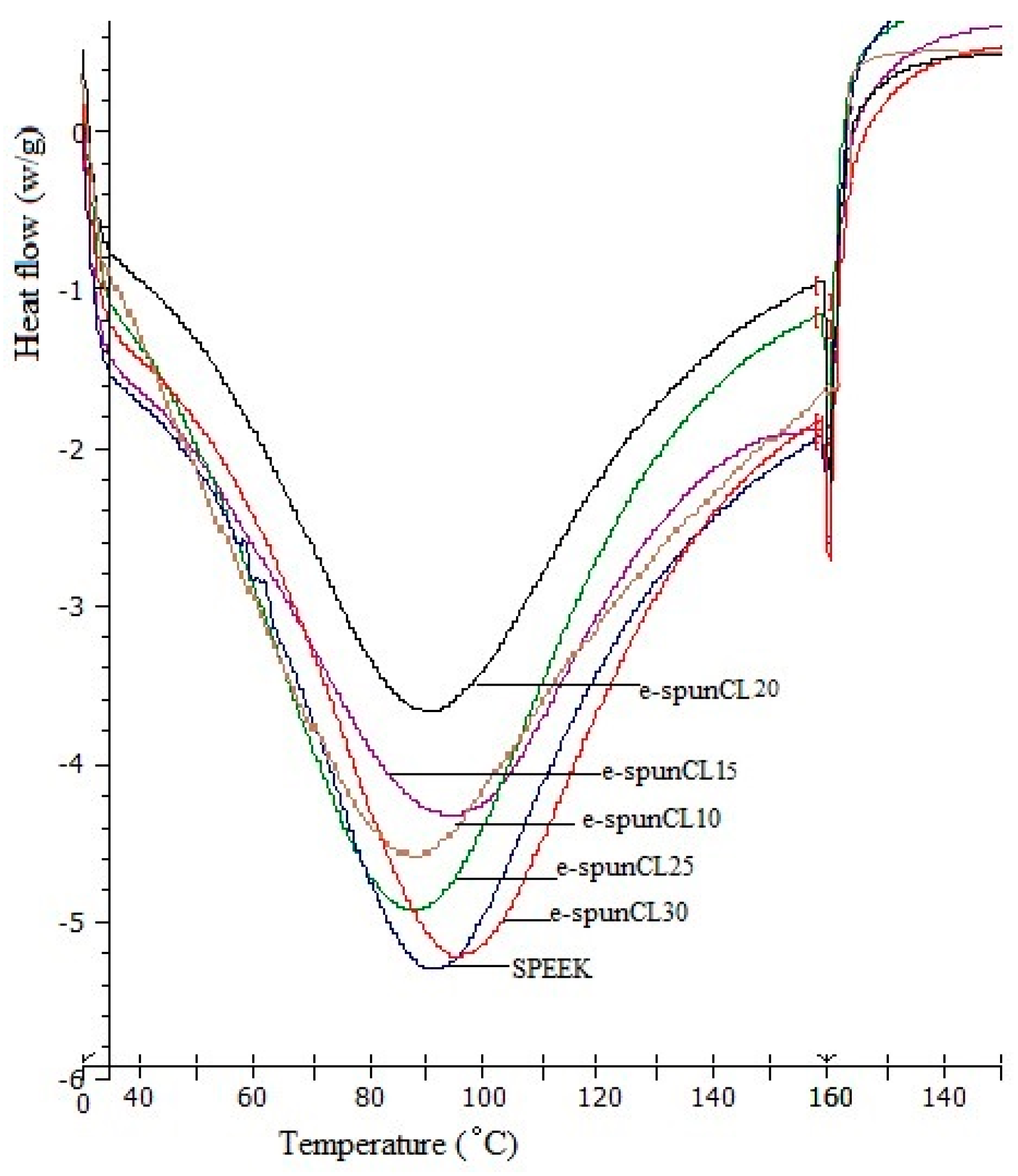
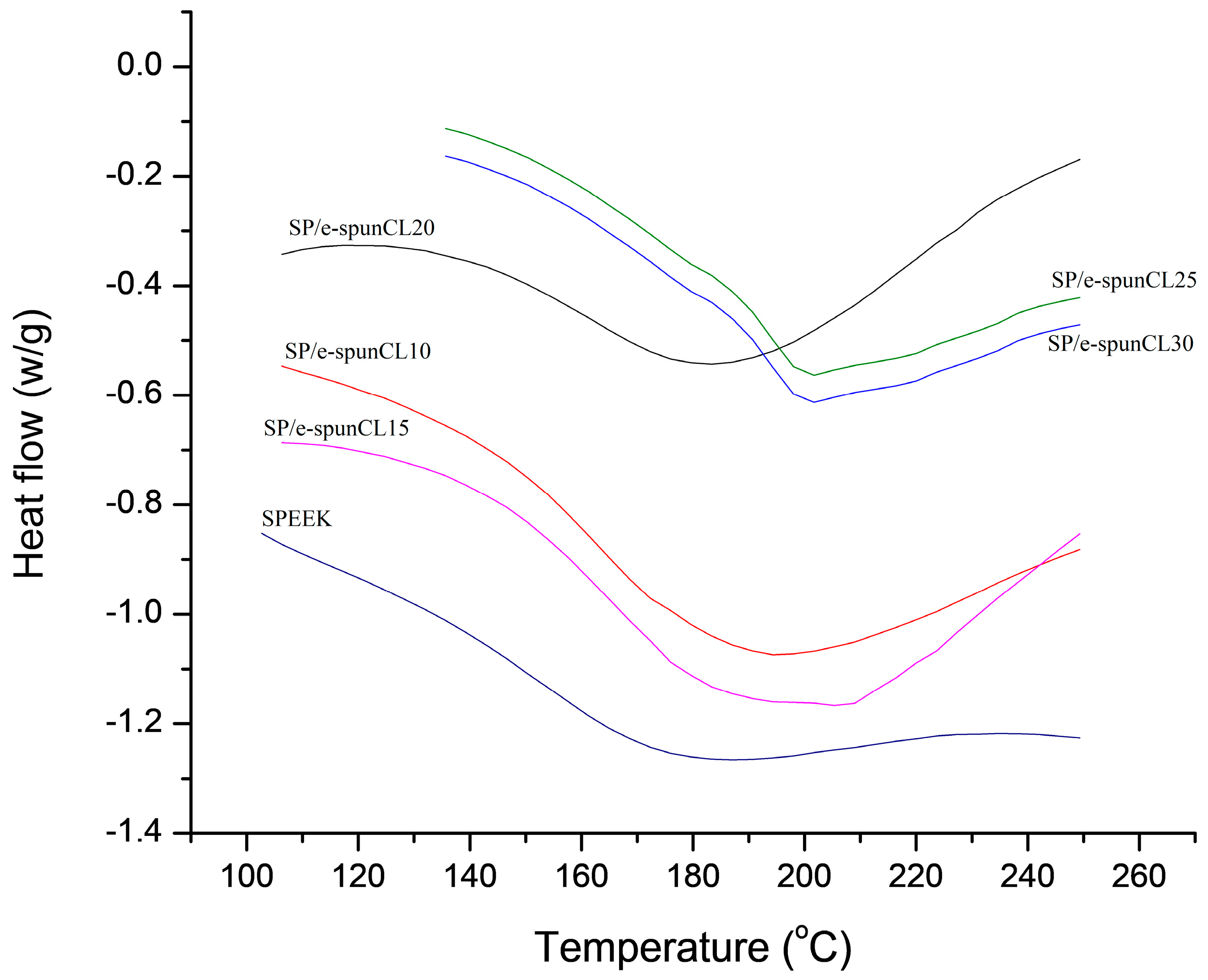
2.7. Differential Scanning Calorimetry (DSC) Analysis
2.8. Water Uptake Measurement
2.9. Proton Conductivity Measurement
- ∂ = proton conductivity (S·cm−1)
- d = membrane thickness (cm)
- R = resistance (ohm) (the value was derived from the low intersection of the high frequency semi-circle on a complex impedance plane with the Re (Z) axis)
- S = membrane cross section area (cm2)
2.10. Methanol Permeability Measurement
- CB (t) = concentration of methanol in compartment B at time, t (M)
- to = time lag, related to the diffusivity (s)
- VB = volume of water in compartment B (cm3) = 200 cm3
- A = membrane cross-section area (cm2)
- L = membrane thickness (cm)
- CA = concentration of methanol in compartment A at time, t (M) = 1 M
2.11. Tensile Test
2.12. Scanning Electron Microscopy Analysis (SEM)
3. Results and Discussion
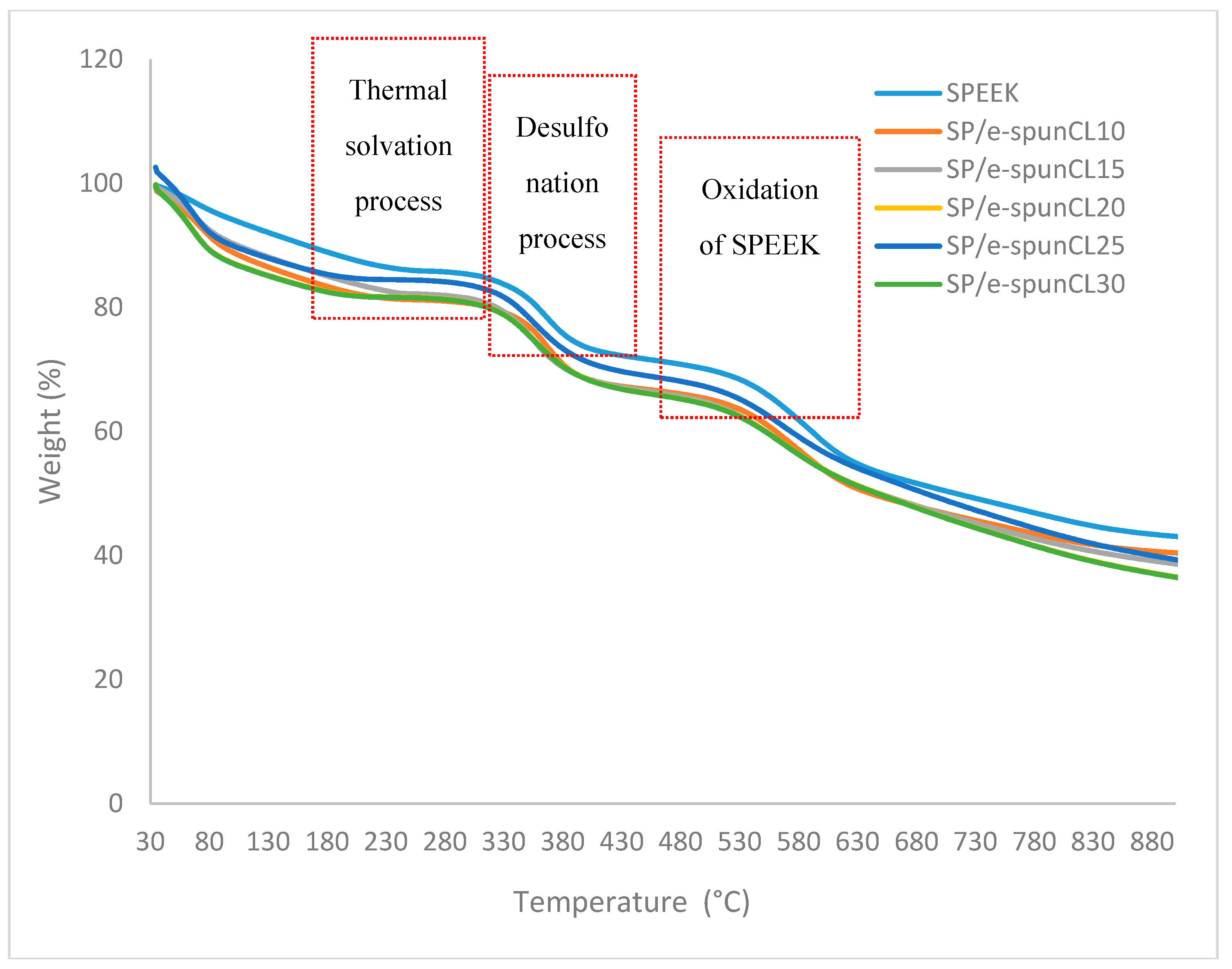
3.1. Thermal Stability Study of Void-Free SP/e-spunCL Membranes
3.2. Wettability Analysis of the Void-Free SP/e-spunCL Membranes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wan, N. High performance direct methanol fuel cell with thin electrolyte membrane. J. Power Sources 2017, 354, 167–171. [Google Scholar] [CrossRef]
- Masdar, M.S.; Zainoodin, A.M.; Rosli, M.I.; Kamarudin, S.K.; Daud, W.R.W. Performance and stability of single and 6-cell stack passive direct methanol fuel cell (DMFC) for long-term operation. Int. J. Hydrog. Energy 2017, 42, 9230–9242. [Google Scholar] [CrossRef]
- Das, V.; Padmanaban, S.; Venkitusamy, K.; Selvamuthukumaran, R.; Blaabjerg, F.; Siano, P. Recent advances and challenges of fuel cell based power system architectures and control—A review. Renew. Sustain. Energy Rev. 2017, 73, 10–18. [Google Scholar] [CrossRef]
- Branco, C.M.; Sharma, S.; de Camargo Forte, M.M.; Steinberger-Wilckens, R. New approaches towards novel composite and multilayer membranes for intermediate temperature-polymer electrolyte fuel cells and direct methanol fuel cells. J. Power Sources 2016, 316, 139–159. [Google Scholar] [CrossRef]
- Dutta, K.; Das, S.; Kumar, P.; Kundu, P.P. Polymer electrolyte membrane with high selectivity ratio for direct methanol fuel cells: A preliminary study based on blends of partially sulfonated polymers polyaniline and PVdF-co-HFP. Appl. Energy 2014, 118, 183–191. [Google Scholar] [CrossRef]
- Costamagna, P.; Yang, C.; Bocarsly, A.B.; Srinivasan, S. Nafion® 115/zirconium phosphate composite membranes for operation of PEMFCs above 100 C. Electrochim. Acta 2002, 47, 1023–1033. [Google Scholar] [CrossRef]
- Radenahmad, N.; Afif, A.; Petra, P.I.; Rahman, S.M.; Eriksson, S.G.; Azad, A.K. Proton-conducting electrolytes for direct methanol and direct urea fuel cells–A state-of-the-art review. Renew. Sustain. Energy Rev. 2016, 57, 1347–1358. [Google Scholar] [CrossRef]
- Mahajan, C.V.; Ganesan, V. Atomistic simulations of structure of solvated sulfonated poly (ether ether ketone) membranes and their comparisons to Nafion: I. Nanophase segregation and hydrophilic domains. J. Phys. Chem. B 2010, 114, 8357–8366. [Google Scholar] [CrossRef] [PubMed]
- Yang, T. Preliminary study of SPEEK/PVA blend membranes for DMFC applications. Int. J. Hydrog. Energy 2008, 33, 6772–6779. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Bi, D.; Lin, H.; Shao, K.; Fu, T.; Na, H. Blend membranes based on disulfonated poly (aryl ether ether ketone) s (SPEEK) and poly (amide imide)(PAI) for direct methanol fuel cell usages. Polymer 2007, 48, 3090–3097. [Google Scholar] [CrossRef]
- Tsai, J.C.; Cheng, H.P.; Kuo, J.F.; Huang, Y.H.; Chen, C.Y. Blended Nafion®/SPEEK direct methanol fuel cell membranes for reduced methanol permeability. J. Power Sources 2009, 189, 958–965. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Sgreccia, E.; Licoccia, S.; Alberti, G.; Tortet, L.; Knauth, P. Analysis of temperature-promoted and solvent-assisted cross-linking in sulfonated poly (ether ether ketone)(SPEEK) proton-conducting membranes. J. Phys. Chem. B 2009, 113, 7505–7512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, S.; Cui, X.; Cai, H.; Fu, T.; Shao, K.; Na, H. Crosslinked SPEEK/AMPS blend membranes with high proton conductivity and low methanol diffusion coefficient for DMFC applications. J. Power Sources 2007, 168, 154–161. [Google Scholar] [CrossRef]
- Zhong, S.; Cui, X.; Cai, H.; Fu, T.; Zhao, C.; Na, H. Crosslinked sulfonated poly (ether ether ketone) proton exchange membranes for direct methanol fuel cell applications. J. Power Sources 2007, 164, 65–72. [Google Scholar] [CrossRef]
- Awang, N.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Yusof, N.; Azman, W.W.M.N. Development of dense void-free electrospun SPEEK-Cloisite15A membrane for direct methanol fuel cell application: Optimization using response surface methodology. Int. J. Hydrog. Energy 2017, 42, 26496–26510. [Google Scholar] [CrossRef]
- Ren, S.; Li, C.; Zhao, X.; Wu, Z.; Wang, S.; Sun, G.; Yang, X. Surface modification of sulfonated poly (ether ether ketone) membranes using Nafion solution for direct methanol fuel cells. J. Membr. Sci. 2005, 247, 59–63. [Google Scholar] [CrossRef]
- Hudiono, Y.; Choi, S.; Shu, S.; Koros, W.J.; Tsapatsis, M.; Nair, S. Porous layered oxide/Nafion® nanocomposite membranes for direct methanol fuel cell applications. Microporous Mesoporous Mater. 2009, 118, 427–434. [Google Scholar] [CrossRef]
- Kawasumi, M. The discovery of polymer-clay hybrids. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 819–824. [Google Scholar] [CrossRef]
- Zhang, S.; He, G.; Gong, X.; Zhu, X.; Wu, X.; Sun, X.; Li, H. Electrospun nanofiber enhanced sulfonated poly (phthalazinone ether sulfone ketone) composite proton exchange membranes. J. Membr. Sci. 2015, 493, 58–65. [Google Scholar] [CrossRef]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Sanchez, C.; Julian, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Jaafar, J.; Ismail, A.F.; Matsuura, T. Preparation and barrier properties of SPEEK/Cloisite 15A®/TAP nanocomposite membrane for DMFC application. J. Membr. Sci. 2009, 345, 119–127. [Google Scholar] [CrossRef]
- Balbaşı, M.; Gözütok, B. Poly (vinyl alcohol)-colloidal silica composite membranes for fuel cells. Synth. Metals 2010, 160, 150–155. [Google Scholar] [CrossRef]
- Mohtar, S.S.; Ismail, A.F.; Matsuura, T. Preparation and characterization of SPEEK/MMT-STA composite membrane for DMFC application. J. Membr. Sci. 2011, 371, 10–19. [Google Scholar] [CrossRef]
- Chou, B. Nano-Scale Modified Inorganic/Organic Hybrid Materials as Proton Conductors. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2006. [Google Scholar]
- Jaafar, J.; Ismail, A.F.; Mustafa, A. Physicochemical study of poly (ether ether ketone) electrolyte membranes sulfonated with mixtures of fuming sulfuric acid and sulfuric acid for direct methanol fuel cell application. Mater. Sci. Eng. A 2007, 460, 475–484. [Google Scholar] [CrossRef]
- Xue, Y.; Fu, R.; Wu, C.; Lee, J.Y.; Xu, T. Acid–base hybrid polymer electrolyte membranes based on SPEEK. J. Membr. Sci. 2010, 350, 148–153. [Google Scholar] [CrossRef]
- Grot, W.G.; Rajendran, G. Membranes Containing Inorganic Fillers and Membrane and Electrode Assemblies and Electrochemical Cells Employing Same. U.S. Patent No. 5,919,583, 1999. [Google Scholar]
- Yamaguchi, T.; Miyata, F.; Nakao, S.I. Pore-filling type polymer electrolyte membranes for a direct methanol fuel cell. J. Membr. Sci. 2003, 214, 283–292. [Google Scholar] [CrossRef]
- Prasad, M.; Mohanty, S.; Nayak, S.K. Study of polymeric nanocomposite membrane made from sulfonated polysulfone and nanoclay for fuel cell applications. High Perform. Polym. 2014, 26, 578–586. [Google Scholar] [CrossRef]
- Dong, B.; Gwee, L.; Salas-de La Cruz, D.; Winey, K.I.; Elabd, Y.A. Super proton conductive high-purity Nafion nanofibers. Nano Lett. 2010, 10, 3785–3790. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Lee, H.J.; Nam, S.Y. Sulfonated poly (arylene ether sulfone) membranes blended with hydrophobic polymers for direct methanol fuel cell applications. Int. J. Hydrog. Energy 2014, 39, 17524–17532. [Google Scholar] [CrossRef]
- Jung, D.H.; Cho, S.Y.; Peck, D.H.; Shin, D.R.; Kim, J.S. Preparation and performance of a Nafion®/montmorillonite nanocomposite membrane for direct methanol fuel cell. J. Power Sources 2003, 118, 205–211. [Google Scholar] [CrossRef]
- Lin, Y.F.; Yen, C.Y.; Hung, C.H.; Hsiao, Y.H.; Ma, C.C.M. A novel composite membranes based on sulfonated montmorillonite modified Nafion® for DMFCs. J. Power Sources 2007, 168, 162–166. [Google Scholar] [CrossRef]
- Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Relationship between hydrogen bonding and bound water in polyhydroxystyrene derivatives. Polymer 1983, 24, 871–876. [Google Scholar] [CrossRef]
- Morishige, K.; Kawano, K. Freezing and melting of water in a single cylindrical pore: The pore-size dependence of freezing and melting behavior. J. Chem. Phys. 1999, 110, 4867–4872. [Google Scholar] [CrossRef]
- Kim, D.S.; Liu, B.; Guiver, M.D. Influence of silica content in sulfonated poly (arylene ether ether ketone ketone)(SPAEEKK) hybrid membranes on properties for fuel cell application. Polymer 2006, 47, 7871–7880. [Google Scholar] [CrossRef]
- Awang, N.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A. Effects of SPEEK/Cloisite Concentration as Electrospinning Parameter on Proton Exchange Membrane for Direct Methanol Fuel Cell Application. Mater. Sci. Forum 2017, 890, 278–284. [Google Scholar] [CrossRef]
- Awang, N.; Ismail, A.F.; Jaafar, J.; Matsuura, T.; Junoh, H.; Othman, M.H.D.; Rahman, M.A. Functionalization of polymeric materials as a high performance membrane for direct methanol fuel cell: A review. React. Funct. Polym. 2015, 86, 248–258. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton conductivity: Materials and applications. Chem. Mater. 1996, 8, 610–641. [Google Scholar] [CrossRef]







| Samples | Voltage (kV) | Distance (cm) | Designation |
|---|---|---|---|
| 0.10 wt % electrospun SPEEK/Cloisite membrane | 22.5 | 20 | SP/e-spunCL10 |
| 0.15 wt % electrospun SPEEK/Cloisite membrane | 22.5 | 20 | SP/e-spunCL15 |
| 0.20 wt % electrospun SPEEK/Cloisite membrane | 22.5 | 20 | SP/e-spunCL20 |
| 0.25 wt % electrospun SPEEK/Cloisite membrane | 22.5 | 20 | SP/e-spunCL25 |
| 0.30 wt % electrospun SPEEK/Cloisite membrane | 22.5 | 20 | SP/e-spunCL30 |
| Membrane | First Weight Loss (%) | Second Weight Loss (%) | Third Weight Loss (%) | Td1 (°C) | Td2 (°C) | Td3 (°C) |
|---|---|---|---|---|---|---|
| SP/e-spunCL10 | 17.85 ± 0.76 | 12.70 ± 0.98 | 15.77 ± 0.76 | 192.1 ± 0.77 | 389.1 ± 0.70 | 602.1 ± 0.77 |
| SP/e-spunCL15 | 16.08 ± 0.56 | 15.26 ± 0.87 | 15.74 ± 0.85 | 201.1 ± 0.87 | 398.1 ± 0.80 | 610.1 ± 0.86 |
| SP/e-spunCL20 | 17.44 ± 0.45 | 13.69 ± 0.08 | 15.72 ± 0.07 | 196.1 ± 0.06 | 395.1 ± 0.08 | 606.1 ± 0.06 |
| SP/e-spunCL25 | 12.61 ± 0.67 | 14.21 ± 0.65 | 16.62 ± 0.64 | 208.1 ± 0.56 | 406.1 ± 0.56 | 613.1 ± 0.58 |
| SP/e-spunCL30 | 9.44 ± 0.34 | 15.31 ± 0.34 | 17.95 ± 0.32 | 209.1 ± 0.34 | 410.1 ± 0.38 | 617.1 ± 0.38 |
| SPEEK | 20.56 ± 0.23 | 14.41 ± 0.12 | 11.12 ± 0.34 | 163.1 ± 0.45 | 388.1 ± 0.89 | 564.1 ± 0.67 |
| Concentration (wt %) | Fiber Diameter, nm |
|---|---|
| SP/e-spunCL10 | 67,680.0 |
| SP/e-spunCL15 | 429.2 |
| SP/e-spunCL20 | 386.17 |
| SP/e-spunCL25 | 495.4 |
| SP/e-spunCL30 | 9257.0 |
| Samples | Tg (°C) |
|---|---|
| SP/e-spunCL10 | 151.00 |
| SP/e-spunCL15 | 156.67 |
| SP/e-spunCL20 | 153.00 |
| SP/e-spunCL25 | 160.33 |
| SP/e-spunCL30 | 164.00 |
| SPEEK | 150.20 |
| Sample | Tensile Strength (MPa) | Young’s Modulus (MPa) |
|---|---|---|
| SP/e-spunCL10 | 29.97 ± 0.78 | 2743.79 ± 0.56 |
| SP/e-spunCL15 | 36.35 ± 0.97 | 3640.74 ± 0.98 |
| SP/e-spunCL20 | 33.40 ± 0.74 | 1713.62 ± 0.35 |
| SP/e-spunCL25 | 28.58 ± 0.67 | 2681.99 ± 0.37 |
| SP/e-spunCL30 | 28.61 ± 0.86 | 1626.70 ± 0.27 |
| Sample | Total Water (%) | ΔHf Normalized (J·g−1 Sample) a | ΔHf Per Mass Water (J·g−1 Water) b | Freezing Water/Total Water (%) c | Non-freezing Water/Total Water (%) | Freezing Water/Sample (%) | Non-freezing Water/Sample (%) |
|---|---|---|---|---|---|---|---|
| SP/e-spunCL10 | 24.56 | 67.04 | 272.96 | 81.97 | 18.03 | 20.13 | 4.43 |
| SP/e-spunCL15 | 25.87 | 50.84 | 196.52 | 59.01 | 40.99 | 15.27 | 10.60 |
| SP/e-spunCL20 | 30.00 | 70.15 | 233.83 | 70.22 | 29.78 | 21.07 | 8.93 |
| SP/e-spunCL25 | 20.00 | 57.55 | 287.75 | 86.41 | 13.59 | 17.28 | 2.72 |
| SP/e-spunCL30 | 19.00 | 59.76 | 314.52 | 94.45 | 5.55 | 17.95 | 1.05 |
| SPEEK | 26.76 | 85.46 | 319.35 | 95.90 | 4.10 | 25.66 | 1.10 |
| Nafion®112 | 21.43 | 64.19 | 299.53 | 89.95 | 10.05 | 19.28 | 2.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awang, N.; Jaafar, J.; Ismail, A.F. Thermal Stability and Water Content Study of Void-Free Electrospun SPEEK/Cloisite Membrane for Direct Methanol Fuel Cell Application. Polymers 2018, 10, 194. https://doi.org/10.3390/polym10020194
Awang N, Jaafar J, Ismail AF. Thermal Stability and Water Content Study of Void-Free Electrospun SPEEK/Cloisite Membrane for Direct Methanol Fuel Cell Application. Polymers. 2018; 10(2):194. https://doi.org/10.3390/polym10020194
Chicago/Turabian StyleAwang, Nuha, Juhana Jaafar, and Ahmad Fauzi Ismail. 2018. "Thermal Stability and Water Content Study of Void-Free Electrospun SPEEK/Cloisite Membrane for Direct Methanol Fuel Cell Application" Polymers 10, no. 2: 194. https://doi.org/10.3390/polym10020194
APA StyleAwang, N., Jaafar, J., & Ismail, A. F. (2018). Thermal Stability and Water Content Study of Void-Free Electrospun SPEEK/Cloisite Membrane for Direct Methanol Fuel Cell Application. Polymers, 10(2), 194. https://doi.org/10.3390/polym10020194