Two Chemically Stable Cd(II) Polymers as Fluorescent Sensor and Photocatalyst for Aromatic Dyes
Abstract
:1. Introduction
2. Materials and Method
2.1. Chemicals and Instrumentation
2.2. X-ray Crystallography
2.3. Synthesis of [Cd2(H2L)2(2,2′-bipy)2]
2.4 Synthesis of [Cd(L)0.5(phen)·0.5H2O]
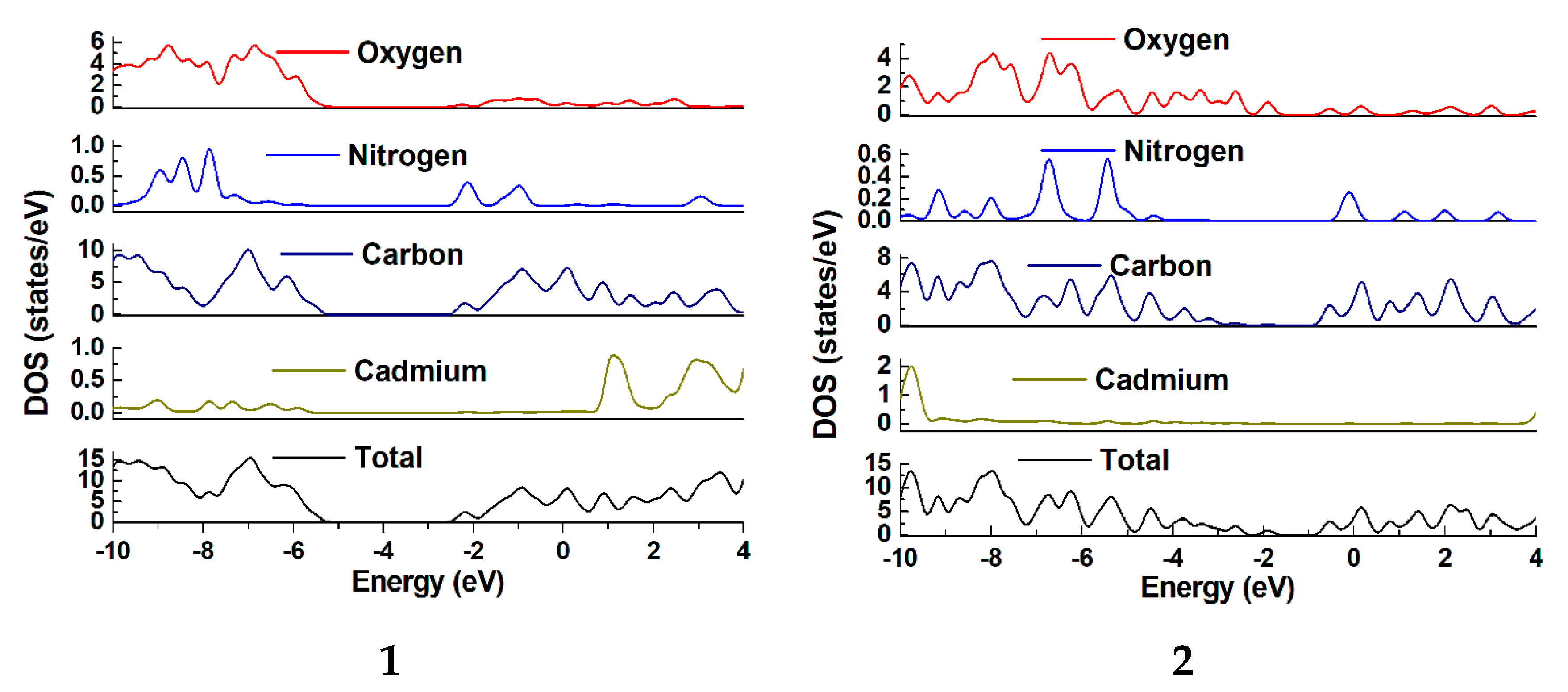
2.5. Computational Protocols
3. Results and Discussion
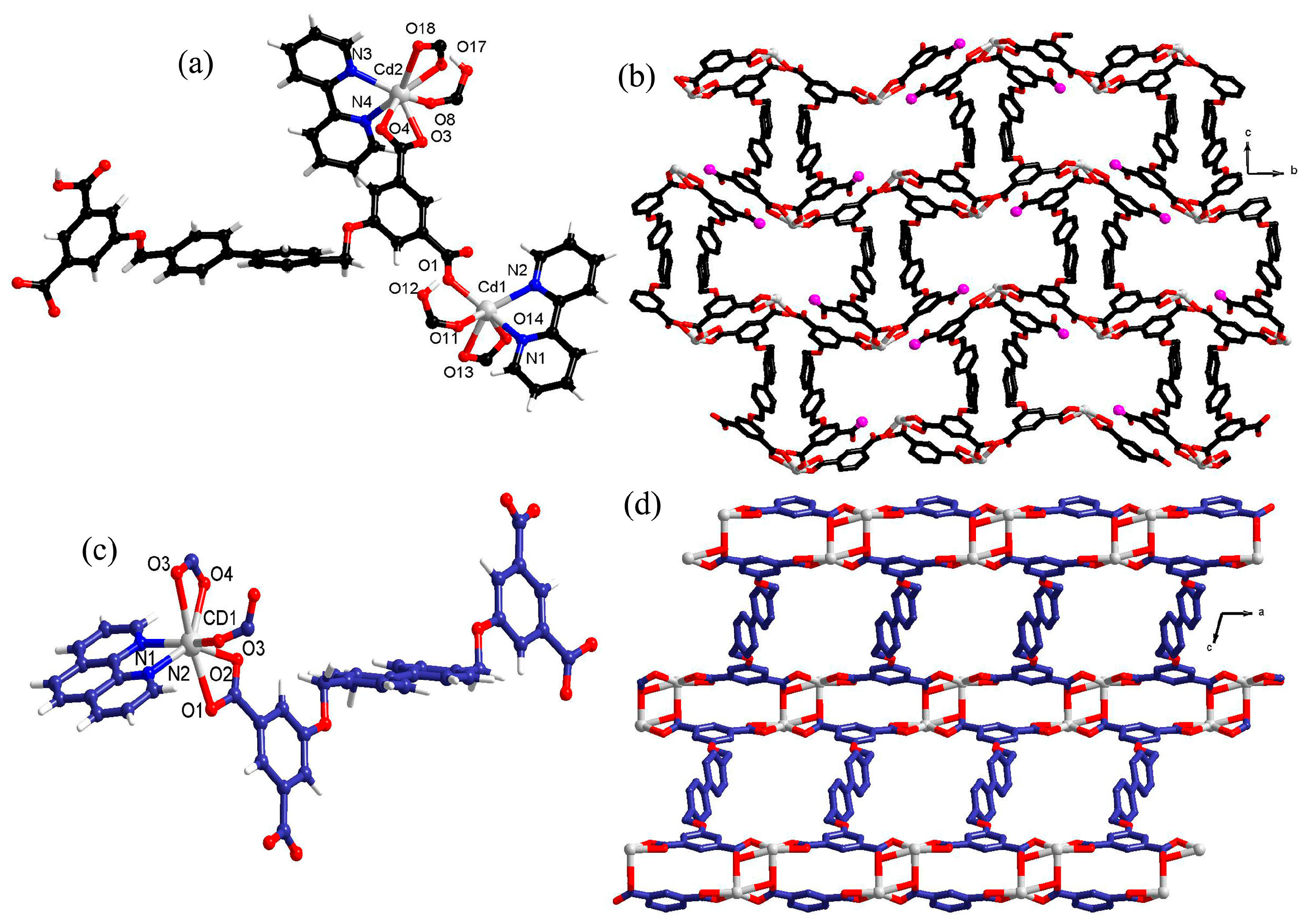
3.1. [Cd2(H2L)2(2,2′-bipy)2]
3.2. [Cd(L)0.5(phen)·0.5H2O]
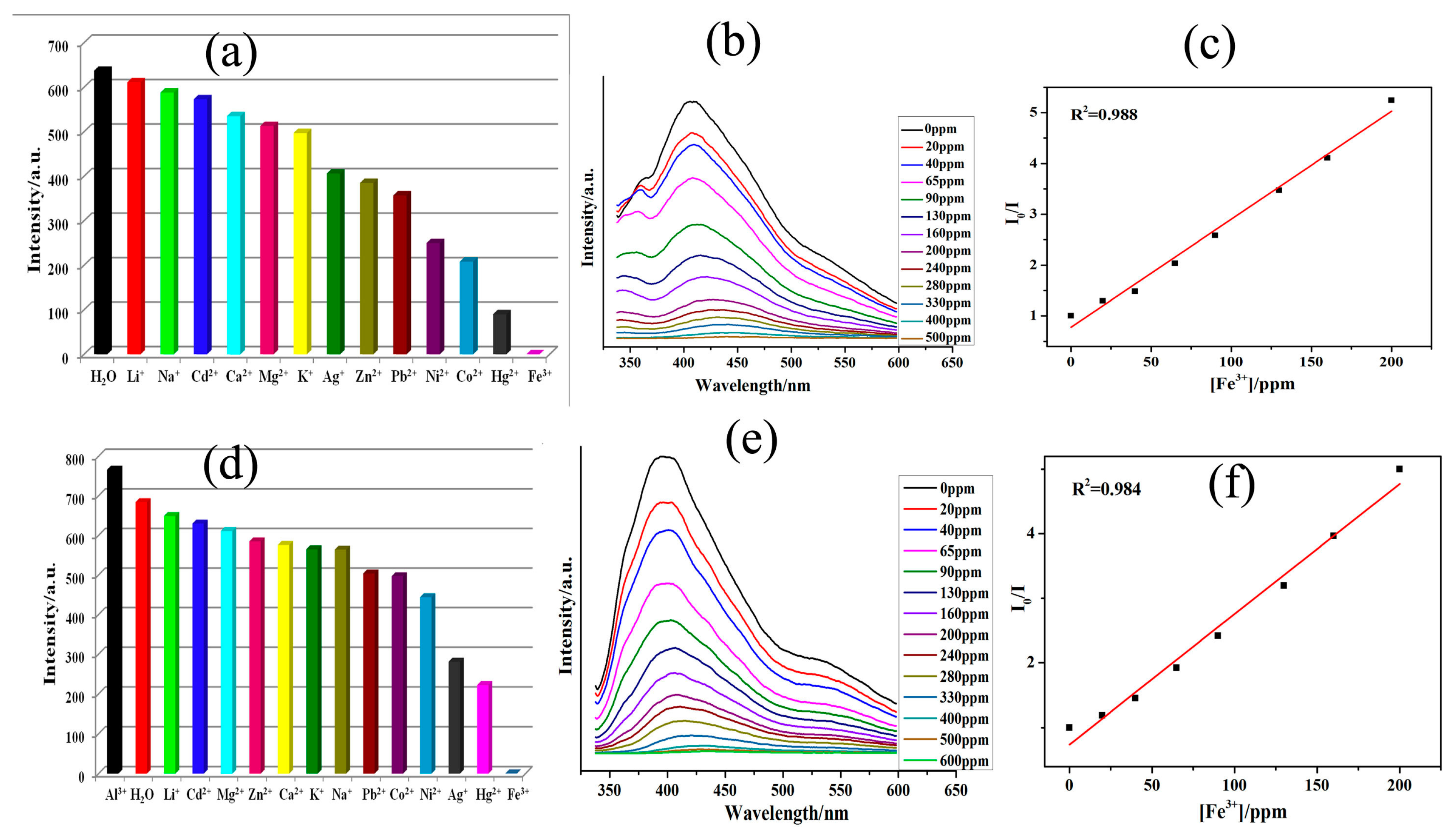
3.3. Luminescence Sensing
3.4. Diffuse-Reflectance UV/Vis Spectroscopy
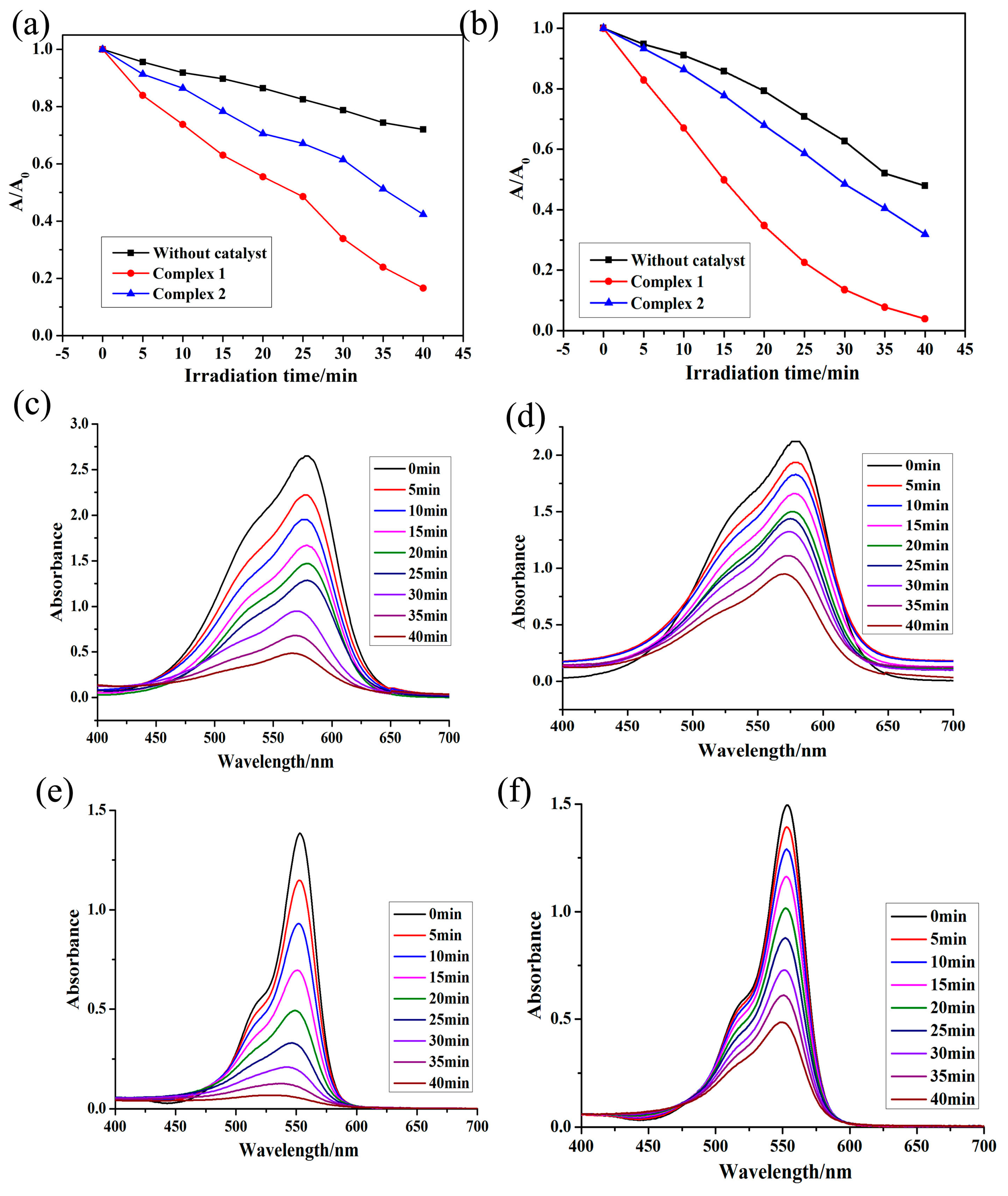
3.5. Photocatalysis
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Xie, Z.G.; Ma, L.Q.; deKrafft, K.E.; Jin, A.; Lin, W.B. Porous phosphorescent coordination polymers for oxygen sensing. J. Am. Chem. Soc. 2010, 132, 922–923. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yang, Y.; Zapata, F.; Lin, G.; Qian, G.; Lobkovsky, E.B. Luminescent open metal sites within a metal-organic framework for sensing small molecules. Adv. Mater. 2007, 19, 1693–1696. [Google Scholar] [CrossRef]
- Bauer, C.A.; Timofeeva, T.V.; Settersten, T.B.; Patterson, B.D.; Liu, V.H.; Simmons, B.A.; Allendrof, M.D. Influence of connectivity and porosity on ligand-based luminescence in zinc metal-organic frameworks. J. Am. Chem. Soc. 2007, 129, 7136–7144. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Wang, L.B.; Zapata, F.; Qian, G.D.; Lobkovsky, E.B. A luminescent microporous metal-organic framework for the recognition and sensing of anions. J. Am. Chem. Soc. 2008, 130, 6718–6719. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.Y.; Liu, Q.; Shen, Y.C.; Wu, X.Z.; Xu, D.D.; Ma, X.L.; Wang, L.H.; Chen, Q.H.; Zhang, Z.J.; et al. Microporous metal-organic framework with lantern-like dodecanuclear metal coordination cages as nodes for selective adsorption of C2/C1 mixtures and sensing of nitrobenzene. Cryst. Growth Des. 2015, 15, 3847–3852. [Google Scholar] [CrossRef]
- Liu, J.Q.; Wu, J.; Li, F.M.; Liu, W.C.; Li, B.H.; Wang, J.; Li, Q.L.; Yadav, R.; Kumar, A. Luminescent sensing from a new Zn (II) metal-organic framework. RSC Adv. 2016, 6, 31161–31166. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.R.; Liu, J.Q.; Li, B.H.; Singh, A.; Kumar, A.; Batten, S.R. An uncommon (5,5)-connected 3D metal organic material for selective and sensitive sensing of nitroaromatics and ferric ion: experimental studies and theoretical analysis. CrystEngComm 2017, 19, 3519–3525. [Google Scholar] [CrossRef]
- Lu, L.; Wu, J.; Wang, J.; Liu, J.Q.; Li, B.H.; Singh, A.; Kumar, A.; Batten, S.R. An uncommon 3D 3,3,4,8-c Cd (II) metal-organic framework for highly efficient luminescent sensing and organic dye adsorption: experimental and theoretical insight. CrystEngComm 2017, 19, 7057–7067. [Google Scholar] [CrossRef]
- Liu, J.Q.; Wang, W.J.; Luo, Z.D.; Li, B.H.; Yuan, D.Q. Microporous metal-organic framework based on ligand-truncation strategy with high performance for gas adsorption and separation. Inorg. Chem. 2017, 56, 10215–10219. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.T.; Yang, G.P.; Liu, B.; Yan, Y.T.; Xi, Z.P.; Wang, Y.Y. Four super water-stable lanthanide-organic frameworks with active uncoordinated carboxylic and pyridyl groups for selective luminescence sensing of Fe3+. Dalton Trans. 2015, 44, 13325–13330. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.C.; Pang, L.Y.; Yang, G.P.; Hou, L.; Wang, Y.Y. Two porous luminescent metal-organic frameworks: quantifiable evaluation of dynamic and static luminescent sensing mechanisms towards Fe3+. Dalton Trans. 2015, 44, 17222–17228. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Sharma, D.; Bera, R.K.; Crisponic, G.; Callan, J.F. Iron (III) selective molecular and supramolecular fluorescent probes. Chem. Soc. Rev. 2012, 41, 7195–7227. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.C.; Wu, J.; He, Y.X.; Li, B.H.; Liu, J.Q.; Prasad, R.; Kumar, A.; Batten, S.R. A 3D luminescent Zn (II) MOF for the detection of high explosives and the degradation of organic dyes: an experimental and computational study. CrystEngComm 2017, 19, 6464–6472. [Google Scholar] [CrossRef]
- Liu, J.Q.; Wu, J.; Luo, Z.D.; Li, B.H.; Singh, A.; Kumar, A. A porous zinc(II) metal–organic framework exhibiting high sensing ability for ferric and nitroaromatics as well as photocatalytic degradation activities against organic dyes. J. Coord. Chem. 2017, 70, 3946–3958. [Google Scholar] [CrossRef]
- Dau, P.V.; Cohen, S.M. Cyclometalated metal-organic frameworks as stable and reusable heterogeneous catalysts for allylic N-alkylation of amines. Chem. Commun. 2013, 49, 6128–6130. [Google Scholar] [CrossRef] [PubMed]
- Dau, P.V.; Cohen, S.M. The influence of nitro groups on the topology and gas sorption property of extended Zn (II)-paddlewheel MOFs. CrystEngComm 2013, 15, 9304–9307. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Yin, Z.; Wang, Q.Q.; Sun, D.; Zeng, M.H.; Kurmoo, M. Tandem postsynthetic modification of a metal-organic framework by thermal elimination and subsequent bromination: Effects on absorption properties and photoluminescence. Angew. Chem. Int. Ed. 2013, 52, 4636–4641. [Google Scholar] [CrossRef]
- Wang, X.X.; Wang, X.Q.; Niu, X.Y.; Hu, T.P. Three novel metal-organic frameworks based on an unsymmetrical rigid carboxylate ligand for luminescence sensing of nitrobenzene derivatives and magnetic properties. CrystEngComm 2016, 18, 7471–7477. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, H.; Yue, Y.; Guo, Z.; Yu, J.; Chen, Z.; Gao, J.; Yang, Y.; Qian, G.; Chen, B. A luminescent mixed-lanthanide metal-organic framework thermometer. J. Am. Chem. Soc. 2012, 134, 3979–3982. [Google Scholar] [CrossRef] [PubMed]
- Dau, P.V.; Kim, M.; Cohen, S.M. Site-selective cyclometalation of a metal-organic framework. Chem. Sci. 2013, 4, 601–605. [Google Scholar] [CrossRef]
- Liu, X.P.; Xiao, Z.Y.; Xu, J.; Xu, W.B.; Sang, P.P.; Zhao, L.M.; Zhu, H.Y.; Sun, D.F.; Guo, W.Y. A NbO-type copper metal-organic framework decorated with carboxylate groups exhibiting highly selective CO2 adsorption and separation of organic dyes. J. Mater. Chem. A 2016, 4, 13844–13851. [Google Scholar] [CrossRef]
- Li, B.H.; Wu, J.; Liu, J.Q.; Gu, C.Y.; Xu, J.W.; Luo, M.M.; Yadav, R.; Kumar, A.; Batten, S.R. A luminescent Zinc (II) metal-organic framework for selective detection of nitroaromatics, Fe3+ and CrO42−: A versatile threefold fluorescent sensor. ChemPlusChem 2016, 81, 885–892. [Google Scholar] [CrossRef]
- Liu, J.Q.; Li, G.P.; Liu, W.C.; Li, Q.L.; Li, B.H.; Gable, R.W.; Batten, S.R. Two unusual nanocage-based Ln-MOFs with triazole sites: Highly fluorescent sensing for Fe3+ and Cr2O72−, and selective CO2 capture. ChemPlusChem 2016, 81, 1299–1304. [Google Scholar] [CrossRef]
- Hou, G.G.; Liu, Y.; Liu, Q.K.; Ma, J.P.; Dong, Y.B. NbO lattice MOFs based on octahedral M (II) and ditopic pyridyl substituted diketonate ligands: Structure, encapsulation and guest-driven luminescent property. Chem. Commun. 2011, 47, 10731–10733. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.N.; Yan, B. Amino-decorated lanthanide (III) organic extended frameworks for multi-color luminescence and fluorescence sensing. J. Mater. Chem. C. 2014, 2, 6758–6764. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Kanga, J.R.R.; Caddy, J.S. Towards conducting metal-organic frameworks. Aust. J. Chem. 2011, 64, 718–722. [Google Scholar] [CrossRef]
- Silva, C.G.; Corma, A.; García, H. Metal-organic frameworks as semiconductors. J. Mater. Chem. 2010, 20, 3141–3156. [Google Scholar] [CrossRef]
- So, M.C.; Wiederrecht, G.P.; Mondloch, J.E.; Hupp, J.T.; Farha, O.K. Metal-organic framework materials for light-harvesting and energy transfer. Chem. Commun. 2015, 51, 3501–3510. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.C.; Wu, J.; Liu, W.C.; Ma, A.Q.; Liu, J.Q.; Singh, A.; Kumar, A. A new Zn(II) metal-organic framework having 3D CdSO4 topology as luminescent sensor and photocatalyst for degradation of organic dyes. New J. Chem. 2018, 42, 2767–2775. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244–245, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous metal-organic framework materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, J.; Sun, L.; Xu, F.; Li, F.; Zhang, H.; Si, X.; Jiao, C.; Li, Z.; Liu, S.; et al. Mesoporous metal-organic frameworks: Design and applications. Energy Environ. Sci. 2012, 5, 7508–7520. [Google Scholar] [CrossRef]
- Lan, Y.Q.; Jiang, H.L.; Li, S.L.; Xu, Q. Mesoporous metal-organic frameworks with size-tunable cages: Selective CO2 uptake, encapsulation of Ln3+ cations for luminescence, and column-chromatographic dye separation. Adv. Mater. 2011, 23, 5015–5020. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1998, 37, 785–789. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T., Jr.; Kudin, K.N.; Burant, J.C.; et al. GAUSSIAN09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. Cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Si, C.D.; Hou, C.; Yao, X.Q.; Hu, D.C.; Yang, Y.X.; Liu, J.C. Three complexes of manganese (II) based on a new semirigid tetracarboxylate and N-containing ligands: Synthesis, crystal structures and magnetic properties. Polyhedron 2015, 98, 64–70. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Q.; Yan, Y.; Pan, Q.H.; Sun, L.B.; He, H.M.; Liu, Y.L.; Liang, Y.Z.Q.; Li, J.Y. A microporous lanthanum metal-organic framework as a bi-functional chemosensor for the detection of picric acid and Fe3+ ions. Dalton Trans. 2015, 44, 13340–13346. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, L.B.; Hao, L.C.Q.; Yan, Y.; Liang, Z.Q. Lanthanide metal–organic frameworks based on a 1,2,3-triazole-containing tricarboxylic acid ligand for luminescence sensing of metal ions and nitroaromatic compounds. RSC Adv. 2016, 6, 57828–57834. [Google Scholar] [CrossRef]
- Singh, A.; Raj, T.; Aree, T.; Singh, N. Fluorescent organic nanoparticles of biginelli-based molecules: recognition of Hg2+ and Cl– in an aqueous medium. Inorg. Chem. 2013, 52, 13830–13832. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Yi, F.Y.; Yu, H.; Jiao, S.H.; Pang, G.S.; Sun, Z.M. Fast response and highly selective sensing of amine vapors using a luminescent coordination polymer. Chem. Commun. 2014, 50, 10506–10509. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.; Li, K.; Wu, H.; Olson, D.H.; Emge, T.J.; Ki, W.; Hong, M.; Li, J. A luminescent microporous metal–organic framework for the fast and reversible detection of high explosives. Angew. Chem. Int. Ed. 2009, 48, 2334–2338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Feng, G.; Song, Z.; Zhou, Y.P.; Chao, H.Y.; Yuan, D.; Tan, T.T.Y.; Guo, Z.; Hu, Z.; Tang, B.Z.; et al. Two-dimensional metal-organic framework with wide channels and responsive turn-on fluorescence for the chemical sensing of volatile organic compounds. J. Am. Chem. Soc. 2014, 136, 7241–7244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Li, Y.; Zhou, W.X.; Liu, R.M.; Ding, Y.J.; Lü, J.; Proserpiocd, D.M. Metal-organic frameworks assembled from flexible alicyclic carboxylate and bipyridyl ligands for sensing of nitroaromatic explosives. CrystEngComm 2016, 18, 4530–4537. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Desai, A.V.; Ghosh, S.K. A fluorescent metal-organic framework for highly selective detection of nitro explosives in the aqueous phase. Chem. Commun. 2014, 50, 8915–8918. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, S.S.; Joarder, B.; Chaudhari, A.K.; Mukherjee, S.; Ghosh, S.K. Highly selective detection of nitro explosives by a luminescent metal-organic framework. Angew. Chem. Int. Ed. 2013, 52, 2881–2885. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.D.; Qiu, L.G.; Ke, F.; Yuan, Y.P.; Xu, G.S.; Wang, Y.M.; Jiang, X. Rapid synthesis of nanoscale terbium-based metal–organic frameworks by a combined ultrasound-vapour phase diffusion method for highly selective sensing of picric acid. J. Mater. Chem. A 2013, 1, 8745–8752. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Sun, L.B.; Du, J.F.; Liu, Y.C.; Shi, H.Z.; Liang, Z.Q.; Li, J.Y. Multifunctional zinc metal-organic framework based on designed H4TCPP ligand with aggregation-induced emission effect: CO2 adsorption, luminescence, and sensing property. Cryst. Growth Des. 2017, 17, 2090–2096. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.H.; Yan, B. An Eu3+ post-functionalized nanosized metal-organic framework for cation exchange-based Fe3+-sensing in an aqueous environment. J. Mater. Chem. A 2014, 2, 13691–132697. [Google Scholar] [CrossRef]
- Yang, C.X.; Ren, H.B.; Yan, X.P. Fluorescent metal-organic framework MIL-53(Al) for highly selective and sensitive detection of Fe3+ in aqueous solution. Anal. Chem. 2013, 85, 7441–7446. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Zhou, W.; Zhang, Z.; Green, M.A.; Liu, Y.; Chen, B. Open metal sites within isostructural metal-organic frameworks for differential recognition of acetylene and extraordinarily high acetylene storage capacity at room temperature. Angew. Chem. Int. Ed. 2010, 49, 4615–4618. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Jiang, M.; Liu, Y.H.; Zhang, L.W.; Wu, P.Y. A highly chemically stable metal-organic framework as a luminescent probe for the regenerable ratiometric sensing of pH. Chem. Eur. J. 2016, 22, 13023–13027. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Liu, S.X.; Liu, Y.W.; Miao, J.; Li, S.J.; Zhang, L.; Shi, Z.; Zheng, Z.P. Cation sensing by a luminescent metal-organic framework with multiple Lewis basic sites. Inorg. Chem. 2013, 52, 2799–2801. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.W.; Liu, J.L.; Jia, J.H.; Leng, J.D.; Lin, W.Q.; Chen, Y.C.; Tong, M.L. Fluorescent single-ion magnets: molecular hybrid (HNEt3)[DyxYb1-x (bpyda)2](x= 0.135-1). Dalton Trans. 2013, 42, 11262–11270. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Eddaoudi, M.; Hyde, S.T.; O’Keeffe, M.; Yaghi, O.M. Interwoven metal-organic framework on a periodic minimal surface with extra-large pores. Science 2001, 291, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.L.; Xu, H.; Cheng, R.R.; Zhao, B. Controlled lanthanide-organic framework nanospheres as reversible and sensitive luminescent sensors for practical applications. Chem. Commun. 2015, 51, 6769–6772. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.C.; Wu, X.R.; Luo, Z.D.; Deng, F.Y.; Liu, J.Q.; Singh, A.; Kumar, A. Luminescent sensing and photocatalytic degradation properties of an uncommon (4,5,5)-connected 3D MOF based on 3,5-Di(3′,5′-dicarboxylphenyl)benzoic acid. CrystEngComm 2017, 19, 4368–4377. [Google Scholar] [CrossRef]
- Pramanik, S.; Zheng, C.; Zhang, X.; Emge, T.J.; Li, J. New microporous metal-organic framework demonstrating unique selectivity for detection of high explosives and aromatic compounds. J. Am. Chem. Soc. 2011, 133, 4153–4155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiang, S.; Rao, X.; Zheng, Q.; Fronczek, F.R.; Qian, G.; Chen, B. A rod packing microporous metal-organic framework with open metal sites for selective guest sorption and sensing of nitrobenzene. Chem. Commun. 2010, 46, 7205–7207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Che, Y.; Zhang, Z.; Yang, X.; Zang, L. Fluorescent nanoscale zinc (II)-carboxylate coordination polymers for explosive sensing. Chem. Commun. 2011, 47, 2336–2338. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.B.; Liu, S.Y.; Ye, J.W.; Li, X.Y.; Zhang, J.P. Photoluminescent metal-organic frameworks for gas sensing. Adv. Sci. 2016, 3, 1500434. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, G.P.; Hou, L.; Cui, L.; Li, Y.P.; Wang, Y.Y.; Shi, Q.Z. Three new solvent-directed 3D lead (II)-MOFs displaying the unique properties of luminescence and selective CO2 sorption. Dalton Trans. 2013, 42, 13590–13598. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Wang, M.J.; Zhang, K.L. A novel photoluminescent Cd(II)-organic framework exhibiting rapid and efficient multi-responsive fluorescence sensing for trace amounts of Fe3+ ions and some NACs, especially for 4-nitroaniline and 2-methyl-4-nitroaniline. J. Mater. Chem. C. 2016, 4, 11404–11418. [Google Scholar] [CrossRef]
- Zhao, S.; Lv, X.X.; Shi, L.L.; Li, B.L.; Wu, B. An unusual (4,4)-connected 3D porous cadmium metal–organic framework as a luminescent sensor for detection of nitrobenzene. RSC Adv. 2016, 6, 56035–56041. [Google Scholar] [CrossRef]
- Zhao, S.; Ding, J.G.; Zheng, T.R.; Li, K.; Li, B.L.; Wu, B. The 3D and 2D cadmium coordination polymers as luminescent sensors for detection of nitroaromatics. J. Lumin. 2017, 188, 356–364. [Google Scholar] [CrossRef]
- Sun, Q.; Lu, J.; Li, J.L.; Jiang, L.; Gu, W.; Liu, X.; Tian, J.L.; Yan, S.P. Synthesis, crystal structures, DNA binding and cleavage properties and protein binding activities of three mononuclear cobalt (II) complexes. Appl. Organomet. Chem. 2014, 28, 259–266. [Google Scholar] [CrossRef]
- Li, D.X.; Ni, C.Y.; Chen, M.M.; Dai, M.; Zhang, W.H.; Yan, W.Y.; Qi, H.X.; Ren, Z.G.; Lang, J.P. Construction of Cd (II) coordination polymers used as catalysts for the photodegradation of organic dyes in polluted water. CrystEngComm 2014, 16, 2158–2167. [Google Scholar] [CrossRef]
- Liu, L.; Ding, J.; Li, M.; Lv, X.F.; Wu, J.; Hou, H.W.; Fan, Y.T. Structural variability, topological analysis and photocatalytic properties of neoteric Cd(II) coordination polymers based on semirigid bis(thiazolylbenzimidazole) and different types of carboxylic acid linkers. Dalton Trans. 2014, 43, 12790–12799. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yu, Z.T.; Yang, J.; Wu, H.; Liu, Y.Y.; Ma, J.F. First three-dimensional inorganic-organic hybrid material constructed from an “inverted Keggin” polyoxometalate and a copper (I)-organic complex. Inorg. Chem. 2011, 50, 8967–8972. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Luo, F.; Dong, F.F. Design synthesis and photocatalytic activity of a novel lilac-like silver-vanadate hybrid solid based on dicyclic rings of [V4O12]4- with {Ag7}7+ cluster. Chem. Commun. 2011, 47, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Kan, W.Q.; Liu, B.; Yang, J.; Liu, Y.Y.; Ma, J.F. Series of Highly Connected Metal-Organic Frameworks Based on Triangular Ligands and d10 Metals: Syntheses, Structures, Photoluminescence, and Photocatalysis. Cryst. Growth Des. 2012, 12, 2288–2298. [Google Scholar] [CrossRef]
- Fu, H.; Li, Y.G.; Lu, Y.; Chen, W.L.; Wu, Q.; Meng, J.X.; Wang, X.L.; Zhang, Z.M.; Wang, E.B. Polyoxometalate-based metal-organic frameworks assembled under the ionothermal conditions. Cryst. Growth Des. 2011, 11, 458–465. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, W.L.; Liu, D.; Liang, C.; Li, Y.G.; Lin, S.W.; Wang, E.B. New class of organic-inorganic hybrid aggregates based on polyoxometalates and Metal-Schiff-base. Dalton Trans. 2011, 40, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.Q.; Xu, Y.M. Photocatalytic degradation of textile dye X3B by heteropolyoxometalate acids. Chemosphere 2004, 54, 431–434. [Google Scholar] [CrossRef]
- Lv, K.L.; Xu, Y.M. Effects of Polyoxometalate and Fluoride on Adsorption and Photocatalytic Degradation of Organic Dye X3B on TiO2: The Difference in the Production of Reactive Species. J. Phys. Chem. B 2006, 110, 6204–6212. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.L.; Zhao, J.B.; Lv, K.L.; Wu, Y.H.; Deng, K.J.; Leng, X.K.; Li, D.F. Visible-light-driven photocatalysts of metal-organic frameworks derived from multi-carboxylic acid and imidazole-based spacer. Cryst. Growth Des. 2012, 12, 1603–1612. [Google Scholar] [CrossRef]

| Ligand/Analyte | HOMO | LUMO |
|---|---|---|
| 1 | −5.49 | –2.23 |
| 2 | –1.89 | –1.55 |
| 2-nitrotoluene (2-NT) | –7.28 | –2.32 |
| 4-nitrotoluene (4-NT) | –7.36 | –2.32 |
| Nitrobenzene (NB) | –7.60 | –2.43 |
| 2,6-dinitrotoluene (2,6-DNT) | –7.91 | –2.87 |
| 2,4-dinitrotoluene (2,4-DNT) | –8.11 | –2.98 |
| 1,3-dinitrobenzene (1,3-DNB) | –8.42 | –3.14 |
| 2,4,6-trinitrophenol (TNP) | –8.54 | –3.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wu, J.; Lu, L.; Ma, A.-Q.; Hu, W.-S.; Wu, W.-P.; Wu, Y.; Sun, Y.-C.; Singh, A.; Kumar, A. Two Chemically Stable Cd(II) Polymers as Fluorescent Sensor and Photocatalyst for Aromatic Dyes. Polymers 2018, 10, 274. https://doi.org/10.3390/polym10030274
Wang J, Wu J, Lu L, Ma A-Q, Hu W-S, Wu W-P, Wu Y, Sun Y-C, Singh A, Kumar A. Two Chemically Stable Cd(II) Polymers as Fluorescent Sensor and Photocatalyst for Aromatic Dyes. Polymers. 2018; 10(3):274. https://doi.org/10.3390/polym10030274
Chicago/Turabian StyleWang, Jun, Jian Wu, Lu Lu, Ai-Qing Ma, Wan-Shan Hu, Wei-Ping Wu, Yu Wu, Yan-Chun Sun, Amita Singh, and Abhinav Kumar. 2018. "Two Chemically Stable Cd(II) Polymers as Fluorescent Sensor and Photocatalyst for Aromatic Dyes" Polymers 10, no. 3: 274. https://doi.org/10.3390/polym10030274




