Microstructure of Copolymers of Norbornene Based on Assignments of 13C NMR Spectra: Evolution of a Methodology
Abstract
:1. Introduction
2. Results and Discussion
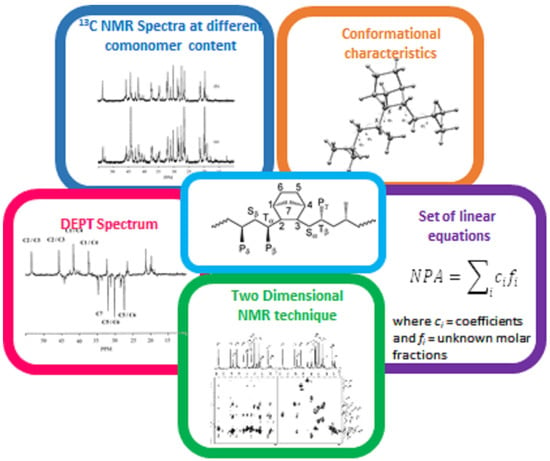
2.1. Methodologies for Signal Assignments
2.1.1. NMR Pulse Sequences
2.1.2. Series of Copolymers with Different Comonomer Content
2.1.3. 13C Enrichment
2.1.4. Conformational Characteristics of the Copolymer Chain
2.1.5. Model Compounds
2.1.6. Two-Dimensional NMR Techniques
2.1.7. Ab Initio Theoretical 13C NMR Chemical Shifts, Combined with R.I.S. (Rotational-Isomeric State) Statistics
2.1.8. Set of Equations and Least-Squares Fitting of Peak Areas
2.2. Microstructural Analysis of E-co-N Copolymers
2.2.1. Microstructure Alternating E-co-N Copolymers with Mid-Low N Content
2.2.2. Microstructure at Tetrad Level of a Random E-co-N Copolymers with Mid N Content
2.2.3. Microstructure at Tetrad Level of a Random E-co-N Copolymers with High N Content
2.3. Microstructural Analysis of P-co-N Copolymers
2.3.1. P-co-N Copolymers with Isolated N Units
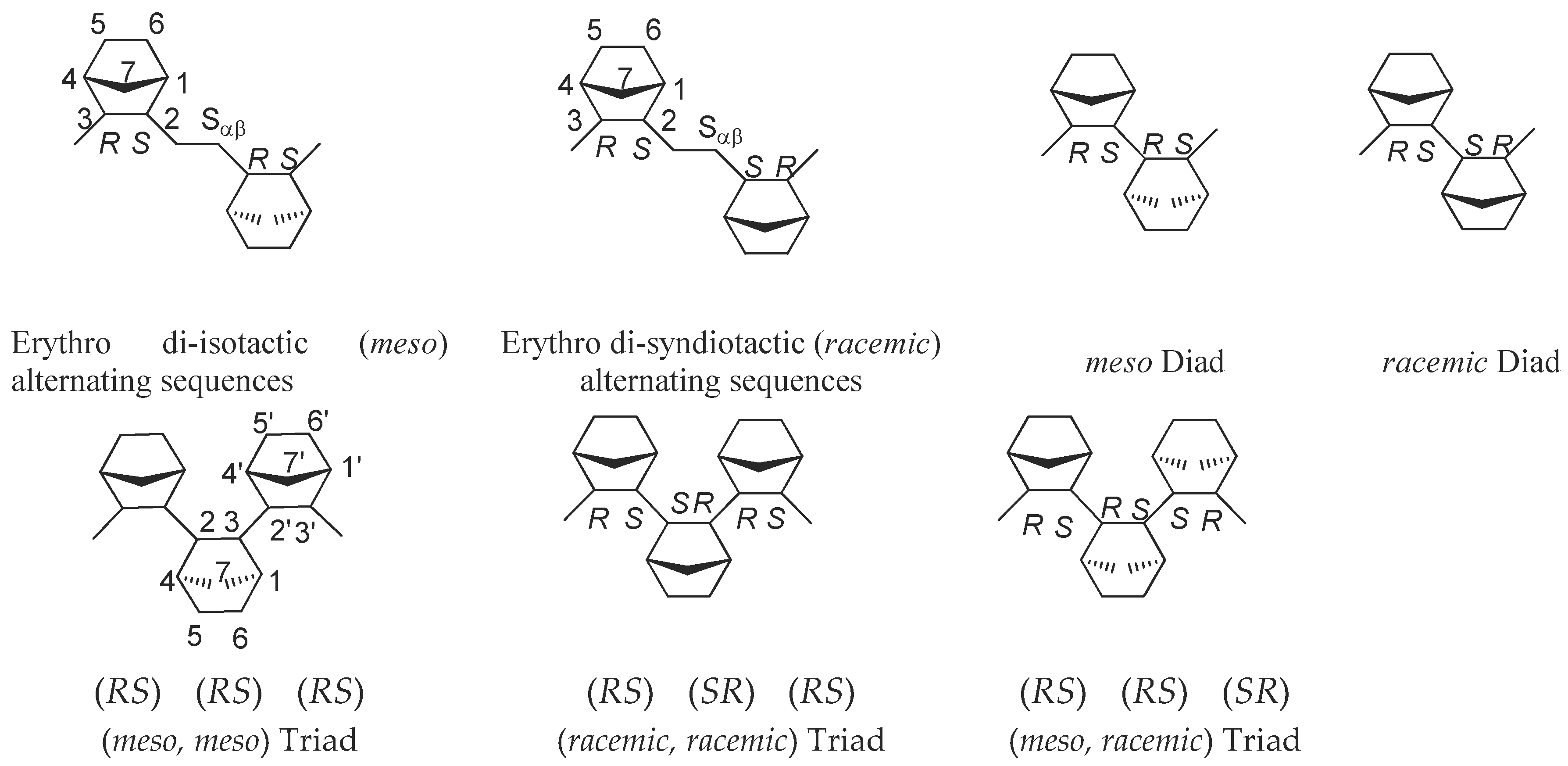
2.3.2. Microstructure at Triad Level P-co-N Copolymers with Mid-Low N Content Synthesized with C2 Symmetric Metallocenes
- (i)
- the resonances at 16.22 and 16.34 ppm due to the methyl carbon atom of central P in P21P12N (S8), and NP21P12 (S23), respectively;
- (ii)
- the signal at 20.05 ppm due to the methyl carbon atom (Pγ) of the P in P12P12N (S2);
- (iii)
- the signal at 21.26 ppm of the Pβγ of alternating triad NP12N (S4);
- (iv)
- the signals from 21.05 to 21.94 ppm to Pβ methyls in triad NP12P12 (S3) adjacent to a variable number of P12 units all in isotactic relationship;
- (v)
- the signal at 20.25 ppm to the methyl of triad NP12P12 (S3) adjacent to a variable number of P12 units having different tacticity;
- (vi)
- (the signal at 25.45 ppm to the C5 of central N in P12NN (S10);
- (vii)
- the signal centered at 32.21 ppm to the CH of central P in NP12N (S4);
- (viii)
- the signals at 33.60 and 33.90 ppm to CH2 of P in the triads NP21P12 (S23) and P21P12N (S8), respectively;
- (ix)
- the signal at 35.70 ppm to the Sαγ methylene of a 1,3 propylene inserted units in NP13P12 triad (S21).
2.4. Microstructural Analysis of Poly(Ethylene-co-4-MechCHE) and Poly(Ethylene-co-MeCPE)
2.5. Microstructural Analysis of Poly(E-ter-N-ter-HED)
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Spiess, H.W. 50th Anniversary Perspective: The Importance of NMR Spectroscopy to Macromolecular Science. Macromolecules 2017, 50, 1761–1777. [Google Scholar] [CrossRef], and references therein
- Tonelli, A.E. NMR Spectroscopy and Polymer Microstructure: The Conformational Connection; VCH: New York, NY, USA, 1989; ISBN 0-89573-737-X. [Google Scholar]
- Grant, D.M.; Paul, E.G. Carbon-13 Magnetic Resonance. II. Chemical Shift Data for the Alkanes. J. Am. Chem. Soc. 1964, 86, 2984–2990. [Google Scholar] [CrossRef]
- Lindeman, L.P.; Adams, J.Q. Carbon-13 nuclear magnetic resonance spectrometry. Chemical shifts for the paraffins through C9. Anal. Chem. 1971, 43, 1245–1252. [Google Scholar] [CrossRef]
- Stothers, J.B. Carbon-13 NMR Spectroscopy; Organic Chemistry, A series of Monographs; Academic Press: New York, NY, USA; London, UK, 1972; Volume 24, ISBN 9780323145503. [Google Scholar]
- Bovey, F.A. Chain Structure and Conformation of Macromolecules; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Provasoli, A.; Ferro, D.R. Correlation between C-13 NMR Chemical-shifts and Conformation of Polymers. 1. Methyl Spectrum of 3,5,7,9,11,13,15-Heptamethylheptadecane. Macromolecules 1977, 10, 874–877. [Google Scholar] [CrossRef]
- Tonelli, A.E. Are the Steric Effects on the C-13 NMR Chemical- shifts of Hydrocarbon Polymers Really Long-range? Macromolecules 1979, 12, 255–256. [Google Scholar] [CrossRef]
- Provasoli, A.; Ferro, D.R.; Zambelli, A.; Provasoli, A.; Locatelli, P.; Rigamonti, E. Correlation between C-13 NMR Chemical-shifts and Conformation of Polymers. 2. Improved Method of Calculation. Macromolecules 1980, 13, 179–186. [Google Scholar] [CrossRef]
- Ferro, D.R.; Ragazzi, M. Correlation between C-13 NMR Chemical-shifts and Conformation of Polymers. 4. Solid-state spectra of poly(3-methyl-1-Pentene). Macromolecules 1984, 17, 485–490. [Google Scholar] [CrossRef]
- Wei, M.; Ivey, D.T.; Tonelli, A.E. What is the source of the microstructural dependence of resonance frequencies observed in the solution NMR of polymers whose local structures and conformations appear to be independent of their longer range microstructures? Macromolecules 2002, 35, 1976–1979. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R. Microstructure of polypropylene. Prog. Polym. Sci. 2001, 26, 443–533. [Google Scholar] [CrossRef]
- Kaminsky, W.; Bark, A.; Arndt, M. New polymers by homogenous zirconocene/aluminoxane catalysts. Makromol. Chem. Macromol. Symp. 1991, 47, 83–93. [Google Scholar] [CrossRef]
- Kaminsky, W.; Arndt-Rosenau, M. Homo- and copolymerization of cycloolefins by metallocene catalysts. In Metallocene-Based Polyolefins; Scheirs, J., Kaminsky, W., Eds.; Wiley: Chichester, UK, 2000; Volume 2, pp. 91–113. [Google Scholar]
- Kaminsky, W.; Boggioni, L.; Tritto, I. 3.26 Cycloolefin polymerization. In Comprehensive Polymer Science. Volume 3. Chain Vinyl Polymerization; Coates, G.W., Sawamoto, M., Eds.; Elsevier: Amsterdam, Netherlands, 2012; pp. 843–873. [Google Scholar], and references therein
- Boggioni, L.; Tritto, I. State of the art of cyclic olefin polymers. MRS Bull. 2013, 38, 245–251. [Google Scholar] [CrossRef]
- Tritto, I.; Boggioni, L.; Ferro, D.R. Metallocene catalyzed ethene- and propene co-norbornene polymerization: Mechanisms from a detailed microstructural analysis. Coord. Chem. Rev. 2006, 250, 212–241. [Google Scholar] [CrossRef], and references therein
- Wendt, R.A.; Fink, G. C-13 NMR studies of ethene/norbornene copolymers using C-13-enriched monomers: Signal assignments of copolymers containing norbornene microblocks of up to a length of three norbornene units. Macromol. Chem. Phys. 2001, 202, 3490. [Google Scholar] [CrossRef]
- Provasoli, A.; Ferro, D.R.; Tritto, I.; Boggioni, L. The conformational characteristics of ethylene−norbornene copolymers and their influence on the 13C NMR spectra. Macromolecules 1999, 32, 6697–6706. [Google Scholar] [CrossRef]
- One of the reviewers pointed out that the conformational models derived by us to determine the conformational populations in the norbornene copolymers have not been independently tested and criticized the relationship employed by us between nuclear shieldings and conformations. The discussion on validity of these objections is out of the scope of this article. We emphasize that the results presented in published papers rely not only on conformational calculations but also on the overall agreement with experimental data, although probably the use of better conformational models would have led to more complete assignments.
- Arndt, M.; Gosmann, M. Transition metal catalyzed polymerisation of norbornene Polym. Bull. 1999, 41, 433–440. [Google Scholar] [CrossRef]
- Bergström, C.H.; Sperlich, B.R.; Ruotoistenmäki, J.; SeppäläJ, V. Investigation of the microstructure of metallocene-catalyzed norbornene-ethylene copolymers using NMR spectroscopy. J. Polym. Sci. Part A 1998, 36, 1633–1638. [Google Scholar] [CrossRef]
- Tritto, I.; Boggioni, L.; Sacchi, M.C.; Locatelli, P. Cyclic olefin polymerization and relationships between addition and ring opening metathesis polymerization. J. Mol. Catal. A Chem. 1998, 133, 139–150. [Google Scholar] [CrossRef]
- Ruchatz, D.; Fink, G. Ethene−Norbornene copolymerization using homogenous metallocene and half-sandwich catalysts: Kinetics and relationships between catalyst structure and polymer structure. 2. Comparative study of different metallocene- and half-sandwich/methylaluminoxane catalysts and analysis of the copolymers by 13C nuclear magnetic resonance spectroscopy. Macromolecules 1998, 31, 4674–4680. [Google Scholar] [CrossRef] [PubMed]
- Tritto, I.; Marestin, C.; Boggioni, L.; Zetta, L.; Provasoli, A.; Ferro, D.R. Ethylene−norbornene copolymer microstructure. Assessment and advances based on assignments of 13C NMR spectra. Macromolecules 2000, 33, 8931–8944. [Google Scholar] [CrossRef]
- Tritto, I.; Marestin, C.; Boggioni, L.; Brintzinger, H.H.; Ferro, D.R. Stereoregular and stereoirregular alternating ethylene−norbornene copolymers. Macromolecules 2001, 34, 5770–5777. [Google Scholar] [CrossRef]
- Thorshaug, K.; Mendichi, R.; Boggioni, L.; Tritto, I.; Trinkle, S.; Friedrich, C.; Mülhaupt, R. Poly(ethene-co-norbornene) obtained with a constrained geometry catalyst. A study of reaction kinetics and copolymer properties. Macromolecules 2002, 35, 2903–2911. [Google Scholar] [CrossRef]
- Herfert, N.; Montag, P.; Fink, G. Elementary processes of the Ziegler catalysis, 7. Ethylene, α-olefin and norbornene copolymerization with the stereorigid catalyst systems Ipr[FluCp]Zrcl2/MAO and Me2Si[Ind]2ZrCl2/MAO. Makromol. Chem. 1993, 194, 3167–3182. [Google Scholar] [CrossRef]
- Rische, T.; Waddon, A.J.; Dickinson, L.C.; MacKnight, W.J. Microstructure and morphology of cycloolefin copolymers. Macromolecules 1998, 31, 1871–1874. [Google Scholar] [CrossRef]
- Ragazzi, M.; Carbone, P.; Ferro, D.R. Ab initio molecular modeling of C-13 NMR chemical shifts of polymers. 1. Ethylene-norbornene copolymers. Int. J. Quantum Chem. 2002, 88, 663–669. [Google Scholar] [CrossRef]
- Tritto, I.; Boggioni, L.; Jansen, J.C.; Thorshaug, K.; Sacchi, M.C.; Ferro, D.R. Ethylene-norbornene copolymers from metallocene-based catalysts: Microstructure at tetrad level and reactivity ratios. Macromolecules 2002, 35, 616–623. [Google Scholar] [CrossRef]
- Tritto, I.; Boggioni, L.; Ferro, D.R. Alternating Isotactic Ethylene−Norbornene Copolymers by C1-Symmetric Metallocenes: Determination of the Copolymerization Parameters and Mechanistic Considerations on the Basis of Pentad Analysis. Macromolecules 2004, 37, 9681–9693. [Google Scholar] [CrossRef]
- Tritto, I.; Boggioni, L.; Zampa, C.; Ferro, D.R. Ethylene-norbornene copolymers by Cs-symmetric metallocenes: Determination of the copolymerization parameters and mechanistic considerations on the basis of tetrad analysis. Macromolecules 2005, 38, 9910–9919. [Google Scholar] [CrossRef]
- The 13C NMR spectra were measured in C2D2Cl4 at 105 °C; chemical shifts are referred to HMDS.
- Carbone, P.; Ragazzi, M.; Tritto, I.; Boggioni, L.; Ferro, D.R. Ab initio molecular modeling of 13C NMR chemical shifts of polymers. 2. Propene-norbornene copolymers. Macromolecules 2003, 36, 891–899. [Google Scholar] [CrossRef]
- Boggioni, L.; Bertini, F.; Zannoni, G.; Tritto, I.; Carbone, P.; Ragazzi, M.; Ferro, D.R. Propene-norbornene copolymers: Synthesis and analysis of polymer structure by C-13 NMR spectroscopy and ab initio chemical shift computations. Macromolecules 2003, 36, 882–890. [Google Scholar] [CrossRef]
- Boggioni, L.; Tritto, I.; Ragazzi, M.; Carbone, P.; Ferro, D.R. Propene-Norbornene Copolymers: Synthesis and Microstructure. Macromol. Symp. 2004, 218, 39–50. [Google Scholar] [CrossRef]
- Boggioni, L.; Zampa, C.; Ravasio, A.; Ferro, D.R.; Tritto, I. Propene-Norbornene Copolymers by C2-symmetric Metallocene rac-Et(Ind)2ZrCl2: Influence of Reaction Conditions on Reactivity and Copolymer Properties. Macromolecules 2008, 41, 5107–5115. [Google Scholar] [CrossRef]
- Boggioni, L.; Ravasio, A.; Zampa, C.; Ferro, D.R.; Tritto, I. Penultimate Effects and Chain Epimerization in Propene-Norbornene Copolymers by rac-Me2Si(2-Me-Ind)2ZrCl2 C-2-Symmetric Metallocene. Macromolecules 2010, 43, 4532–4542. [Google Scholar] [CrossRef]
- Boggioni, L.; Ravasio, A.; Boccia, A.C.; Ferro, D.R.; Tritto, I. Propene-Norbornene Copolymers. Toward a Description of Microstructure at Triad Level Based on Assignments of C-13 NMR Spectra. Macromolecules 2010, 43, 4543–4556. [Google Scholar] [CrossRef]
- Itagaki, K.; Fujiki, M.; Nomura, K. Effect of cyclopentadienyl and anionic donor ligands on monomer reactivities in copolymerization of ethylene with 2-methyl-1-pentene by nonbridged half-titanocenes- cocatalyst systems. Macromolecules 2007, 40, 6489–6499. [Google Scholar] [CrossRef]
- Nomura, K.; Itagaki, K. Efficient incorporation of vinylcylohexane in ethylene/vinylcyclohexane copolymerization catalyzed by nonbridged half-titanocenes. Macromolecules 2005, 38, 8121–8123. [Google Scholar] [CrossRef]
- Khan, F.Z.; Kakinuki, K.; Nomura, K. Copolymerization of ethylene with t-buytlethylene using nonbridged half-titanocene-cocatalyst systems. Macromolecules 2009, 42, 3767–3773. [Google Scholar] [CrossRef]
- Kakinuki, K.; Fujiki, M.; Nomura, K. Copolymerization of ethylene with α-olefins containing various substituents catalyzed by half-titanocenes: Factors affecting the monomer reactivities. Macromolecules 2009, 42, 4585–4595. [Google Scholar] [CrossRef]
- Nomura, K.; Kakinuki, K.; Fujiki, M.; Itagaki, K. Direct precise functional group introduction into polyolefins: Efficient incorporation of vinyltrialkylsilanes in ethylene copolymerizations by nonbridged half-titanocenes. Macromolecules 2008, 41, 8974–8976. [Google Scholar] [CrossRef]
- Harakawa, H.; Patamma, S.; Boccia, A.C.; Boggioni, L.; Ferro, D.R.; Losio, S.; Nomura, K.; Tritto, I. Ethylene Copolymerization with 4-Methyl-cyclohexene, 1-Methylcyclopentene by Half-Titanocene Catalysts: Effect of Ligands and Microstructural Analysis in the Copolymers. Macromolecules 2018, 51, 853–863. [Google Scholar] [CrossRef]
- Naga, N.; Imanishi, Y. Copolymerization of ethylene and cyclopentene with zirconocene catalysts: Effect of ligand structure of zirconocenes. Macromol. Chem. Phys. 2002, 203, 159–165. [Google Scholar] [CrossRef]
- Lavoie, A.R.; Waymouth, R.M. Catalytic syntheses of alternating, stereoregular ethylene/cycloolefin copolymers. Tetrahedron 2004, 60, 7147–7155. [Google Scholar] [CrossRef]
- Fujita, M.; Coates, G.W. Synthesis and characterization of alternating and multiblock copolymers from ethylene and cyclopentene. Macromolecules 2002, 35, 9640–9647. [Google Scholar] [CrossRef]
- Tang, L.-M.; Duan, Y.-Q.; Pan, L.; Li, Y.-S. Copolymerization of ethylene and cyclopentene with bis(β-enaminoketonato) titanium complexes. J. Polym. Sci. Part A 2005, 43, 1681–1689. [Google Scholar] [CrossRef]
- Catalyst X is considered for patent applications, so the structures of this catalyst cannot be disclosed.
- Naga, N. Copolymerization of ethylene with cycloolefins or cyclodiolefins by a constrained-geometry catalyst. J. Polym. Sci. Part A 2005, 43, 1285–1291. [Google Scholar] [CrossRef]
- Liu, J.; Nomura, K. Highly efficient ethylene/cyclopentene copolymerization with exclusive 1,2-cyclopentene incorporation by (cyclopentadienyl)-(ketimide)titanium(IV complex–MAO catalysts. Adv. Synth. Catal. 2007, 349, 2235–2240. [Google Scholar] [CrossRef]
- Cheng, H.N.; Khasat, N.P. C-13-NMR Characterization of poly(1,5-hexadiene). J. Appl. Polym. Sci. 1988, 35, 825–829. [Google Scholar] [CrossRef]



























| Carbon | Chemical shift | Carbon | Chemical shift | Carbon | Chemical shift | Carbon | Chemical shift |
|---|---|---|---|---|---|---|---|
| C5′ m,m | 26.59 | C7 m,m | 33.07 | C1/C4 m,m | 34.92 | C2/C3 T | 45.42/47.13 |
| C5′ r,m | 27.20–27.70 | C7 T | 34.34 | C1/C4 T | 35.18 | C2/C3 T | 49.34 |
| C5/C6 T | 29.37 | C7 T | 35.70 | C1/C4 T | 36.94 | C2/C3 m,m | 49.80 |
| C5/C6 T | 29.49 | C7 m,m | 36.74 | C1/C4 T | 37.87 | C2/C3 T | 50.00 |
| C5/C6 T | 30.50 | C7 m,m | 36.94 | C1/C4 T | 39.28–39.39 | C2/C3 m,m | 52.70–52.84 |
| C5/C6 m,m | 30.58 | C1/C4 T | 40.80 | C2/C3 T | 53.44 | ||
| C1/C4 T | 41.32 | ||||||
| C1/C4 T | 41.45 | ||||||
| C1/C4 T | 41.56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boggioni, L.; Losio, S.; Tritto, I. Microstructure of Copolymers of Norbornene Based on Assignments of 13C NMR Spectra: Evolution of a Methodology. Polymers 2018, 10, 647. https://doi.org/10.3390/polym10060647
Boggioni L, Losio S, Tritto I. Microstructure of Copolymers of Norbornene Based on Assignments of 13C NMR Spectra: Evolution of a Methodology. Polymers. 2018; 10(6):647. https://doi.org/10.3390/polym10060647
Chicago/Turabian StyleBoggioni, Laura, Simona Losio, and Incoronata Tritto. 2018. "Microstructure of Copolymers of Norbornene Based on Assignments of 13C NMR Spectra: Evolution of a Methodology" Polymers 10, no. 6: 647. https://doi.org/10.3390/polym10060647