Modifying an Active Compound’s Release Kinetic Using a Supercritical Impregnation Process to Incorporate an Active Agent into PLA Electrospun Mats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
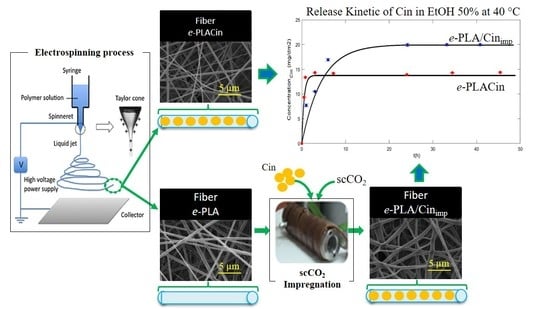
2.2. Electrospinning of e-PLA and e-PLACIN Nanofibers
2.3. Supercritical Fluid Impregnation of CIN in e-PLA Mats
2.4. Characterization of Active Electrospun PLA Mats
2.4.1. Quantification of CIN in e-PLA Mats
2.4.2. Scanning Electronic Microscopy (SEM) Analysis
2.4.3. Fourier Transform Infrared (FTIR)–Attenuated Total Reflectance (ATR) Spectroscopy
2.4.4. Thermal Properties
2.5. Study of the Release Kinetic of CIN from Active e-PLA Electrospun Mats
2.5.1. Experimental Procedure for CIN Release Rate Quantification in e-PLA Mats
2.6. Statistical Analysis
3. Results and Discussion
3.1. Incorporation of CIN in e-PLA Mats by the scCO2 Impregnation Process
3.2. Morphological Results of e-PLA Mats
3.3. FTIR Analysis Results
3.4. Thermal Characterization of e-PLA Mats
3.5. Study of the Release Kinetic of CIN from e-PLA-CIN and e-PLA/CINimp Mats
Determination of Partition and Diffusion Coefficients of CIN in e-PLA Mats
Supplementary Files
Supplementary File 1Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kalluri, S.; Seng, K.H.; Guo, Z.; Liu, H.K.; Dou, S.X. Electrospun lithium metal oxide cathode materials for lithium-ion batteries. RSC Adv. 2013, 3, 25576. [Google Scholar] [CrossRef]
- Srinivasu, P.; Islam, A.; Singh, S.P.; Han, L.; Kantam, M.L.; Bhargava, S.K. Highly efficient nanoporous graphitic carbon with tunable textural properties for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 20866. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, J.; Zhang, L.; Qiu, Y. Recent advances in energy materials by electrospinning. Renew. Sustain. Energy Rev. 2018, 81, 1825–1858. [Google Scholar] [CrossRef]
- Gangwal, S.; Wright, M. Nanofibres: New scalable technology platform for producing polymeric nanofibres. Filtr. Sep. 2013, 50, 30–33. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, F.; Tarascon, J.-M.; Kim, J.-K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531. [Google Scholar] [CrossRef]
- Ingrao, C.; Tricase, C.; Cholewa-Wójcik, A.; Kawecka, A.; Rana, R.; Siracusa, V. Polylactic acid trays for fresh-food packaging: A Carbon Footprint assessment. Sci. Total Environ. 2015, 537, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly(lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Alazab, M.; Mitchell, G.R.; Davis, F.J.; Mohan, S.D. Sustainable Electrospinning of Nanoscale Fibres. Procedia Manuf. 2017, 12, 66–78. [Google Scholar] [CrossRef]
- Li, Y.; Lim, C.T.; Kotaki, M. Study on structural and mechanical properties of porous PLA nanofibers electrospun by channel-based electrospinning system. Polymer 2015, 56, 572–580. [Google Scholar] [CrossRef]
- Casasola, R.; Thomas, N.L.; Trybala, A.; Georgiadou, S. Electrospun poly lactic acid (PLA) fibres: Effect of different solvent systems on fibre morphology and diameter. Polymer 2014, 55, 4728–4737. [Google Scholar] [CrossRef] [Green Version]
- Pisani, S.; Dorati, R.; Conti, B.; Modena, T.; Bruni, G.; Genta, I. Design of copolymer PLA-PCL electrospun matrix for biomedical applications. React. Funct. Polym. 2018, 124, 77–89. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Wang, Y.; Yu, H.; Chen, X.; Jing, X. Biodegradable electrospun poly(l-lactide) fibers containing antibacterial silver nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar] [CrossRef]
- Vega-Lugo, A.-C.; Lim, L.-T. Controlled release of allyl isothiocyanate using soy protein and poly(lactic acid) electrospun fibers. Food Res. Int. 2009, 42, 933–940. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Sanguansri, P.; Augustin, M.A. Nanoscale materials development—A food industry perspective. Trends Food Sci. Technol. 2006, 17, 547–556. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, Z.; Xu, H.; Li, F.; Zhang, G. Interaction between Amylose and β-Cyclodextrin Investigated by Complexing with Conjugated Linoleic Acid. J. Agric. Food Chem. 2010, 58, 5620–5624. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Men, K.; Shi, H.; Xiang, M.; Zhang, J.; Song, J.; Long, J.; Wan, Y.; Luo, F.; Zhao, X.; et al. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale 2011, 3, 1558. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Zong, M.-H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A novel nano-encapsulation approach for bioactive compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Jacobsen, C.; García-Moreno, P.J.; Mendes, A.C.; Mateiu, R.V.; Chronakis, I.S. Use of Electrohydrodynamic Processing for Encapsulation of Sensitive Bioactive Compounds and Applications in Food. Annu. Rev. Food Sci. Technol. 2018, 9, 525–549. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of processing conditions on the physical, chemical and transport properties of polylactic acid films containing thymol incorporated by supercritical impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Lauw, S.J.L.; Zhong, C.; Webster, R.D. Studies on the electrochemical reduction and coupled homogeneous reactions of cinnamaldehyde in acetonitrile. J. Electroanal. Chem. 2016, 779, 220–228. [Google Scholar] [CrossRef]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical impregnation of cinnamaldehyde into polylactic acid as a route to develop antibacterial food packaging materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Moreira, R.G.; Castell-Perez, E. Poly(dl-lactide-co-glycolide) (PLGA) Nanoparticles with Entrapped trans-Cinnamaldehyde and Eugenol for Antimicrobial Delivery Applications. J. Food Sci. 2011, 76, N16–N24. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Scaffaro, R.; D’Arrigo, M.; Botta, L.; Filocamo, A.; Marino, A.; Bisignano, G. Study on carvacrol and cinnamaldehyde polymeric films: Mechanical properties, release kinetics and antibacterial and antibiofilm activities. Appl. Microbiol. Biotechnol. 2012, 96, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.; Tagliazucchi, D. Impact of in-vitro gastro-pancreatic digestion on polyphenols and cinnamaldehyde bioaccessibility and antioxidant activity in stirred cinnamon-fortified yogurt. LWT 2018, 89, 164–170. [Google Scholar] [CrossRef]
- Rieger, K.A.; Schiffman, J.D. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibers. Carbohydr. Polym. 2014, 113, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Cui, W.; Zhou, S.; Tan, R.; Wang, C. Structural stability and release profiles of proteins from core-shell poly(dl-lactide) ultrafine fibers prepared by emulsion electrospinning. J. Biomed. Mater. Res. Part A 2008, 86A, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Yang, L.; Liang, Q.; Zhang, X.; Guan, H.; Xu, X.; Chen, X.; Jing, X. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J. Control. Release 2005, 105, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Torres, A.; Martínez, F.; Salazar, L.; Villegas, C.; Galotto, M.J.; Guarda, A.; Romero, J. Assessment of kinetic release of thymol from LDPE nanocomposites obtained by supercritical impregnation: Effect of depressurization rate and nanoclay content. Eur. Polym. J. 2017, 93, 294–306. [Google Scholar] [CrossRef]
- Kikic, I.; Vecchione, F. Supercritical impregnation of polymer. Curr. Opin. Solid State Mater. Sci. 2003, 7, 399–405. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.-M.; Tassaing, T.; Jérôme, C. Drug loading of polymer implants by supercritical CO2 assisted impregnation: A review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Development of flexible materials based on plasticized electrospun PLA–PHB blends: Structural, thermal, mechanical and disintegration properties. Eur. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Stoffers, N.H.; Störmer, A.; Bradley, E.L.; Brandsch, R.; Cooper, I.; Linssen, J.P.H.; Franz, R. Feasibility study for the development of certified reference materials for specific migration testing. Part 1: Initial migrant concentration and specific migration. Food Addit. Contam. 2004, 21, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garcia, M.D.; Lagaron, J.M. On the use of plant cellulose nanowhiskers to enhance the barrier properties of polylactic acid. Cellulose 2010, 17, 987–1004. [Google Scholar] [CrossRef]
- De Dicastillo, C.L.; Rodríguez, F.; Guarda, A.; Galotto, M.J. Antioxidant films based on cross-linked methyl cellulose and native Chilean berry for food packaging applications. Carbohydr. Polym. 2016, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Ikushima, Y.; Chatterjee, M.; Sato, O.; Arai, M. Hydrogenation of an α,β-unsaturated aldehyde catalyzed with ruthenium complexes with different fluorinated phosphine compounds in supercritical carbon dioxide and conventional organic solvents. J. Supercrit. Fluids 2003, 27, 65–72. [Google Scholar] [CrossRef]
- De Souza, A.C.; Dias, A.M.A.; Sousa, H.C.; Tadini, C.C. Impregnation of cinnamaldehyde into cassava starch biocomposite films using supercritical fluid technology for the development of food active packaging. Carbohydr. Polym. 2014, 102, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Ayodeji, O.; Graham, E.; Kniss, D.; Lannutti, J.; Tomasko, D. Carbon dioxide impregnation of electrospun polycaprolactone fibers. J. Supercrit. Fluids 2007, 41, 173–178. [Google Scholar] [CrossRef]
- Molinaro, S.; Cruz Romero, M.; Boaro, M.; Sensidoni, A.; Lagazio, C.; Morris, M.; Kerry, J. Effect of nanoclay-type and PLA optical purity on the characteristics of PLA-based nanocomposite films. J. Food Eng. 2013, 117, 113–123. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Torkamani, A.E.; Syahariza, Z.A.; Norziah, M.H.; Wan, A.K.M.; Juliano, P. Encapsulation of polyphenolic antioxidants obtained from Momordica charantia fruit within zein/gelatin shell core fibers via coaxial electrospinning. Food Biosci. 2018, 21, 60–71. [Google Scholar] [CrossRef]
- De Dicastillo, C.L.; Garrido, L.; Alvarado, N.; Romero, J.; Palma, J.; Galotto, M. Improvement of Polylactide Properties through Cellulose Nanocrystals Embedded in Poly(Vinyl Alcohol) Electrospun Nanofibers. Nanomaterials 2017, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- De-Dicastillo, C.L.; Gómez-Estaca, J.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Active antioxidant packaging films: Development and effect on lipid stability of brined sardines. Food Chem. 2012, 131. [Google Scholar] [CrossRef]
- Jamshidian, M.; Arab Tehrany, E.; Cleymand, F.; Leconte, S.; Falher, T.; Desobry, S. Effects of synthetic phenolic antioxidants on physical, structural, mechanical and barrier properties of poly lactic acid film. Carbohydr. Polym. 2012, 87, 1763–1773. [Google Scholar] [CrossRef]
- Samsudin, H.; Soto-Valdez, H.; Auras, R. Poly(lactic acid) film incorporated with marigold flower extract (Tagetes erecta) intended for fatty-food application. Food Control 2014, 46, 55–66. [Google Scholar] [CrossRef]
- Piorkowska, E.; Kulinski, Z.; Galeski, A.; Masirek, R. Plasticization of semicrystalline poly(l-lactide) with poly(propylene glycol). Polymer 2006, 47, 7178–7188. [Google Scholar] [CrossRef]
- Narayanan, G.; Gupta, B.S.; Tonelli, A.E. Poly(ε-caprolactone) Nanowebs Functionalized with α- and γ-Cyclodextrins. Biomacromolecules 2014, 15, 4122–4133. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Aguda, R.; Hartman, M.; Chung, C.-C.; Boy, R.; Gupta, B.S.; Tonelli, A.E. Fabrication and Characterization of Poly(ε-caprolactone)/α-Cyclodextrin Pseudorotaxane Nanofibers. Biomacromolecules 2016, 17, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, N.; Romero, J.; Torres, A.; de Dicastillo, C.L.; Rojas, A.; Galotto, M.J.; Guarda, A. Supercritical impregnation of thymol in poly(lactic acid) filled with electrospun poly(vinyl alcohol)-cellulose nanocrystals nanofibers: Development an active food packaging material. J. Food Eng. 2018, 217. [Google Scholar] [CrossRef]
- Galotto, M.J.; Torres, A.; Guarda, A.; Moraga, N.; Romero, J. Experimental and theoretical study of LDPE versus different concentrations of Irganox 1076 and different thickness. Food Res. Int. 2011, 44, 566–574. [Google Scholar] [CrossRef]
- Helmroth, E.; Rijk, R.; Dekker, M.; Jongen, W. Predictive modelling of migration from packaging materials into food products for regulatory purposes. Trends Food Sci. Technol. 2002, 13, 102–109. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Strumia, M.C.; Martini, R.E. Eugenol-loaded LLDPE films with antioxidant activity by supercritical carbon dioxide impregnation. J. Supercrit. Fluids 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Ivanovic, J.; Zizovic, I. Supercritical impregnation of cellulose acetate with thymol. J. Supercrit. Fluids 2015, 97, 107–115. [Google Scholar] [CrossRef]
- Neo, Y.P.; Swift, S.; Ray, S.; Gizdavic-Nikolaidis, M.; Jin, J.; Perera, C.O. Evaluation of gallic acid loaded zein sub-micron electrospun fibre mats as novel active packaging materials. Food Chem. 2013, 141, 3192–3200. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Bhattacharyya, S.; Laurencin, C.T. Development of novel tissue engineering scaffolds via electrospinning. Expert Opin. Biol. Ther. 2004, 4, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- He, C.; Huang, Z.; Han, X.; Liu, L.; Zhang, H.; Chen, L. Coaxial Electrospun Poly(l-Lactic Acid) Ultrafine Fibers for Sustained Drug Delivery. J. Macromol. Sci. Part B 2006, 45, 515–524. [Google Scholar] [CrossRef]






| e-Mats | Fiber Diameter (nm) | CIN Content (%) |
|---|---|---|
| e-PLA | 495 ± 147 | 0 |
| e- | 609 ± 218 | 0 |
| e-PLA/CINimp | 384 ± 149 | 3.29 ± 0.26 |
| e-PLA-CIN | 362 ± 102 | 1.78 ± 0.03 |
| Peaks | PLA ext | e-PLA | e- | e-PLA/CINimp | e-PLA-CIN | Assignment |
|---|---|---|---|---|---|---|
| a | - | - | - | 691 | 691 | CH=CH bending in alkene of CIN |
| b | 867 | 867 | 870 | 870 | 867 | PLA amorphous zone |
| c | 1040 | 1044 | - | - | - | C-O stretching |
| d | 1080 | 1085 | - | - | - | C=O and C-O symmetric stretching |
| e | 1180 | 1182 | - | - | - | C-O-C stretching |
| f | - | - | - | 1681 | 1679 | aromatic ring and aldehyde group of CIN |
| g | 1747 | 1751 | 1752 | 1754 | 1753 | C=O carbonyl stretching |
| Materials | Tdeg | Tg (°C) | Tcc (°C) | ΔHcc (J/g) | Tm (°C) | ΔHm (J/g) | Xc’ (%) |
|---|---|---|---|---|---|---|---|
| PLA ext | 365.1 ± 1.5 b | 63.2 ± 0.7 d | 117.2 ± 0.3 b | 22.3 ± 0.1 b | 155.6 ± 1.7 c | 26.5 ± 0.5 b | 4.6 ± 0.4 b |
| e-PLA | 334.0 ± 11.1 a | 53.1 ± 0.2 c | 113.8 ± 0.2 b | 24.7 ± 4.5 b | 153.2 ± 0.3 bc | 25.8 ± 4.2 b | 1.1 ± 0.4 a |
| e- | 334.6 ± 8.3 a | 56.7 ± 0.1 c | 122.9 ± 0.5 b | 8.0 ± 1.6 a | 150.9 ± 0.8 b | 9.3 ± 2.3 a | 1.4 ± 0.8 a |
| e-PLA/CINimp | 350.8 ± 8.2 ab | 46.5 ± 2.7 b | 103.7 ± 7.2 a | 24.8 ± 0.2 b | 151.2 ± 0.4 b | 27.0 ± 0.4 b | 2.4 ± 0.2 a |
| e-PLA-CIN | 349.8 ± 5.9 ab | 38.2 ± 3.9 a | 100.3 ± 4.1 a | 25.3 ± 0.8 b | 147.3 ± 1.5 a | 30.3 ± 0.2 b | 5.4 ± 0.6 b |
| e-Mats | KPLA/SS | DCin (m−2 s−1) | RMSE (%) |
|---|---|---|---|
| e-PLACIN | 470 | 1 × 10−12 | 0.65 |
| e-PLA/CINimp | 133 | 6 × 10−14 | 0.71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López de Dicastillo, C.; Villegas, C.; Garrido, L.; Roa, K.; Torres, A.; Galotto, M.J.; Rojas, A.; Romero, J. Modifying an Active Compound’s Release Kinetic Using a Supercritical Impregnation Process to Incorporate an Active Agent into PLA Electrospun Mats. Polymers 2018, 10, 479. https://doi.org/10.3390/polym10050479
López de Dicastillo C, Villegas C, Garrido L, Roa K, Torres A, Galotto MJ, Rojas A, Romero J. Modifying an Active Compound’s Release Kinetic Using a Supercritical Impregnation Process to Incorporate an Active Agent into PLA Electrospun Mats. Polymers. 2018; 10(5):479. https://doi.org/10.3390/polym10050479
Chicago/Turabian StyleLópez de Dicastillo, Carol, Carolina Villegas, Luan Garrido, Karina Roa, Alejandra Torres, María José Galotto, Adrián Rojas, and Julio Romero. 2018. "Modifying an Active Compound’s Release Kinetic Using a Supercritical Impregnation Process to Incorporate an Active Agent into PLA Electrospun Mats" Polymers 10, no. 5: 479. https://doi.org/10.3390/polym10050479