High Permittivity Nanocomposites Embedded with Ag/TiO2 Core–Shell Nanoparticles Modified by Phosphonic Acid
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Conclusions
3.1. Characterization of Surfactant-Coated Ag/TiO2 Core–Shell NPs
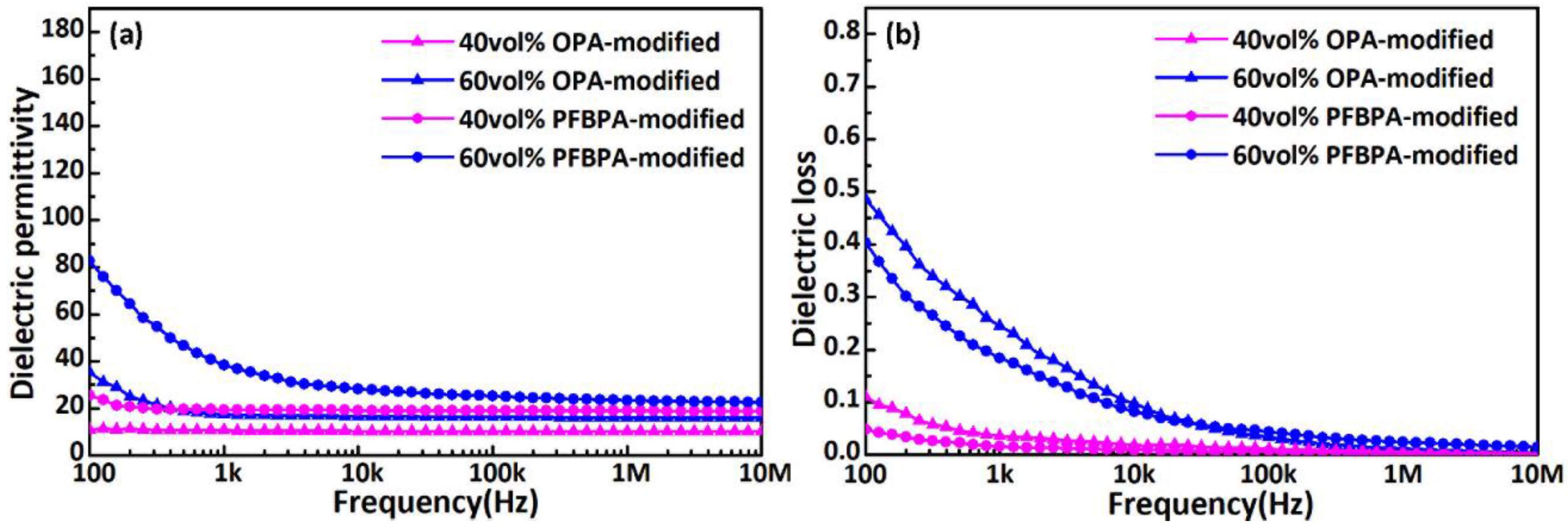
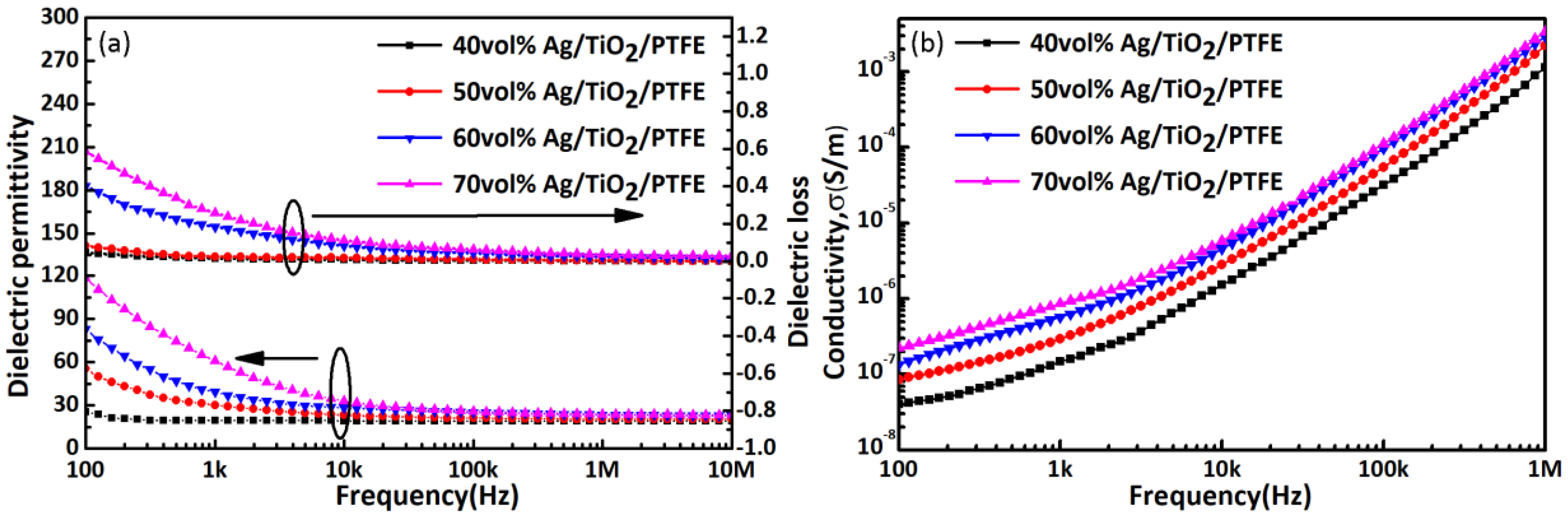
3.2. Morphology and Dielectric Properties of Modified Ag/TiO2/PTFE Nanocomposite
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.M.; Li, H.F.; Poh, M.; Xia, F.; Cheng, Z.Y.; Xu, H.S.; Huang, C. An all-organic composite actuator material with a high dielectric constant. Nature 2002, 419, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.B.; Yao, L.G.; Zhai, J.W.; Shen, B.; Wang, H.T. Significantly improved dielectric properties and energy density of polymer nanocomposites via small loaded of BaTiO3 nanotubes. Compos. Sci. Technol. 2017, 147, 30–38. [Google Scholar] [CrossRef]
- Shiri, H.M.; Ehsani, A. A novel and facile route for the electrosynthesis of Ho2O3 nanoparticles and its nanocomposite with p-Type conductive polymer: Characterisation and electrochemical performance. Bull. Chem. Soc. Jpn. 2016, 89, 1201–1206. [Google Scholar] [CrossRef]
- Wang, Y.; Mayorga-Martinez, C.C.; Pumera, M. Polyaniline/MoSX supercapacitor by electrodeposition. Bull. Chem. Soc. Jpn. 2017, 90, 847–853. [Google Scholar] [CrossRef]
- Li, A.; Zhang, C.; Zhang, Y.F. Thermal Conductivity of Graphene-Polymer Composites: Mechanisms, Properties, and Applications. Polymer 2017, 9, 437. [Google Scholar]
- Chen, X.Z.; Liang, F.; Lu, W.Z.; Zhao, Y.F.; Fan, G.F.; Wang, X.C. Improved dielectric properties of Ag@TiO2/PVDF nanocomposites induced by interfacial polarization and modifiers with different carbon chain lengths. Appl. Phys. Lett. 2018, 112, 162902. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.C.; Xu, P.; Qiang, R.; Han, X.J. Recent Advances in conjugated polymer-based microwave absorbing materials. Polymer 2017, 9, 29. [Google Scholar] [CrossRef]
- Dang, Z.M.; Yuan, J.K.; Yao, S.H.; Liao, R.J. Flexible Nanodielectric Materials with High Permittivity for Power Energy Storage. Adv. Mater. 2013, 25, 6334–6365. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, H.L.; Yin, D.; Lu, Z.H.; Wei, J.H.; Xiong, R.; Shi, J.; Wang, Z.Y.; Liu, Z.Y.; Lei, Q.Q. High performance of polyimide/CaCu3Ti4O12@Ag hybrid films with enhanced dielectric permittivity and low dielectric loss. J. Mater. Chem. A 2015, 3, 4916–4921. [Google Scholar] [CrossRef]
- Kuang, X.W.; Liu, Z.; Zhu, H. Dielectric properties of Ag@C/PVDF composites. J. Appl. Polym. Sci. 2013, 129, 3411–3416. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, S.; Chen, S.; Wang, D.R.; Han, B.Z.; Dang, Z.M. Preparation and dielectric properties of core–shell structured Ag@polydopamine/poly(vinylidene fluoride) composites. Compos. Sci. Technol. 2015, 110, 126–131. [Google Scholar] [CrossRef]
- Shen, Y.; Lin, Y.; Li, M.; Nan, C.W. High Dielectric Performance of Polymer Composite Films Induced by a Percolating Interparticle Barrier Layer. Adv. Mater. 2007, 19, 1418–1422. [Google Scholar] [CrossRef]
- Huang, X.; Feng, M.N.; Liu, X.B. The interfacial effect of TiO2-Ag core-shell micro-/nanowires on poly(arylene ether nitrile). Polym. Int. 2014, 63, 1324–1331. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Wang, L.; Zhang, H.; Bai, Y.Y.; Niu, Y.J.; Wang, H. Enhanced high thermal conductivity and low permittivity of polyimide based composites by core-shell Ag@SiO2 nanoparticle fillers. Appl. Phys. Lett. 2012, 101, 12903. [Google Scholar] [CrossRef]
- Xiao, X.R.; Yang, H.; Xu, N.X.; Hu, L.; Zhang, Q.L. High performance of P(VDF-HFP)/Ag@TiO2 hybrid films with enhanced dielectric permittivity and low dielectric loss. RSC Adv. 2015, 5, 79342. [Google Scholar] [CrossRef]
- Chen, H.Z.; Tian, X.X.; Liu, J. Unsaturated Polyester Resin Nanocomposites Containing ZnO Modified with Oleic Acid Activated by N,N’-Carbonyldiimidazole. Polymers 2018, 10, 362. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Gao, F.; Zhang, C.C.; Wang, L.; Wang, M.; Qin, M.J.; Hu, G.X.; Kong, J. Enhanced dielectric tunability of Ba0.6Sr0.4TiO3/Poly(vinylidenefluoride) composites via interface modification by silane coupling agent. Compos. Sci. Technol. 2016, 129, 93–100. [Google Scholar] [CrossRef]
- Niu, Y.J.; Bai, Y.Y.; Yu, K.; Wang, Y.F.; Xiang, F.; Wang, H. Effect of the Modifier Structure on the Performance of Barium Titanate/Poly(vinylidene fluoride) Nanocomposites for Energy Storage Applications. ACS Appl. Mater. Interfaces 2015, 7, 24168–24176. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, C.; Fettkenhauer, C.; Glenneberg, J.; Munchgesang, W.; Leipner, H.S.; Diestelhorst, M.; Lemm, S.; Beige, H.; Ebbinghaus, S.G. A solution-based approach to composite dielectric films of surface functionalized CaCu3Ti4O12 and P(VDF-HFP). J. Mater. Chem. A 2014, 2, 2266–2274. [Google Scholar] [CrossRef]
- Kim, P.; Jones, S.C.; Hotchkiss, P.J.; Haddock, J.N.; Kippelen, B.; Marder, S.R.; Perry, J.W. Phosphonic Acid modified Barium Titanate Polymer Nanocomposites with High Permittivity and Dielectric Strength. Adv. Mater. 2010, 19, 1001–1005. [Google Scholar] [CrossRef]
- Qi, L.; Lee, B.; Chen, S.; Samuels, W.D.; Exarhos, G.J. High-Dielectric-Constant Silver-Epoxy Composites as Embedded Dielectrics. Adv. Mater. 2005, 17, 1777–1781. [Google Scholar] [CrossRef]
- Ruiterkamp, G.J.; Hempenius, M.A.; Wormeester, H.; Vancso, G.J. Surface functionalization of titanium dioxide nanoparticles with alkanephosphonic acids for transparent nanocomposites. J. Nanopart. Res. 2011, 13, 2779–2790. [Google Scholar] [CrossRef]
- Dang, Z.M.; You, S.S.; Zha, J.W.; Song, H.T.; Li, S.T. Effect of shell-layer thickness on dielectric properties in Ag@TiO2 core@shell nanoparticles filled ferroelectric poly(vinylidene fluoride) composites. Phys. Status Solidi A 2010, 207, 739–742. [Google Scholar] [CrossRef]
- Yang, X.H.; Fu, H.T.; Wong, K.; Jiang, X.C.; Yu, A.B. Hybrid Ag@TiO2 core-shell nanostructures with highly enhanced photocatalytic performance. Nanotechnology 2013, 24, 415601. [Google Scholar] [CrossRef] [PubMed]
- Angkaew, S.; Limsuwan, P. Preparation of silver-titanium dioxide core-shell (Ag@TiO2) nanoparticles Effect of Ti-Ag mole ratio. Procedia Eng. 2012, 32, 649–655. [Google Scholar] [CrossRef]
- Saberi, A.A. Recent advances in percolation theory and its applications. Phys. Rep. 2015, 578, 1–32. [Google Scholar] [CrossRef]
- Yang, W.H.; Yu, S.H.; Sun, R.; Ke, S.M.; Huang, H.T.; Du, R.X. Electrical modulus analysis on the Ni/CCTO/PVDF system near the percolation threshold. J. Phys. D Appl. Phys. 2011, 44, 475305–475312. [Google Scholar] [CrossRef]
- Fang, F.; Yang, W.H.; Yu, S.H.; Luo, S.B.; Sun, R. Mechanism of high dielectric performance of polymer composites induced by BaTiO3 supporting Ag hydrid fillers. Appl. Phys. Lett. 2014, 104, 132909. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, L.; Lu, W.Z.; Wan, Q.X.; Fan, G.F. Dielectric performance of polymer-based composites containing core-shell Ag@TiO2 nanoparticle fillers. Appl. Phys. Lett. 2016, 108, 284–287. [Google Scholar] [CrossRef]
- Shafee, E.E.; Gamal, M.E.; Isa, M. Electrical properties of multi walled carbon nanotubes/poly(vinylidene fluoride/trifluoroethylene) nanocomposites. J. Polym. Res. 2012, 19, 9805–9808. [Google Scholar] [CrossRef]
- Rajesh, S.; Murali, K.P.; Priyadarsini, V. Rutile filled PTFE composites for flexible microwave substrate applications. Mater. Sci. Eng. 2009, 163, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Huang, E.Q.; Zha, J.W.; Dang, Z.M. Preparation and Dielectric Properties of (Ba0.5Sr0.4Ca0.1)TiO3/Polystyrene Composites. J. Appl. Polym. Sci. 2015, 132, 41398. [Google Scholar] [CrossRef]
- Sadie, I.W.; Rose, M.M.; Patrick, M.V.; David, J.; Sam, H.; James, M.K.; Ju, L. Electrical Percolation Behavior in Silver Nanowire–Polystyrene Composites: Simulation and Experiment. Adv. Funct. Mater. 2010, 20, 2709–2716. [Google Scholar]
- Yu, K.; Niu, Y.J.; Bai, Y.Y.; Zhou, Y.C.; Wang, H. Poly(vinylidene fluoride) polymer based nanocomposites with significantly reduced energy loss by filling with core-shell structured BaTiO3/SiO2 nanoparticles. Appl. Phys. Lett. 2013, 102, 102903. [Google Scholar] [CrossRef]
- Hernandez, Y.R.; Gryson, A.; Blighe, F.M.; Cadek, M.; Nicolosi, V.; Blau, W.J.; Gun’ko, Y.K.; Coleman, J.N. Comparison of carbon nanotubes and nanodisks as percolative fillers in electrically conductive composites. Scr. Mater. 2008, 58, 69–72. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liang, F.; Lu, W.; Jin, Z.; Zhao, Y.; Fu, M. High Permittivity Nanocomposites Embedded with Ag/TiO2 Core–Shell Nanoparticles Modified by Phosphonic Acid. Polymers 2018, 10, 586. https://doi.org/10.3390/polym10060586
Chen X, Liang F, Lu W, Jin Z, Zhao Y, Fu M. High Permittivity Nanocomposites Embedded with Ag/TiO2 Core–Shell Nanoparticles Modified by Phosphonic Acid. Polymers. 2018; 10(6):586. https://doi.org/10.3390/polym10060586
Chicago/Turabian StyleChen, Xizi, Fei Liang, Wenzhong Lu, Zheng Jin, Yifei Zhao, and Ming Fu. 2018. "High Permittivity Nanocomposites Embedded with Ag/TiO2 Core–Shell Nanoparticles Modified by Phosphonic Acid" Polymers 10, no. 6: 586. https://doi.org/10.3390/polym10060586




