Thermoresponsive Microgel Coatings as Versatile Functional Compounds for Novel Cell Manipulation Tools
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
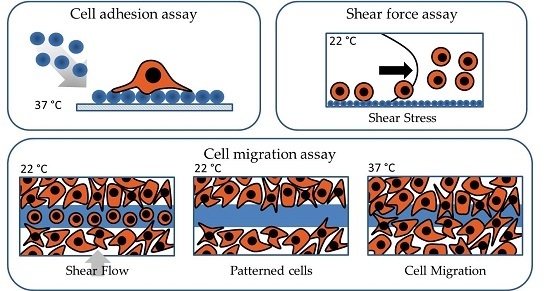
3.1. Shear Force Assay
3.2. Cell Migration Assay
3.3. Cell Adhesion Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FACS | fluorescence-activated cell sorting |
| BIS | N,N′-methylenebis(acrylamide) |
| PCS | photon correlation spectroscopy |
| DMEM | Dulbecco’s Modified Eagle Medium |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| FCS | fetal calf serum |
| COP | cyclo-olefin polymer |
| RGD | arginylglycylaspartic acid (Arg-Gly-Asp) |
References
- Canavan, H.E.; Cheng, X.; Graham, D.J.; Ratner, B.D.; Castner, D.G. Cell sheet detachment affects the extracellular matrix: A surface science study comparing thermal liftoff, enzymatic, and mechanical methods. J. Biomed. Mater. Res. A 2005, 75, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H.; Sakurai, Y. Mechanism of cell detachment from temperature-modulated, hydrophilic–hydrophobic polymer surfaces. Biomaterials 1995, 16, 297–303. [Google Scholar] [CrossRef]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Macromol. Chem. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Wischerhoff, E.; Uhlig, K.; Lankenau, A.; Börner, H.G.; Laschewsky, A.; Duschl, C.; Lutz, J.-F. Controlled cell adhesion on PEG-based switchable surfaces. Angew. Chem. Int. Ed. 2008, 47, 5666–5668. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, J.; Nitschke, M.; Pette, D.; Valtink, M.; Gramm, S.; Härtel, F.V.; Noll, T.; Funk, R.H.W.; Engelmann, K.; Werner, C. Thermo-responsive cell culture carriers based on poly(vinyl methyl ether)—The effect of biomolecular ligands to balance cell adhesion and stimulated detachment. Sci. Technol. Adv. Mater. 2015, 16, 45003. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Zeiser, M.; Hellweg, T.; Duschl, C.; Fery, A.; Möhwald, H. Adhesion and Mechanical Properties of PNIPAM Microgel Films and Their Potential Use as Switchable Cell Culture Substrates. Adv. Funct. Mater. 2010, 20, 3235–3243. [Google Scholar] [CrossRef]
- Uhlig, K.; Boysen, B.; Lankenau, A.; Jaeger, M.; Wischerhoff, E.; Lutz, J.F.; Laschewsky, A.; Duschl, C. On the influence of the architecture of poly(ethylene glycol)-based thermoresponsive polymers on cell adhesion. Biomicrofluidics 2012, 6, 024129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer-Soenen, H.; Moerkerke, R.; Berghmans, H.; Koningsveld, R.; Dušek, K.; Šolc, K. Zero and off-zero critical concentrations in systems containing polydisperse polymers with very high molar masses. 2. The system water-poly(vinyl methyl ether). Macromolecules 1997, 30, 410–416. [Google Scholar] [CrossRef]
- Wellert, S.; Richter, M.; Hellweg, T.; von Klitzing, R.; Hertle, Y. Responsive Microgels at Surfaces and Interfaces. Z. Phys. Chem. 2015, 229, 1225–1250. [Google Scholar] [CrossRef]
- Uhlig, K.; Wegener, T.; He, J.; Zeiser, M.; Bookhold, J.; Dewald, I.; Godino, N.; Jaeger, M.; Hellweg, T.; Fery, A.; et al. Patterned Thermoresponsive Microgel Coatings for Noninvasive Processing of Adherent Cells. Biomacromolecules 2016, 17, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Ernst, O.; Lieske, A.; Jaeger, M.; Lankenau, A.; Duschl, C. Control of cell detachment in a microfluidic device using a thermo-responsive copolymer on a gold substrate. Lab Chip 2007, 7, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Truskey, G.A.; Pirone, J.S. The effect of fluid shear stress upon cell adhesion to fibronectin-treated surfaces. J. Biomed. Mater. Res. 1990, 24, 1333–1353. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, K.; Boerner, H.G.; Wischerhoff, E.; Lutz, J.-F.; Jaeger, M.S.; Laschewsky, A.; Duschl, C. On the Interaction of Adherent Cells with Thermoresponsive Polymer Coatings. Polymers 2014, 6, 1164–1177. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wu, J.; Gao, C. Gradient immobilization of a cell adhesion RGD peptide on thermal responsive surface for regulating cell adhesion and detachment. Colloids Surf. B Biointerfaces 2011, 85, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Kikuchi, A.; Yamato, M.; Okano, T. Bio-functionalized thermoresponsive interfaces facilitating cell adhesion and proliferation. Biomaterials 2006, 27, 5069–5078. [Google Scholar] [CrossRef] [PubMed]
- Trzebicka, B.; Szweda, R.; Kosowski, D.; Szweda, D.; Otulakowski, Ł.; Haladjova, E.; Dworak, A. Thermoresponsive polymer-peptide/protein conjugates. Prog. Polym. Sci. 2017, 68, 35–76. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhlig, K.; Wegener, T.; Hertle, Y.; Bookhold, J.; Jaeger, M.; Hellweg, T.; Fery, A.; Duschl, C. Thermoresponsive Microgel Coatings as Versatile Functional Compounds for Novel Cell Manipulation Tools. Polymers 2018, 10, 656. https://doi.org/10.3390/polym10060656
Uhlig K, Wegener T, Hertle Y, Bookhold J, Jaeger M, Hellweg T, Fery A, Duschl C. Thermoresponsive Microgel Coatings as Versatile Functional Compounds for Novel Cell Manipulation Tools. Polymers. 2018; 10(6):656. https://doi.org/10.3390/polym10060656
Chicago/Turabian StyleUhlig, Katja, Thomas Wegener, Yvonne Hertle, Johannes Bookhold, Magnus Jaeger, Thomas Hellweg, Andreas Fery, and Claus Duschl. 2018. "Thermoresponsive Microgel Coatings as Versatile Functional Compounds for Novel Cell Manipulation Tools" Polymers 10, no. 6: 656. https://doi.org/10.3390/polym10060656