Low-Dissipation Thermosets Derived from Oligo(2,6-Dimethyl Phenylene Oxide)-Containing Benzoxazines
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
2.3. Synthesis of Nitro End-Capped Oligo (2,6-Dimethyl Phenylene Oxide) (NPPO)
2.4. Synthesis of Amine End-Capped Oligo (2,6-Dimethyl Phenylene Oxide) (APPO)
2.5. Two-Pot Synthesis of Telechelic Oligomer-Type Benzoxazine (P-APPO-2)
2.6. One-Pot Synthesis of Telechelic Oligomer-Type Benzoxazine (P-APPO-1)
2.7. Syntheis of Main-Chain Type Benzoxazine Polymer (BPA-APPO)
2.8. Sample Preparation and Curing Procedure
2.8.1. The Self-Curing of P-APPO-2, P-APPO-1, and BPA-APPO
2.8.2. The Co-Curing of HP7200 with P-APPO-2, P-APPO-1, and BPA-APPO
2.8.3. The Co-Curing of HP7200 with SA90
3. Results and Discussion
3.1. Synthesis and Characterization of APPO
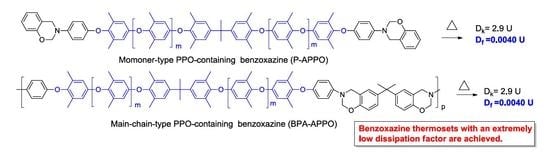
3.2. Synthesis and Characterization of Benzoxazine P-APPO
3.3. Synthesis and Characterization of Benzoxazine BPA-APPO
3.4. Solubility
3.5. DSC Thermograms
3.6. Thermal Properties
3.7. Dielectric Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ning, X.; Ishida, H. Phenolic materials via ring-opening polymerization: Synthesis and characterization of bisphenol-a based benzoxazines and their polymers. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 1121–1129. [Google Scholar] [CrossRef]
- Ohashi, S.; Cassidy, F.; Huang, S.; Chiou, K.; Ishida, H. Synthesis and ring-opening polymerization of 2-substituted 1,3-benzoxazine: The first observation of the polymerization of oxazine ring-substituted benzoxazines. Polym. Chem. 2016, 7, 7177–7184. [Google Scholar] [CrossRef]
- Liu, J.; Ishida, H. Anomalous isomeric effect on the properties of bisphenol f-based benzoxazines: Toward the molecular design for higher performance. Macromolecules 2014, 47, 5682–5690. [Google Scholar] [CrossRef]
- Yagci, Y.; Kiskan, B.; Ghosh, N.N. Recent advancement on polybenzoxazine—A newly developed high performance thermoset. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5565–5576. [Google Scholar] [CrossRef]
- Ghosh, N.N.; Kiskan, B.; Yagci, Y. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Endo, T. Toward elucidating the role of number of oxazine rings and intermediates in the benzoxazine backbone on their thermal characteristics. Macromolecules 2016, 49, 8466–8478. [Google Scholar]
- Baranek, A.D.; Kendrick, L.L.; Narayanan, J.; Tyson, G.E.; Wand, S.; Patton, D.L. Flexible aliphatic-bridged bisphenol-based polybenzoxazines. Polym. Chem. 2012, 3, 2892–2900. [Google Scholar] [CrossRef]
- Rodriguez Arza, C.; Froimowicz, P.; Ishida, H. Smart chemical design incorporating umbelliferone as natural renewable resource toward the preparation of thermally stable thermosets materials based on benzoxazine chemistry. RSC Adv. 2015, 5, 97855–97861. [Google Scholar] [CrossRef]
- Ye, Y.S.; Huang, Y.J.; Chang, F.C.; Xue, Z.G.; Xie, X.L. Synthesis and characterization of thermally cured polytriazole polymers incorporating main or side chain benzoxazine crosslinking moieties. Polym. Chem. 2014, 5, 2863–2871. [Google Scholar] [CrossRef]
- Lligadas, G.; Tuzun, A.; Ronda, J.C.; Galia, M.; Cadiz, V. Polybenzoxazines: New players in the bio-based polymer arena. Polym. Chem. 2014, 5, 6636–6644. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, R.; Yang, P.; Gu, Y. A study on the chain propagation of benzoxazine. Polym. Chem. 2016, 7, 860–866. [Google Scholar] [CrossRef]
- Kawaguchi, A.W.; Sudo, A.; Endo, T. Functional 1,3-benzoxazine bearing 4-pyridyl group: Synthesis and thermally induced polymerization behavior. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 410–416. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Benzoxazine-based thermosets with autonomous self-healing ability. Macromolecules 2015, 48, 1329–1334. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, P.; Bai, Y.; Zhou, T.; Zhu, R.; Gu, Y. Polybenzoxazines: Thermal responsiveness of hydrogen bonds and application as latent curing agents for thermosetting resins. ACS Omega 2017, 2, 1529–1534. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Ring-opening polymerization of 1,3-benzoxazines via borane catalyst. Polymers 2018, 10, 239. [Google Scholar] [CrossRef]
- Soto, M.; Hiller, M.; Oschkinat, H.; Koschek, K. Multifunctional benzoxazines feature low polymerization temperature and diverse polymer structures. Polymers 2016, 8, 278. [Google Scholar] [CrossRef]
- Hamerton, I.; McNamara, L.T.; Howlin, B.J.; Smith, P.A.; Cross, P.; Ward, S. Examining the initiation of the polymerization mechanism and network development in aromatic polybenzoxazines. Macromolecules 2013, 46, 5117–5132. [Google Scholar] [CrossRef] [PubMed]
- Wan Hassan, W.A.; Liu, J.; Howlin, B.J.; Ishida, H.; Hamerton, I. Examining the influence of bisphenol a on the polymerisation and network properties of an aromatic benzoxazine. Polymer 2016, 88, 52–62. [Google Scholar] [CrossRef]
- Kim, S.-K.; Choi, S.-W.; Jeon, W.S.; Park, J.O.; Ko, T.; Chang, H.; Lee, J.-C. Cross-linked benzoxazine–benzimidazole copolymer electrolyte membranes for fuel cells at elevated temperature. Macromolecules 2012, 45, 1438–1446. [Google Scholar] [CrossRef]
- Ishida, H.; Allen, D.J. Physical and mechanical characterization of near-zero shrinkage polybenzoxazines. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1019–1030. [Google Scholar] [CrossRef]
- Wang, C.F.; Su, Y.C.; Kuo, S.W.; Huang, C.F.; Sheen, Y.C.; Chang, F.C. Low-surface-free-energy materials based on polybenzoxazines. Angew. Chem. Int. Ed. 2006, 45, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-C.; Chang, F.-C. Synthesis and characterization of fluorinated polybenzoxazine material with low dielectric constant. Polymer 2003, 44, 7989–7996. [Google Scholar] [CrossRef]
- Tseng, M.-C.; Liu, Y.-L. Preparation, morphology, and ultra-low dielectric constants of benzoxazine-based polymers/polyhedral oligomeric silsesquioxane (poss) nanocomposites. Polymer 2010, 51, 5567–5575. [Google Scholar] [CrossRef]
- Zhang, K.; Zhuang, Q.; Liu, X.; Cai, R.; Yang, G.; Han, Z. Synthesis and copolymerization of benzoxazines with low-dielectric constants and high thermal stability. RSC Adv. 2013, 3, 5261–5270. [Google Scholar] [CrossRef]
- Zhang, K.; Zhuang, Q.; Zhou, Y.; Liu, X.; Yang, G.; Han, Z. Preparation and properties of novel low dielectric constant benzoxazole-based polybenzoxazine. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 5115–5123. [Google Scholar] [CrossRef]
- Vengatesan, M.R.; Devaraju, S.; Dinakaran, K.; Alagar, M. Sba-15 filled polybenzoxazine nanocomposites for low-k dielectric applications. J. Mater. Chem. 2012, 22, 7559–7566. [Google Scholar] [CrossRef]
- Velez-Herrera, P.; Doyama, K.; Abe, H.; Ishida, H. Synthesis and characterization of highly fluorinated polymer with the benzoxazine moiety in the main chain. Macromolecules 2008, 41, 9704–9714. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, S.L.; Lee, H.H.; Chang, H.C.; Hwang, K.Y.; Tu, A.P.; Su, W.C. Fluorinated benzoxazines and the structure-property relationship of resulting polybenzoxazines. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4970–4983. [Google Scholar] [CrossRef]
- Hay, A.S. Poly(phenylene oxides)s and poly(arylene ether)s derived from 2,6-diarylphenols. Prog. Polym. Sci. 1999, 24, 45–80. [Google Scholar] [CrossRef]
- Hay, A.S.; Blanchard, H.S.; Endres, G.F.; Eustance, J.W. Polymerization by oxidative coupling. J. Am. Chem. Soc. 1959, 81, 6335–6336. [Google Scholar] [CrossRef]
- Nunoshige, J.; Akahoshi, H.; Shibasaki, Y.; Ueda, M. Efficient oxidative coupling polymerization for synthesis of thermosetting poly(phenylene ether) copolymer with a low dielectric loss. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 5278–5282. [Google Scholar] [CrossRef]
- Fukuhara, T.; Shibasaki, Y.; Ando, S.; Ueda, M. Synthesis of thermosetting poly(phenylene ether) containing allyl groups. Polymer 2004, 45, 843–847. [Google Scholar] [CrossRef]
- White, D.M. Polymerization by oxidative coupling. Ii. Co-redistribution of poly(2,6-diphenyl-1,4-phenylene ether) with phenols. J. Polym. Sci. Part A-1 Polym. Chem. 1971, 9, 663–675. [Google Scholar] [CrossRef]
- White, D.M. Reactions of poly(phenylene oxide)s with quinones. I. The quinone-coupling reaction between low-molecular-weight poly(2,6-dimethyl-1,4-phenylene oxide) and 3,3′,5,5′-tetramethyl-4,4′-diphenoquinone. J. Polym. Sci. Polym. Chem. Ed. 1981, 19, 1367–1383. [Google Scholar] [CrossRef]
- White, D.M. Synthesis of 4-hydroxyarylene ethers by the equilibration of phenols with poly(2,6-dimethyl-1,4-phenylene ether). J. Org. Chem. 1969, 34, 297–303. [Google Scholar] [CrossRef]
- Jayakannan, M.; Smitha, T.R. Synthesis and characterization of new telechelic poly(phenyleneoxide)s. Eur. Polym. J. 2004, 40, 1169–1175. [Google Scholar] [CrossRef]
- Tingerthal, J.M.; Nava, H.; Percec, V. Synthesis and characterization of aba triblock copolymers containing poly(2,6-dimethyl-1,4-phenylene oxide) as a blocks and tetramethyl bisphenol-a polysulfone as b blocks. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 2043–2062. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, S.L.; Hsieh, C.W.; Lee, H.H. Aromatic diamine-based benzoxazines and their high performance thermosets. Polymer 2008, 49, 1220–1229. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, S.L.; Shen, T.Y.; Shih, Y.S.; Lin, H.T.; Wang, C.F. Flexible polybenzoxazine thermosets with high glass transition temperatures and low surface free energies. Polym. Chem. 2012, 3, 935–945. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, Q.; Liu, X.; Zhu, R.; Ran, Q.; Gu, Y. Phase separation in benzoxazine/epoxy resin blending systems. Polym. J. 2012, 45, 637. [Google Scholar] [CrossRef]
- Wang, M.W.; Jeng, R.J.; Lin, C.H. The robustness of a thermoset of a main-chain type polybenzoxazine precursor prepared through a strategy of a-a and b-b polycondensation. RSC Adv. 2016, 6, 18678–18684. [Google Scholar] [CrossRef]
- Tao, Z.; Yang, S.; Ge, Z.; Chen, J.; Fan, L. Synthesis and properties of novel fluorinated epoxy resins based on 1,1-bis(4-glycidylesterphenyl)-1-(3′-trifluoromethylphenyl)-2,2,2-trifluoroethane. Eur. Polym. J. 2007, 43, 550–560. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Liu, H.-J. Review of polymer materials with low dielectric constant. Polym. Int. 2010, 59, 597–606. [Google Scholar] [CrossRef]
- Lin, C.H.; Jiang, Z.R.; Wang, C.S. Low dielectric thermoset. Ii. Synthesis and properties of novel 2,6-dimethyl phenol–dipentene epoxy. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 4084–4097. [Google Scholar] [CrossRef]
- Lin, C.H.; Hsiao, C.N.; Li, C.H.; Wang, C.S. Low dielectric thermoset. Iv. Synthesis and properties of a dipentene-containing cyanate ester and its copolymerization with bisphenol a dicyanate ester. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3986–3995. [Google Scholar] [CrossRef]
- Cai, S.X.; Lin, C.H. Flame-retardant epoxy resins with high glass-transition temperatures from a novel trifunctional curing agent: Dopotriol. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 2862–2873. [Google Scholar] [CrossRef]
- Ishii, K.; Norisue, Y.; Ohno, D.; Miyamoto, M. Vinyl Compound and Cured Product Thereof. U.S. Patent US6,995,195 B992, 25 July 2006. [Google Scholar]












| Sample a | NMP | DMSO | DMAc | DMF | MEK | THF | M-Cresol | Xylene | Toluene | CH2Cl2 | CHCl3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SA90 | ++ b | +− | ++ | ++ | ++ | ++ | +h | ++ | ++ | ++ | ++ |
| P-APPO-2 | ++ | − | ++ | ++ | ++ | ++ | +h | ++ | ++ | ++ | ++ |
| P-APPO-1 | ++ | − | ++ | ++ | ++ | ++ | +h | ++ | ++ | ++ | ++ |
| BPA-APPO | ++ | − | ++ | ++ | ++ | ++ | +h | ++ | ++ | ++ | ++ |
| Sample ID | Tg (°C) (DMA) a | Tg (°C) (TMA) b | CTE (ppm/°C) c | Td5% (°C) d | CR (%) e |
|---|---|---|---|---|---|
| C-P-APPO-2 | 222 | 194 | 60 | 443 | 28 |
| C-P-APPO-1 | 218 | 185 | 56 | 442 | 28 |
| C-BPA-APPO | 225 | 197 | 60 | 437 | 28 |
| E-P-APPO-2 | 230 | 195 | 71 | 435 | 22 |
| E-P-APPO-1 | 227 | 185 | 75 | 430 | 22 |
| E-BPA-APPO | 232 | 203 | 66 | 433 | 21 |
| E-SA90 | 207 | 174 | 70 | 416 | 22 |
| Sample | Dk (1 G Hz) | Df (1 G Hz) |
|---|---|---|
| C-P-APPO-2 | 2.9 ± 0.01 | 0.0040 ± 0.00002 |
| C-P-APPO-1 | 2.9 ± 0.02 | 0.0043 ± 0.00006 |
| C-BPA-APPO | 2.9 ± 0.05 | 0.0040 ± 0.00005 |
| E-P-APPO-2 | 2.9 ± 0.05 | 0.0051 ± 0.00003 |
| E-P-APPO-1 | 2.9 ± 0.02 | 0.0053 ± 0.00006 |
| E-BPA-APPO | 2.9 ± 0.03 | 0.0050 ± 0.00006 |
| E-SA90 | 3.0 ± 0.02 | 0.0060 ± 0.00001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Lee, K.-W.; Lin, C.-H.; Juang, T.-Y. Low-Dissipation Thermosets Derived from Oligo(2,6-Dimethyl Phenylene Oxide)-Containing Benzoxazines. Polymers 2018, 10, 411. https://doi.org/10.3390/polym10040411
Chen C-H, Lee K-W, Lin C-H, Juang T-Y. Low-Dissipation Thermosets Derived from Oligo(2,6-Dimethyl Phenylene Oxide)-Containing Benzoxazines. Polymers. 2018; 10(4):411. https://doi.org/10.3390/polym10040411
Chicago/Turabian StyleChen, Chien-Han, Kuan-Wei Lee, Ching-Hsuan Lin, and Tzong-Yuan Juang. 2018. "Low-Dissipation Thermosets Derived from Oligo(2,6-Dimethyl Phenylene Oxide)-Containing Benzoxazines" Polymers 10, no. 4: 411. https://doi.org/10.3390/polym10040411
APA StyleChen, C.-H., Lee, K.-W., Lin, C.-H., & Juang, T.-Y. (2018). Low-Dissipation Thermosets Derived from Oligo(2,6-Dimethyl Phenylene Oxide)-Containing Benzoxazines. Polymers, 10(4), 411. https://doi.org/10.3390/polym10040411