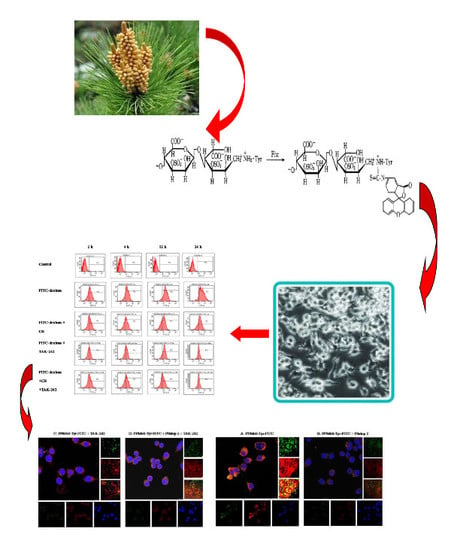
Fluorescent Labeling of Polysaccharides from Masson Pine Pollen and Its Effect on RAW264.7 Macrophages
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. Purification, Detection, and Fluorescent Labeling of PPM60
3.1.1. Preparation of Polysaccharides
3.1.2. Purification of PPM60-Tyr-FITC
3.1.3. Determination of Fluorescent Substitution of PPM60-Tyr-FITC
3.2. Effect of PPM60-Tyr-FITC on RAW264.7 Cell Viability
3.3. Influences of PPM60-Tyr-FITC on RAW264.7 Macrophages
3.3.1. Observation of the Fluorescence Changes by CLSM
Cell Climbing Experiment
Observation of Each Group with CLSM
Analysis by Flow Cytometer
4. Results
4.1. Fluorescent Labeling of Polysaccharides from Masson Pine Pollen
4.1.1. Tyramine Labeling of Polysaccharides
4.1.2. The Results of FITC Labeling
4.1.3. Determination of Substitution Degree and Molecular Weight of PPM60-Tyr-FITC
4.2. Effects of PPM60, PPM60-Tyr, and PPM60-Tyr-FITC on RAW264.7 Macrophages
4.3. Immunofluorescence Research on the Changes of Fluorescence after FITC-Dextran Entersthe RAW264.7 Macrophages
4.4. FCM Detection of the Effect of Pitstop 2 and TAK-242 on FITC-Dextran Entering the RAW264.7 Macrophagesin Various Time Periods
4.5. The Changes of Fluorescence after PPM60-Tyr-FITC Entersinto RAW264.7 Macrophages
5. Discussion
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Glabe, C.G.; Harty, P.K.; Rosen, S.D. Preparation and properties of fluorescent polysaccharides. Anal. Biochem. 1983, 130, 287–294. [Google Scholar] [CrossRef]
- Gordon, G.W.; Berry, G.; Liang, X.H.; Levine, B.; Herman, B. Quantitative fluorescence resonance energy transfer measurements using fluorescence microscopy. Biophys. J. 1998, 74, 2702–2713. [Google Scholar] [CrossRef]
- Lee, S.G.; Jung, J.Y.; Shin, J.S.; Shin, K.S.; Cho, C.W.; Rhee, Y.K.; Hong, H.D.; Lee, K.T. Immunostimulatory polysaccharide isolated from the leaves of Diospyros kaki Thumb modulate macrophage via TLR2. Int. J. Biol. Macromol. 2015, 79, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Shuai, X.X.; Li, W.J.; Liu, D.F.; Wang, Y.T.; Zhu, K.X.; Xie, M.Y. Effect of Polysaccharides from Ganoderma atrum on Mannose Receptor of Mouse Peritoneal Macrophages. Food Sci. 2014, 35, 262–267. [Google Scholar]
- Wang, C.L.; Lu, C.Y.; Pi, C.C.; Zhuang, Y.J.; Chu, C.L.; Liu, W.H.; Chen, C.J. Extracellular polysaccharides produced by Ganoderma formosanum stimulate macrophage activation via multiple pattern-recognition receptors. BMC Complement. Altern. Med. 2012, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Suzuki, H.; Wada, Y.; Kodama, T.; Doi, T. Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and NF-κB-dependent signaling pathways through macrophage scavenger receptors. Biochem. Biophys. Res. Commun. 2006, 343, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Sharshiner, R.; Brace, R.A.; Cheung, C.Y. Vesicular uptake of macromolecules by human placental amniotic epithelial cells. Placenta 2017, 57, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Scita, G.; Fiore, P.D. The endocytic matrix. Nature 2010, 463, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Parton, R.G.; Bassereau, P.; Mayor, S. Building endocytic pits without clathrin. Nat. Rev. Mol. Cell Biol. 2015, 16, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; Pust, S.; Skotland, T.; Bo, V.D. Clathrin-independent endocytosis: Mechanisms and function. Curr. Opin. Cell Biol. 2011, 23, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.P.; Gleeson, P.A. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol. Cell Biol. 2011, 89, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Neil, T.K.; Fearnley, D.B.; Mclellan, A.D.; Vuckovic, S.; Hart, D.N.J. Expression of multilectin receptors and comparative FITC-dextran uptake by human dendritic cells. Int. Immunol. 2000, 12, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Gekle, M.; Mildenberger, S.; Freudinger, R.; Silbemagl, S. Endosomal alkalinization reduces Jmax and Km of albumin receptor-mediated endocytosis in OK cells. Am. J. Physiol. 1995, 268, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Hu, J.; Li, Y.T.; Jin, Z.Y.; Yang, Y.; Yang, Y.M.; Song, H. Preparation of fluorescent polysaccharides from Ganoderma applanatum and cellular localization on splenic lymphocytes of mice. J. China Agric. Univ. 2013, 18, 147–152. [Google Scholar]
- Hua, K.F.; Hsu, H.Y.; Chao, L.K.; Chen, S.T.; Yang, W.B.; Hsu, J.; Wong, C.H. Ganoderma lucidum polysaccharides enhance CD14 endocytosis of LPS and promote TLR4 signal transduction of cytokine expression. J. Cell. Physiol. 2007, 212, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Yang, Y.; Liu, X.; Wang, N.; Cao, H.W.; Lu, Y.L.; Zhou, H.; Zheng, J. Inhibition of clathrin/dynamin-dependent internalization interferes with LPS-mediated TRAM-TRIF-dependent signaling pathway. Cell. Immunol. 2012, 274, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Xing, L.; Sun, M.; Su, F. Immunomodulatory effects of sulfated polysaccharides of pine pollen on mouse macrophages. Int. J. Biol. Macromol. 2016, 91, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, N.; Geng, Y. Influences of Sulfated Polysaccharide from Pine (Pinus massoniana) Pollen on the Immunomodulatory Effects of B Lymphocytes in Mice. Chin. J. Cell Biol. 2014, 36, 461–469. [Google Scholar]
- Yang, S.F.; Zhuang, T.F.; Si, Y.M.; Qi, K.Y.; Zhao, J. Coriolus versicolor mushroom polysaccharides exert immunoregulatory effects on mouse B cells via membrane Ig and TLR-4 to activate the MAPK and NF-κB signaling pathways. Mol. Immunol. 2015, 64, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Mi, M.; Jiang, F.; Sun, Y.; Li, Y.; Yang, L.; Fan, L.; Li, Q.; Meng, J.; Yue, Z.; et al. Apple Polysaccharide Reduces NF-κB Mediated Colitis-Associated Colon Carcinogenesis. Nutr. Cancer 2015, 67, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Nie, S.; Jiang, L.; Xie, M. A novel polysaccharide from the seeds of Plantago asiatica L. induces dendritic cells maturation through toll-like receptor 4. Int. Immunopharmacol. 2014, 18, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Li, F.C.; Geng, M.Y.; Li, Y.X.; Li, J.; Miao, B.C.; Guan, H.S. Studies on Fluorescent Labeling of Marine Sulfated Polysaccharide 911. Chem. J. Chin. Univ. 2002, 23, 1704–1708. [Google Scholar]
- Cuesta, G.; Suarez, N.; Bessio, M.I.; Ferreira, F.; Massaldi, H. Quantitative determination of pneumococcal capsular polysaccharide serotype 14 using a modification of phenol-sulfuric acid method. J. Microbiol. Methods 2003, 52, 69–73. [Google Scholar] [CrossRef]
- Kagan, J.C.; Su, T.; Horng, T.; Chow, A.; Akira, S.; Medzhitov, R. TRAM couples endocytosis of Toll-like receptor 4 to theinduction of interferon-beta. Nat. Immunol. 2008, 9, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Han, S.B.; Ahn, K.S.; Kim, H.M. Activation of NF-κB/Relin angelan-stimulated macrophages. Immunopharmacology 1999, 43, 1–9. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Husebye, H.; Halaas, O.; Stenmark, H.; Tunheim, G.; Sandanger, O.; Bogen, B.; Brech, A.; Latz, E.; Espevik, T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006, 25, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Ostuni, R.; Marek, L.R.; Barrest, S.; Barbalat, R.; Barton, G.M. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 2011, 14, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, T.; Han, C.; He, D.; Liu, H.; An, H.; Cai, Z.; Cao, X. Lysosome-associated small RabGTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood 2007, 110, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lou, J.; Ouyang, C.; Chen, W.L.; Liu, Y.Q.; Liu, X.Y.; Cao, X.T.; Wang, J.L.; Lu, L.R. Ras-related protein Rab10 facilitates TLR4 signaling by promoting replenishment of TLR4 onto the plasma membrane. Proc. Natl. Acad. Sci. USA 2010, 107, 13806–13811. [Google Scholar] [CrossRef] [PubMed]









| Group | Percent (%) |
|---|---|
| Control | 0.3 |
| FITC-dextran | 46.1 |
| FITC-dextran + Pitstop 2 | 37.5 |
| FITC-dextran + TAK-242 | 29.3 |
| Group | Percent (%) |
|---|---|
| Control | 0.4 |
| PPM60-Tyr-FITC | 46.2 |
| PPM60-Tyr-FITC + Pitstop 2 | 30.5 |
| PPM60-Tyr-FITC + TAK-242 | 31.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Su, F.; Yang, J.; Gao, Z.; Geng, Y. Fluorescent Labeling of Polysaccharides from Masson Pine Pollen and Its Effect on RAW264.7 Macrophages. Polymers 2018, 10, 372. https://doi.org/10.3390/polym10040372
Sun M, Su F, Yang J, Gao Z, Geng Y. Fluorescent Labeling of Polysaccharides from Masson Pine Pollen and Its Effect on RAW264.7 Macrophages. Polymers. 2018; 10(4):372. https://doi.org/10.3390/polym10040372
Chicago/Turabian StyleSun, Mengmeng, Fangchen Su, Jinxin Yang, Zheng Gao, and Yue Geng. 2018. "Fluorescent Labeling of Polysaccharides from Masson Pine Pollen and Its Effect on RAW264.7 Macrophages" Polymers 10, no. 4: 372. https://doi.org/10.3390/polym10040372