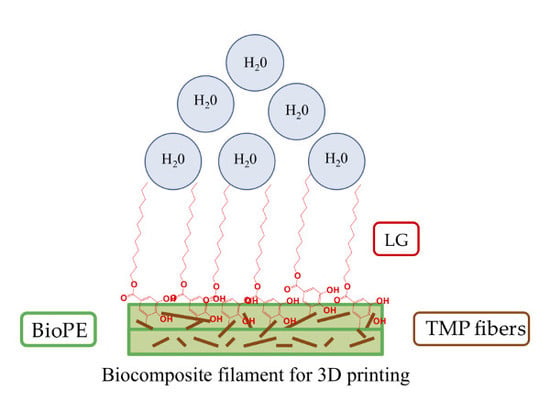
3D Printable Filaments Made of Biobased Polyethylene Biocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TMP Fibers Modification
2.3. Extrusion of Biocomposite Filaments
2.4. Filament Morphology
2.5. SEM Analysis
2.6. Water Uptake
2.7. 3D Printing
3. Results and Discussion
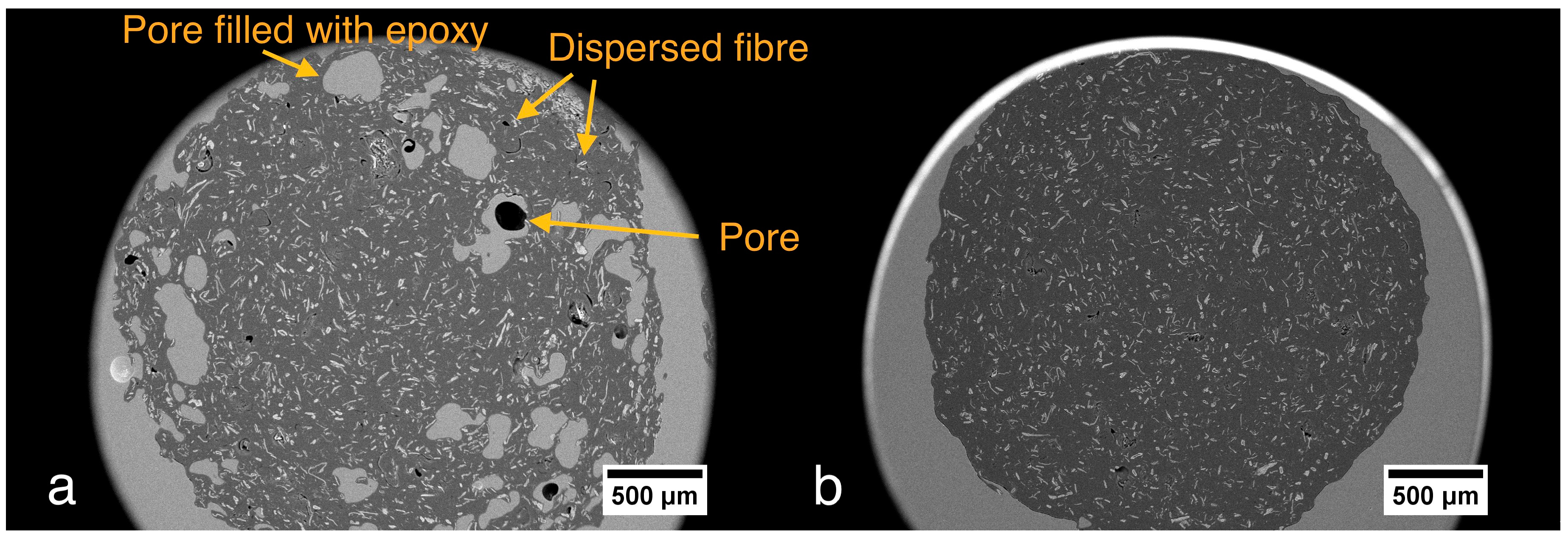
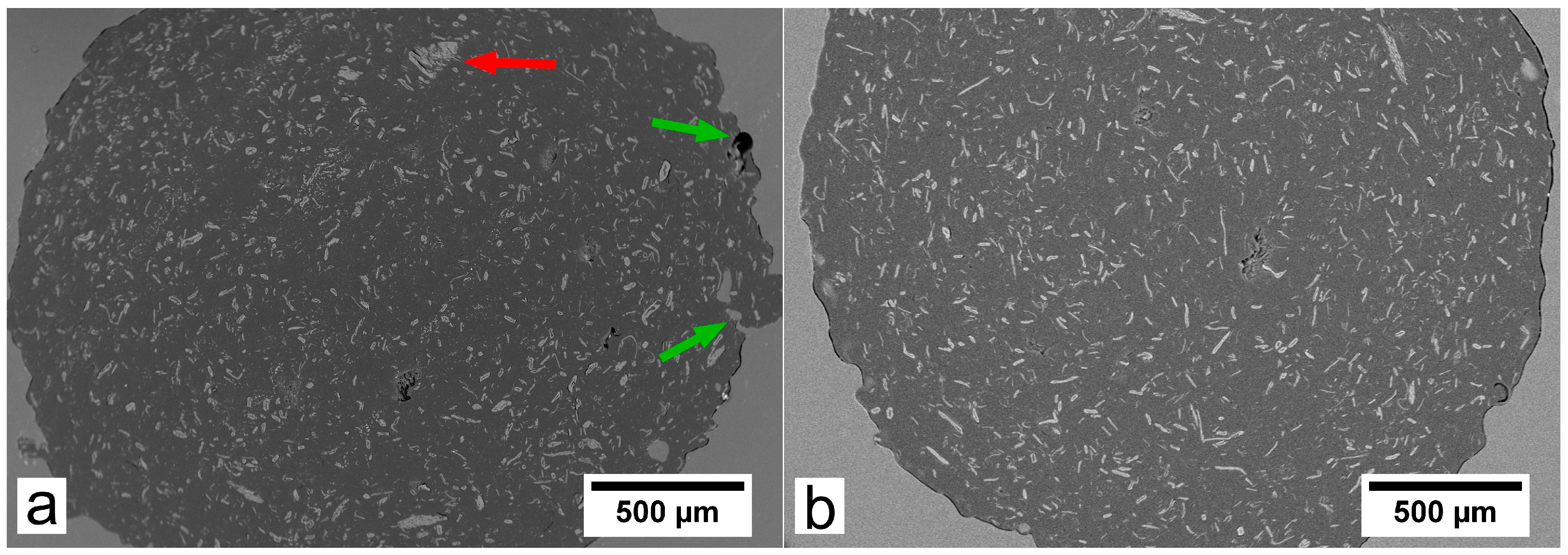
3.1. Filaments Morphology and Porosity
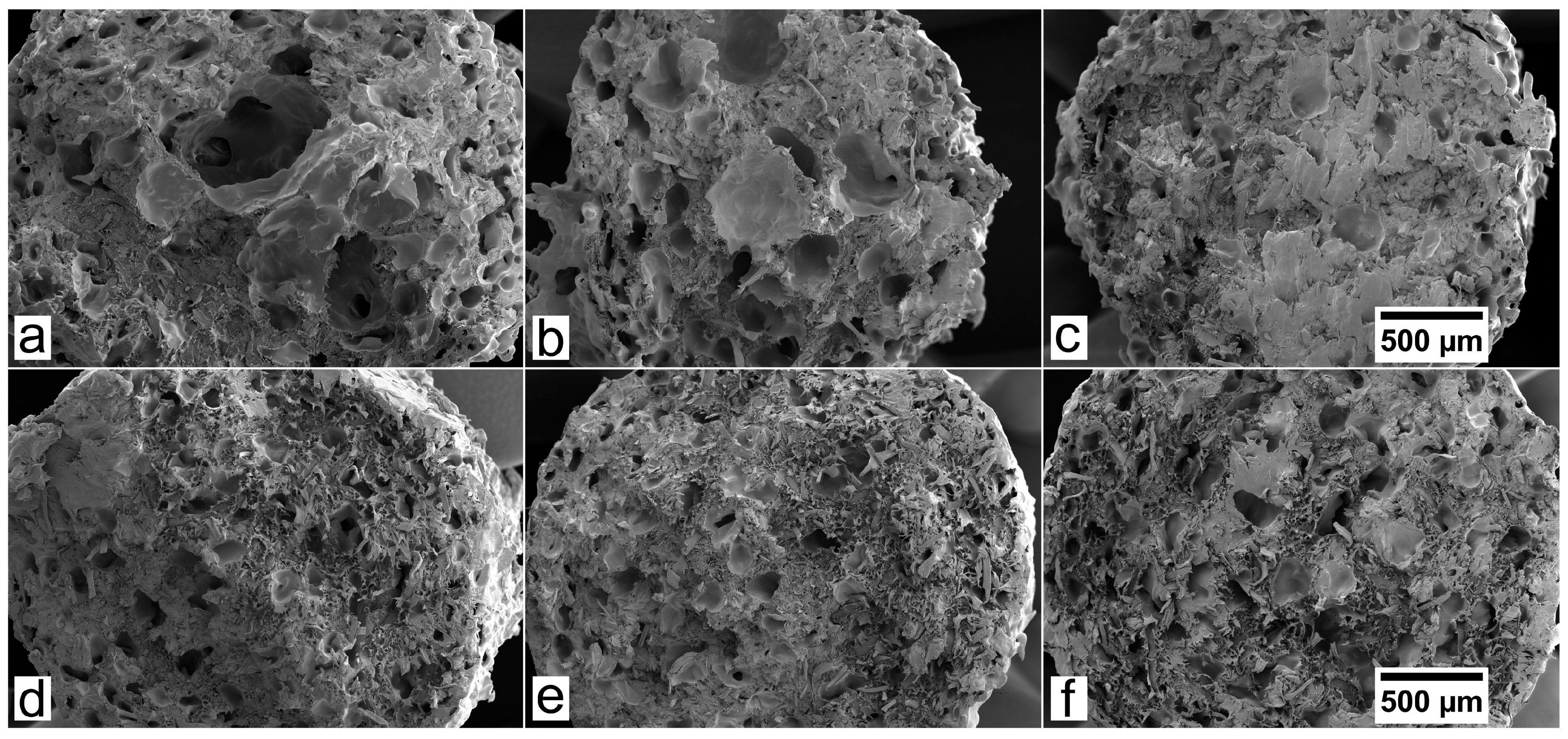
3.2. SEM Analysis
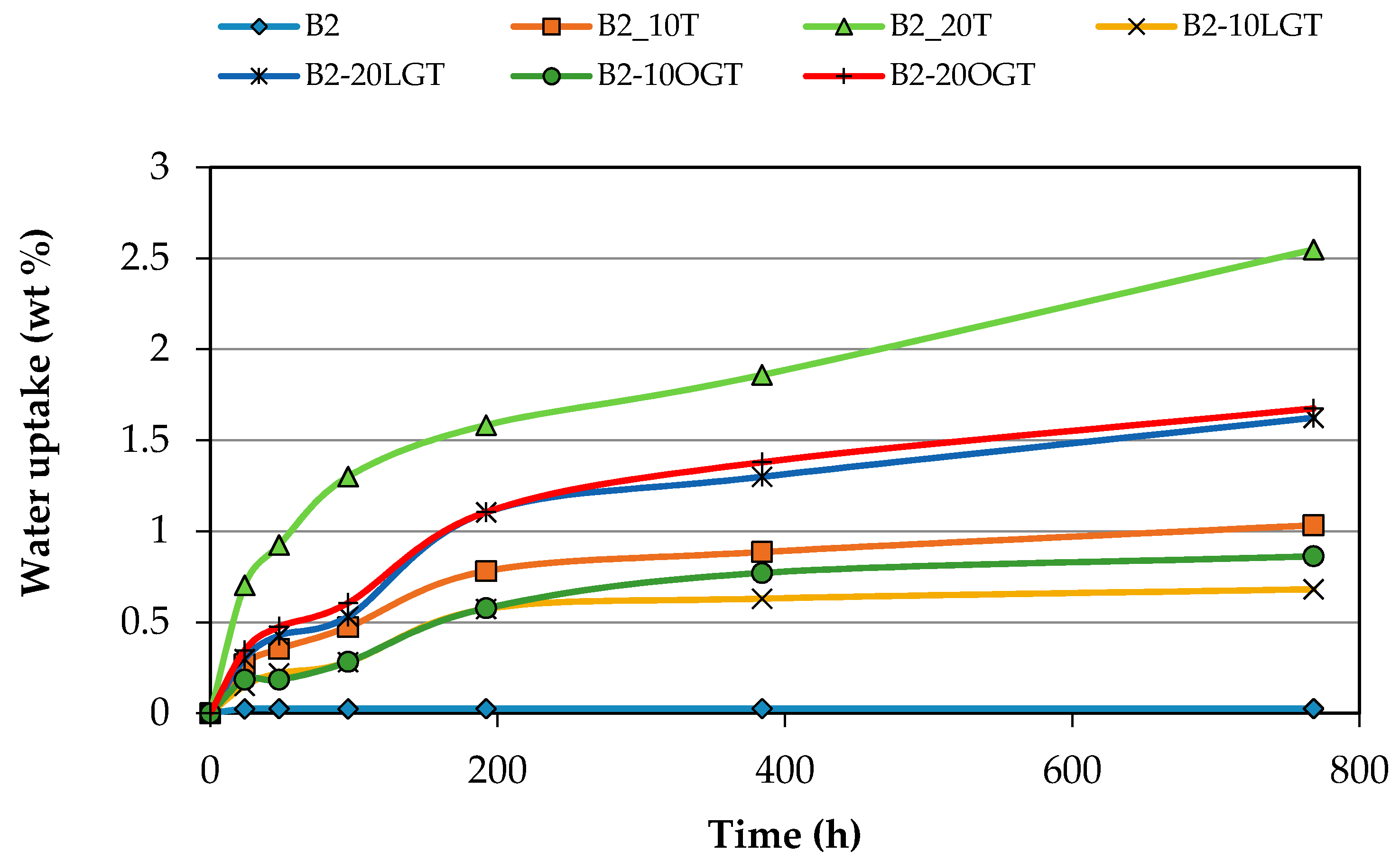
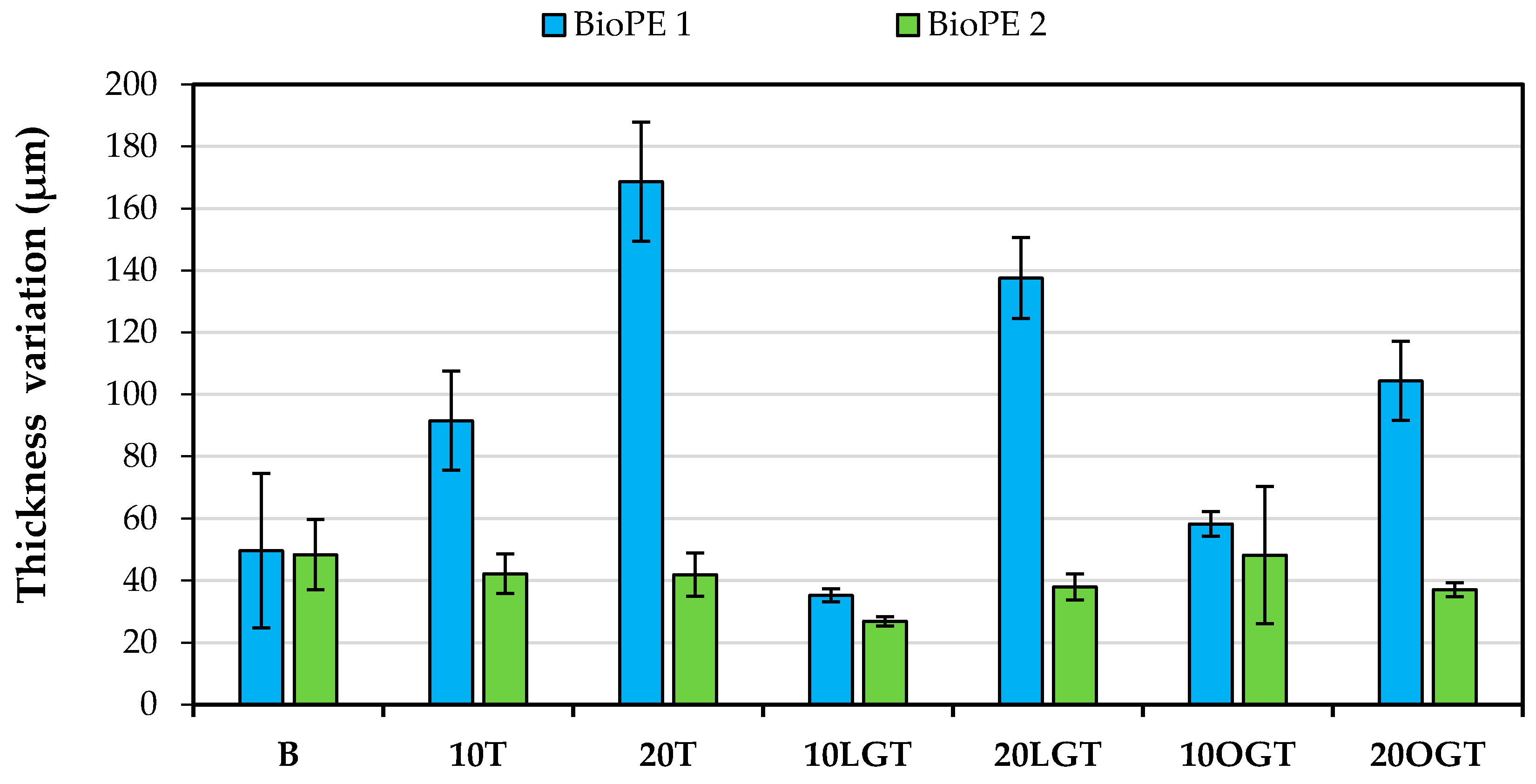
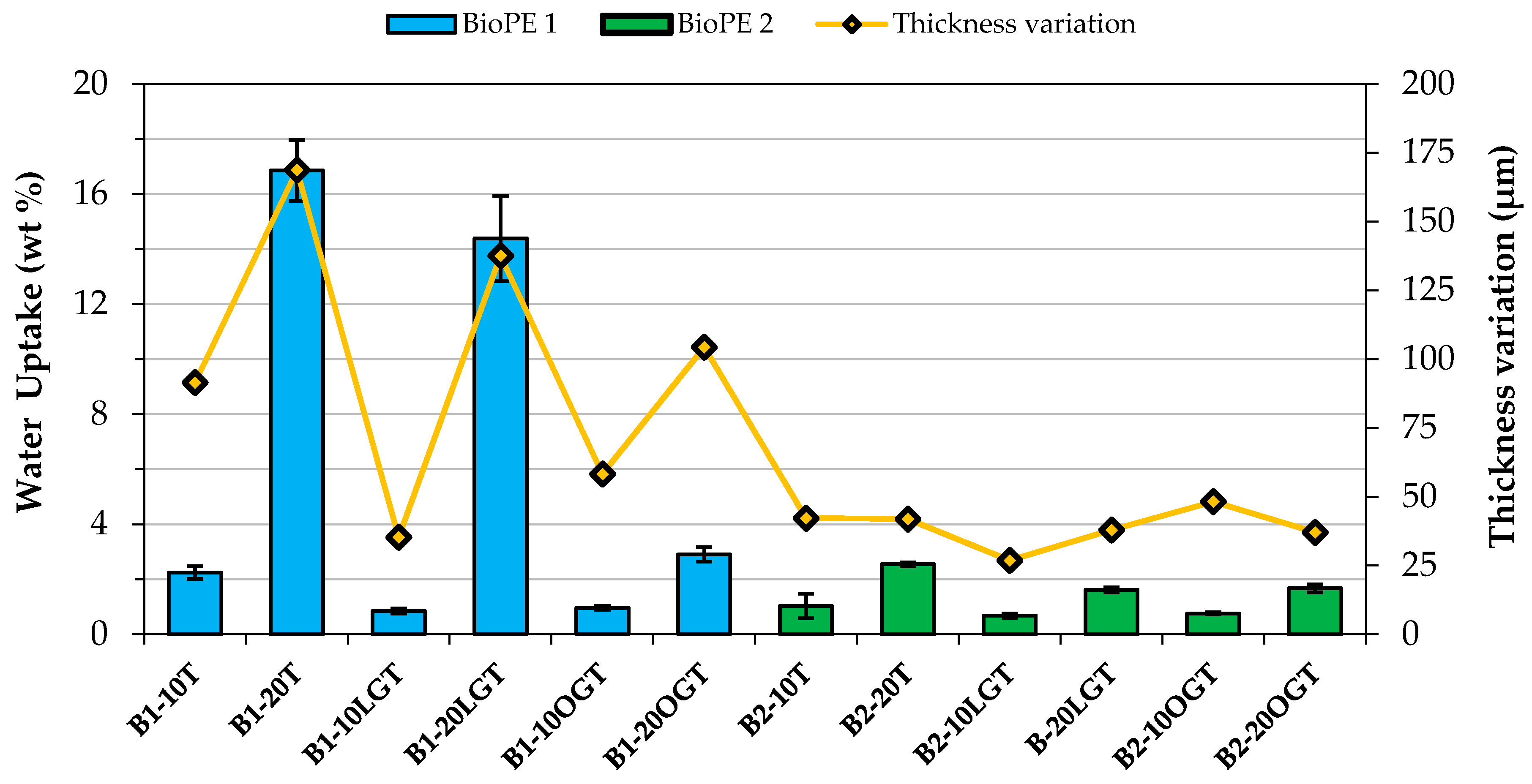
3.3. Water Uptake
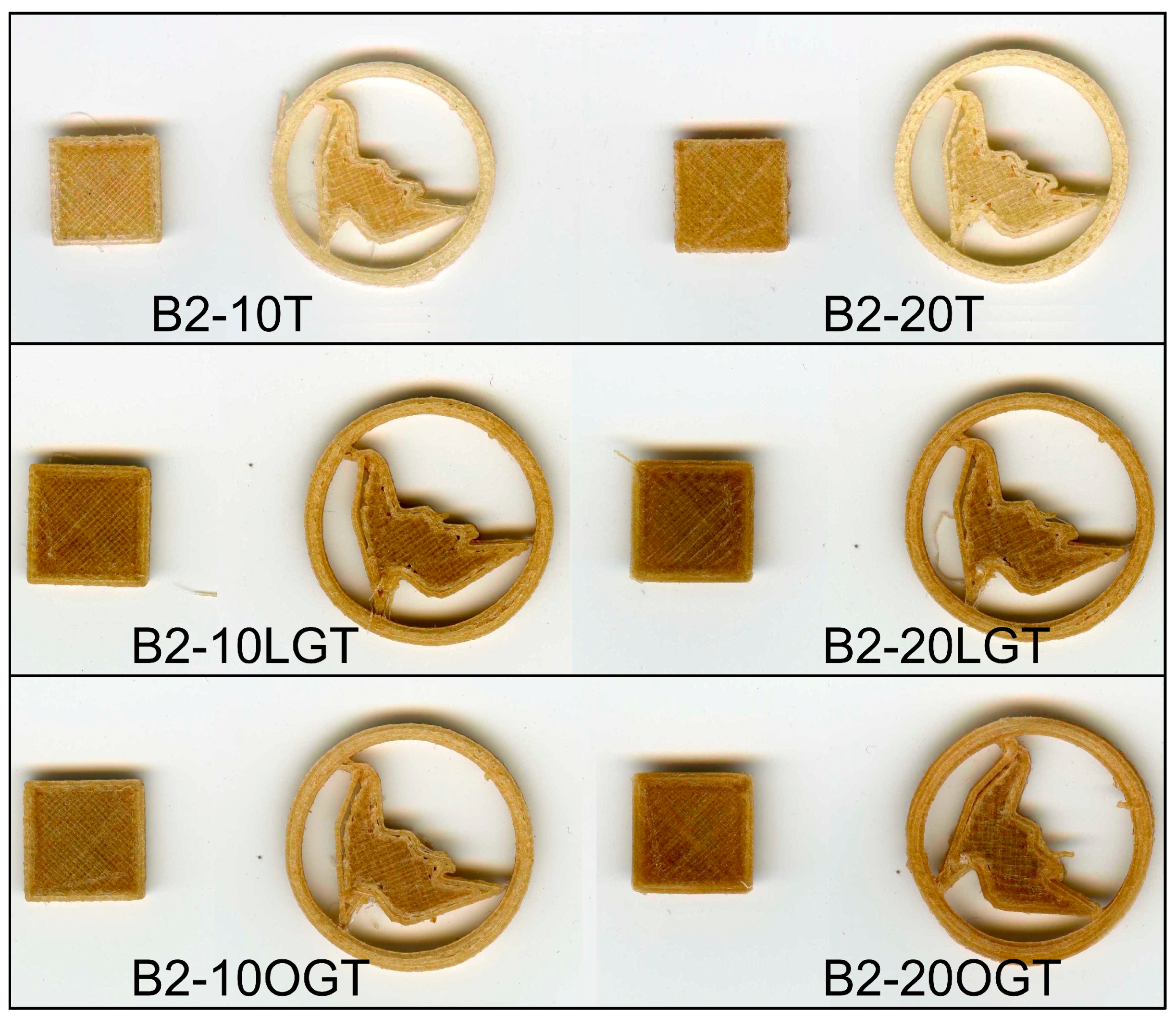
3.4. 3D Printing
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A

References
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable Bio-Composites from renewable resources: Opportunities and challenges in the green materials world. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Abhilash, M.; Thomas, D. Biopolymers for Biocomposites and Chemical Sensor Applications. In Biopolymer Composites in Electronics; Sadasivuni, K.K., Ponnamma, D., Cabibihan, J.J., Al-Maadeed, M.A., Kim, J., Eds.; Elsevier Inc.: Oxford, UK, 2017; pp. 405–435. ISBN 9780128092613. [Google Scholar]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part A 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Ho, M.; Wang, H.; Lee, J.-H.; Ho, C.-K.; Lau, K.-T.; Leng, J.; Hui, D. Critical factors on manufacturing processes of natural fibre composites. Compos. Part B 2012, 8, 3549–3562. [Google Scholar] [CrossRef]
- Saheb, D.N.; Jog, J.P. Natural Fiber Polymer Composites: A Review. Adv. Polym. Technol. 1999, 18, 351–363. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Forte, M.M.C.; Santana, R.M.C. Thermal decomposition of wood: Influence of wood components and cellulose crystallite size. Bioresour. Technol. 2012, 109, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kakroodi, A.R.; Kazemi, Y.; Cloutier, A.; Rodrigue, D. Mechanical performance of polyethylene (PE)-based biocomposites. In Biocomposites: Design and Mechanical Performance; Manjusri, M., Pandey, J.K., Mohanty, A.K., Eds.; Elsevier Inc.: Cambridge, UK, 2015; pp. 237–256. ISBN 9781782423942. [Google Scholar]
- Narayan, R. Carbon footprint of bioplastics using biocarbon content analysis and life-cycle assessment. MRS Bull. 2011, 36, 716–721. [Google Scholar] [CrossRef]
- Morschbacker, L.; Siqueira Campos, C.E.; Cassiano, L.C.; Roza, L.; Almada, F.; Werneck do Carmo, R. Bio-polyethylene. In Handbook of Green Materials: Processing Technologies, Properties and Applications (in 4 Volumes): Materials Science; Oksman, K., Mathew, A.P., Bismarck, A., Rojas, O., Sain, M., Eds.; World Scientific Publishing Co.: Singapore, 2014; Volume 4, pp. 89–104. ISBN 9789814566476. [Google Scholar]
- Shinoj, S.; Panigrahi, S.; Visvanathan, R. Water absorption pattern and dimensional stability of oil palm fiber-linear low density polyethylene composites. J. Appl. Polym. Sci. 2010, 117, 1064–1075. [Google Scholar] [CrossRef]
- Nygård, P.; Tanem, B.S.; Karlsen, T.; Brachet, P.; Leinsvang, B. Extrusion-based wood fibre-PP composites: Wood powder and pelletized wood fibres—A comparative study. Compos. Sci. Technol. 2008, 68, 3418–3424. [Google Scholar] [CrossRef]
- Migneault, S.; Koubaa, A.; Erchiqui, F.; Chaala, A.; Englund, K.; Krause, C.; Wolcott, M. Effect of fiber length on processing and properties of extruded wood-fiber/HDPE composites. J. Appl. Polym. Sci. 2008, 110, 1085–1092. [Google Scholar] [CrossRef]
- Agarwal, B.D.; Broutman, L.J. Short fiber composites. In Analysis and Performance of FIber Composites; John Wiley: New York, NY, USA, 1990; pp. 193–196. ISBN 0-471-511528. [Google Scholar]
- Mertens, O.; Gurr, J.; Krause, A. The utilization of thermomechanical pulp fibers in WPC: A review. J. Appl. Polym. Sci. 2017, 134, 45161. [Google Scholar] [CrossRef]
- Peltola, H.; Pääkkönen, E.; Jetsu, P.; Heinemann, S. Wood based PLA and PP composites: Effect of fibre type and matrix polymer on fibre morphology, dispersion and composite properties. Compos. Part A 2014, 61, 13–22. [Google Scholar] [CrossRef]
- Koljonen, K.; Österberg, M.; Johansson, L.S.; Stenius, P. Surface chemistry and morphology of different mechanical pulps determined by ESCA and AFM. Colloids Surf. A Physicochem. Eng. Asp. 2003, 228, 143–158. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M. Composites: Part A Green composites: A brief review. Compos. Part A 2011, 42, 579–588. [Google Scholar] [CrossRef]
- Grönqvist, S.; Rantanen, K.; Alén, R.; Mattinen, M.L.; Buchert, J.; Viikari, L. Laccase-catalysed functionalisation of TMP with tyramine. Holzforschung 2006, 60, 503–508. [Google Scholar] [CrossRef]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, Y.I. Laccase-mediator systems and their applications: A review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Rencoret, J.; Ibarra, D.; Molina, S.; Camarero, S.; Romero, J.; Del Río, J.C.; Martínez, Á.T. Removal of lipophilic extractives from paper pulp by laccase and lignin-derived phenols as natural mediators. Environ. Sci. Technol. 2007, 41, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, D.; Holmen, S.; Melbø, J.K.; Moldes, D.; Echtermeyer, A.; Chinga-Carrasco, G. Enzymatic-assisted modification of Thermomechanical Pulp Fibres To Improve the Interfacial Adhesion with Poly(lactic acid) for 3D printing. ACS Sustain. Chem. Eng. 2017, 5, 9338–9346. [Google Scholar] [CrossRef]
- Euring, M.; Rühl, M.; Ritter, N.; Kües, U.; Kharazipour, A. Laccase mediator systems for eco-friendly production of medium-density fiberboard (MDF) on a pilot scale: Physicochemical analysis of the reaction mechanism. Biotechnol. J. 2011, 6, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Ruedin, P.; Civardi, C.; Richter, M.; Hach, A.; Christen, H. Laccase-catalyzed surface modification of thermo-mechanical pulp (TMP) for the production of wood fiber insulation boards using industrial process water. PLoS ONE 2015, 10, e0128623. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; Del Río, J.C.; Rencoret, J.; Ibarra, D.; Martínez, Á.T. Main lipophilic extractives in different paper pulp types can be removed using the laccase-mediator system. Appl. Microbiol. Biotechnol. 2006, 72, 845–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarzkopf, M.J.; Burnard, M.D. Wood-Plastic Composites—Performance and Environmental Impacts. In Environmental Impacts of Traditional and Innovative Forest-Based Products; Kutnar, A., Muthu, S.S., Eds.; Springer: Singapore, 2016; pp. 19–43. ISBN 9789811006555. [Google Scholar]
- Chinga-Carrasco, G.; Ehman, N.V.; Pettersson, J.; Vallejos, M.; Brodin, M.; Felissia, F.E.; Hakansson, J.; Area, M.C. Pulping and pretreatment affect the characteristics of bagasse inks for 3D printing. ACS Sustain. Chem. Eng. 2018, 6, 4068–4075. [Google Scholar] [CrossRef]
- Chiulan, I.; Frone, A.; Brandabur, C.; Panaitescu, D. Recent Advances in 3D Printing of Aliphatic Polyesters. Bioengineering 2018, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Bose, S.; Das, S. 3D printing of biomaterials. MRS Bull. 2015, 40, 108–114. [Google Scholar] [CrossRef]
- Gbureck, U.; Vorndran, E.; Müller, F.A.; Barralet, J.E. Low temperature direct 3D printed bioceramics and biocomposites as drug release matrices. J. Controll. Release 2007, 122, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar] [CrossRef]
- Shafiee, A.; Atala, A. Printing Technologies for Medical Applications. Trends Mol. Med. 2016, 22, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Z.; Sparks, T.; Liou, F.; Newkirk, J.; States, U. Aerospace applications of laser additive manufacturing. In Laser Additive Manufacturing: Materials, Design, Technologies, and Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 351–371. ISBN 9780081004333. [Google Scholar]
- Guo, G.; Rizvi, G.M.; Park, C.B.; Lin, W.S. Critical Processing Temperature in the Manufacture of Fine-Celled Plastic/Wood-Fiber Composite Foams. J. Appl. Polym. Sci. 2004, 91, 621–629. [Google Scholar] [CrossRef]
- Reme, P.A.; Helle, T. Assessment of transverse dimensions of wood tracheids using SEM and image analysis. J. Pulp Pap. Sci. 2002, 28, 122–128. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science, 4th ed.; John Wiley and Sons Inc.: Chicester, UK, 2011; ISBN 9780471706069. [Google Scholar]
- High-Density Polyethylene (HDPE) 3D Printer Filament. Available online: https://filaments.ca/products/hdpe-filament-natural-1-75mm?variant=42590589320 (accessed on 12 March 2018).
- Tazi, M.; Erchiqui, F.; Godard, F.; Kaddami, H.; Ajji, A. Characterization of rheological and thermophysical properties of HDPE-wood composite. J. Appl. Polym. Sci. 2014, 131, 1–11. [Google Scholar] [CrossRef]
- Shofner, M.L.; Lozano, K.; Rodríguez-Macías, F.J.; Barrera, E.V. Nanofiber-Reinforced Polymers Prepared by Fused Deposition Modeling. J. Appl. Polym. Sci. 2003, 89, 3081–3090. [Google Scholar] [CrossRef]
- Chang, T.C.; Faison, E.I. Shrinkage Behavior and Optimization of Injection Molded Parts Studies by the Taguchi Method. Polym. Eng. Sci. 2001, 41, 703–710. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B. Additive Manufacturing Technologies Rapid Prototyping to Direct Digital Manufacturing; Springer: New York, NY, USA, 2010; ISBN 9781441911193. [Google Scholar]
- D Printing a Functional Boat with Post-Consumer Milk Jugs. Available online: https://makezine.com/2013/05/30/large-format-3d-printing/ (accessed on 12 March 2018).



| Code | BioPE | BioPE (Weight %) | TMP fiber (Weight %) | TMP fiber modification |
|---|---|---|---|---|
| B1 | BioPE1 | 100 | - | - |
| B1-10T | BioPE1 | 90 | 10 | - |
| B1-20T | BioPE1 | 80 | 20 | - |
| B1-10LGT | BioPE1 | 90 | 10 | LG |
| B1-20LGT | BioPE1 | 80 | 20 | LG |
| B1-10OGT | BioPE1 | 90 | 10 | OG |
| B1-20OGT | BioPE1 | 80 | 20 | OG |
| B2 | BioPE2 | 100 | - | - |
| B2-10T | BioPE2 | 90 | 10 | - |
| B2-20T | BioPE2 | 80 | 20 | - |
| B2-10LGT | BioPE2 | 90 | 10 | LG |
| B2-20LGT | BioPE2 | 80 | 20 | LG |
| B2-10OGT | BioPE2 | 90 | 10 | OG |
| B2-20OGT | BioPE2 | 80 | 20 | OG |
| Sample | Theoretical density (g/cm3) | Measured density, Equations (1) and (2) (g/cm3) | Void fraction (vol %) |
|---|---|---|---|
| B1 | 0.955 | 0.9538 | 0.1 |
| B1-10T | 0.8595 | 0.6761 | 27.1 |
| B1-20T | 0.764 | 0.5199 | 47.0 |
| B1-10LGT | 0.8595 | 0.7930 | 8.4 |
| B1-20LGT | 0.764 | 0.5922 | 29.0 |
| B1-10OGT | 0.8595 | 0.7746 | 11.0 |
| B1-20OGT | 0.764 | 0.6466 | 18.2 |
| B2 | 0.954 | 0.953 | 0.1 |
| B2-10T | 0.8586 | 0.697 | 23.2 |
| B2-20T | 0.763 | 0.6143 | 24.2 |
| B2-10LGT | 0.8586 | 0.7709 | 11.4 |
| B2-20LGT | 0.763 | 0.6741 | 13.2 |
| B2-10OGT | 0.8586 | 0.739 | 16.2 |
| B2-20OGT | 0.763 | 0.5994 | 27.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filgueira, D.; Holmen, S.; Melbø, J.K.; Moldes, D.; Echtermeyer, A.T.; Chinga-Carrasco, G. 3D Printable Filaments Made of Biobased Polyethylene Biocomposites. Polymers 2018, 10, 314. https://doi.org/10.3390/polym10030314
Filgueira D, Holmen S, Melbø JK, Moldes D, Echtermeyer AT, Chinga-Carrasco G. 3D Printable Filaments Made of Biobased Polyethylene Biocomposites. Polymers. 2018; 10(3):314. https://doi.org/10.3390/polym10030314
Chicago/Turabian StyleFilgueira, Daniel, Solveig Holmen, Johnny K. Melbø, Diego Moldes, Andreas T. Echtermeyer, and Gary Chinga-Carrasco. 2018. "3D Printable Filaments Made of Biobased Polyethylene Biocomposites" Polymers 10, no. 3: 314. https://doi.org/10.3390/polym10030314