Effective Reduction of Volumetric Thermal Expansion of Aromatic Polyimide Films by Incorporating Interchain Crosslinking
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Measurements
2.3. Quantum Chemical Calculations
3. Results and Discussion
3.1. Estimation of Degrees of Thermal Crosslinking Reactions
3.2. Aggregation Structure and Molecular Chain Orientation of Polyimide Films
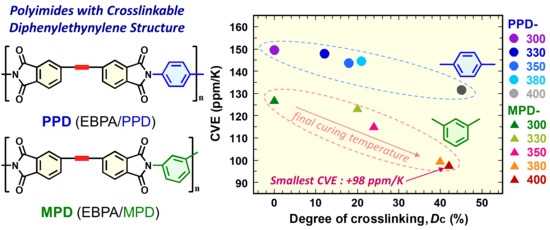
3.3. Effects of Crosslinking on the Coefficient of Volumetric Thermal Expansion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sroog, C.E. Polyimides. J. Polym. Sci. Macromol. Rev. 1976, 11, 161–208. [Google Scholar] [CrossRef]
- Dan, T.; Connie, T. Sex education and sexual awareness building for autistic children and youth: Some viewpoints and considerations. J. Autism Dev. Disord. 1985, 15, 213–227. [Google Scholar] [CrossRef]
- Rabilloud, G. High-Performance Polymers : Chemistry and Applications; Editions Technip: Paris, France, 1997; ISBN 9782710807209. [Google Scholar]
- Sekiguchi, K.; Takizawa, K.; Ando, S. Thermal expansion behavior of the ordered domain in polyimide films investigated by variable temperature WAXD measurements. J. Photopolym. Sci. Technol. 2013, 26, 327–332. [Google Scholar] [CrossRef]
- Ando, S.; Sekiguchi, K.; Mizoroki, M.; Okada, T.; Ishige, R. Anisotropic linear and volumetric thermal-expansion behaviors of self-standing polyimide films analyzed by thermomechanical analysis (TMA) and optical interferometry. Macromol. Chem. Phys. 2018, 219, 1700354. [Google Scholar] [CrossRef]
- Okada, T.; Ishige, R.; Ando, S. Effects of chain packing and structural isomerism on the anisotropic linear and volumetric thermal expansion behaviors of polyimide films. Polymer 2018, 146, 386–395. [Google Scholar] [CrossRef]
- Hergenrother, P.M.; Connell, J.W.; Smith, J.G. Phenylethynyl containing imide oligomers. Polymer 2000, 41, 5073–5081. [Google Scholar] [CrossRef]
- Sun, H.; Huo, H.; Nie, H.; Yang, S.; Fan, L. Phenylethynyl terminated oligoimides derived from 3,3′,4,4′-diphenylsulfonetetracarboxylic dianhydride and their adhesive properties. Eur. Polym. J. 2009, 45, 1169–1178. [Google Scholar] [CrossRef]
- Meng, X.; Yan, J.; Fan, W.; Liu, J.; Wang, Z.; Li, G. Thermosetting polyimides and composites based on highly soluble phenylethynyl-terminated isoimide oligomers. RSC Adv. 2014, 4, 37458–37469. [Google Scholar] [CrossRef]
- Liou, H.C.; Ho, P.S.; Tung, B. Structure-property correlation for thin films of semi-interpenetrating polyimide networks. I. Miscibility, curing, and morphology studies. J. Appl. Polym. Sci. 1998, 70, 261–272. [Google Scholar] [CrossRef]
- Nakamura, K.; Ando, S.; Takeichi, T. Thermal analysis and solid-state 13C NMR study of crosslink in polyimides containing acetylene groups in the main chain. Polymer 2001, 42, 4045–4054. [Google Scholar] [CrossRef]
- Lin, A.A.; Sastri, V.R.; Tesoro, G.; Reiser, A.; Eachus, R. On the Crosslinking Mechanism of Benzophenone-Containing Polyimides. Macromolecules 1988, 21, 1165–1169. [Google Scholar] [CrossRef]
- Terui, Y.; Ando, S. Coefficients of molecular packing and intrinsic birefringence of aromatic polyimides estimated using refractive indices and molecular polarizabilities. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 2354–2366. [Google Scholar] [CrossRef]
- Hermans, J.J.; Hermans, P.H.; Vermaas, D.; Weidinger, A. Quantitative evaluation of orientation in cellulose fibres from the X-ray fibre diagram. Recueil des Travaux Chimiques des Pays-Bas et de la Belgique 1946, 65, 427–447. [Google Scholar] [CrossRef]
- Terui, Y.; Matsuda, S.I.; Ando, S. Molecular structure and thickness dependence of chain orientation in aromatic polyimide films. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 2109–2120. [Google Scholar] [CrossRef]
- Fang, X.; Hutcheon, R.; Scola, D.A. Study of the kinetics of the microwave cure of a phenylethynyl-terminated imide model compound and imide oligomer (PETI-5). J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2526–2535. [Google Scholar] [CrossRef]
- Okada, T.; Ando, S. Conformational characterization of imide compounds and polyimides using far-infrared spectroscopy and DFT calculations. Polymer 2016, 86, 83–90. [Google Scholar] [CrossRef]
- Terui, Y.; Ando, S. Polarization dependence of thermo-optic coefficients in polyimide films originating from chain orientation and residual thermal stress. J. Appl. Phys. 2014, 116, 053524. [Google Scholar] [CrossRef]
- Ishige, R.; Masuda, T.; Kozaki, Y.; Fujiwara, E.; Okada, T.; Ando, S. Precise analysis of thermal volume expansion of crystal lattice for fully aromatic Crystalline polyimides by X-ray diffraction method: Relationship between molecular structure and linear/volumetric thermal expansion. Macromolecules 2017, 50, 2112–2123. [Google Scholar] [CrossRef]
- Takizawa, K.; Wakita, J.; Sekiguchi, K.; Ando, S. Variations in aggregation structures and fluorescence properties of a semialiphatic fluorinated polyimide induced by very high pressure. Macromolecules 2012, 45, 4764–4771. [Google Scholar] [CrossRef]
- Matsuura, T.; Hasuda, Y.; Nishi, S.; Yamada, N. Polyimide derived from 2,2′-bis(trifluoromethyl)-4,4′-diaminobiphenyl. 1. Synthesis and characterization of polyimides prepared with 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride or pyromellitic dianhydride. Macromolecules 1991, 24, 5001–5005. [Google Scholar] [CrossRef]
- Matsuura, T.; Ishizawa, M.; Hasuda, Y.; Nishi, S. Polyimides derived from 2,2′-bis(trifluoromethyl)-4,4′-diaminobiphenyl. 2. Synthesis and characterization of polyimides prepared from fluorinated benzenetetracarboxylic dianhydrides. Macromolecules 1992, 25, 3540–3545. [Google Scholar] [CrossRef]
- Ishii, J.; Takata, A.; Oami, Y.; Yokota, R.; Vladimirov, L.; Hasegawa, M. Spontaneous molecular orientation of polyimides induced by thermal imidization (6). Mechanism of negative in-plane CTE generation in non-stretched polyimide films. Eur. Polym. J. 2010, 46, 681–693. [Google Scholar] [CrossRef]
- Pottiger, M.T.; Coburn, J.C.; Edman, J.R. The effect of orientation on thermal expansion behavior in polyimide films. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 825–837. [Google Scholar] [CrossRef]











| Polyimide | Diamine | Post baking | Thermal | Final Curing Condition |
|---|---|---|---|---|
| Imidization | ||||
| PPD-300 | PPD | 70 °C ×30 min | 300 °C ×1 h | 300 °C × 1 h |
| PPD-330 | 330 °C × 1 h | |||
| PPD-350 | 350 °C × 1 h | |||
| PPD-380 | 380 °C × 1 h | |||
| PPD-400 | 400 °C × 1 h | |||
| MPD-300 | MPD | 300 °C × 1 h | ||
| MPD-330 | 330 °C × 1 h | |||
| MPD-350 | 350 °C × 1 h | |||
| MPD-380 | 380 °C × 1 h | |||
| MPD-400 | 400 °C × 1 h |
| Polyimide | Final Curing Temperature (°C) | ||||
|---|---|---|---|---|---|
| 300 | 330 | 350 | 380 | 400 | |
| PPD | 0 | 12 | 18 | 21 | 45 |
| MPD | 0 | 20 | 24 | 40 | 42 |
| Polyimide | nav | Δn | P200 | Kp |
|---|---|---|---|---|
| PPD-300 | 1.729 | 0.200 | −0.317 | 0.568 |
| PPD-330 | 1.755 | 0.197 | −0.313 | 0.577 |
| PPD-350 | 1.722 | 0.191 | −0.304 | 0.580 |
| PPD-380 | 1.710 | 0.165 | −0.268 | 0.575 |
| PPD-400 | 1.694 | 0.135 | −0.222 | 0.585 |
| MPD-300 | 1.691 | 0.022 | −0.036 | 0.545 |
| MPD-330 | 1.683 | 0.019 | −0.032 | 0.558 |
| MPD-350 | 1.682 | 0.016 | −0.027 | 0.560 |
| MPD-380 | 1.672 | 0.012 | −0.020 | 0.566 |
| MPD-400 | 1.677 | 0.011 | −0.018 | 0.572 |
| Polyimide | CTE// (ppm/K) | CTE⊥ (ppm/K) | CVE (ppm/K) | η | Tβ (°C) |
|---|---|---|---|---|---|
| PPD-300 | 3 | 144 | 150 | 0.944 | 169 ± 10 |
| PPD-330 | 2 | 144 | 148 | 0.963 | 132 ± 10 |
| PPD-350 | 0 | 144 | 144 | 0.994 | 192 ± 10 |
| PPD-380 | 0 | 144 | 144 | 0.994 | - |
| PPD-400 | 3 | 126 | 132 | 0.939 | - |
| MPD-300 | 41 | 46 | 127 | 0.042 | 162 ± 10 |
| MPD-330 | 43 | 38 | 124 | −0.044 | 169 ± 10 |
| MPD-350 | 38 | 39 | 115 | 0.000 | 132 ± 10 |
| MPD-380 | 34 | 33 | 100 | −0.010 | - |
| MPD-400 | 33 | 32 | 98 | −0.005 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, S.; Harada, M.; Okada, T.; Ishige, R. Effective Reduction of Volumetric Thermal Expansion of Aromatic Polyimide Films by Incorporating Interchain Crosslinking. Polymers 2018, 10, 761. https://doi.org/10.3390/polym10070761
Ando S, Harada M, Okada T, Ishige R. Effective Reduction of Volumetric Thermal Expansion of Aromatic Polyimide Films by Incorporating Interchain Crosslinking. Polymers. 2018; 10(7):761. https://doi.org/10.3390/polym10070761
Chicago/Turabian StyleAndo, Shinji, Mari Harada, Tomohiro Okada, and Ryohei Ishige. 2018. "Effective Reduction of Volumetric Thermal Expansion of Aromatic Polyimide Films by Incorporating Interchain Crosslinking" Polymers 10, no. 7: 761. https://doi.org/10.3390/polym10070761