Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review
Abstract
:1. Introduction
2. Structure of Pectins
2.1. The Pectin Family
2.2. Pectin Sources
2.3. Structure of Pectins Related to Solubility
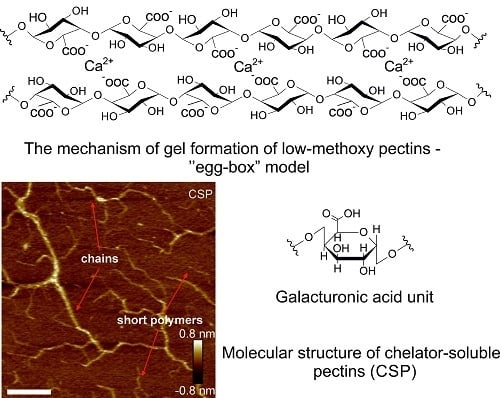
3. Gelling Capacity
4. Crosslinking Agents
5. Interactions of Pectins with Other Natural Compounds
5.1. Cellulose
5.2. Hemicellulose
5.3. Ferulic Acid
5.4. Protein
5.5. Starch
5.6. Chitosan
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Anderson, C.T. Roles of pectin in biomass yield and processing for biofuels. Front. Plant Sci. 2013, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Sila, D.N.; Van Buggenhout, S.; Duvetter, T.; Fraeye, I.; de Roeck, A.; van Loey, A.; Hendrickx, M. Pectins in processed fruits and vegetables: Part II—Structure–function relationships. Compr. Rev. Food Sci. Food Saf. 2009, 8, 86–104. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Bacic, A.; Harris, P.; Stone, B. Structure and function of plant cell walls. In The Biochemistry of Plants: A Comprehensive Treatise; Preiss, J., Ed.; Academic Press: London, UK, 1988; Volume 14, pp. 297–369. ISBN 9780080926155. [Google Scholar]
- Baldwin, L.; Domon, J.-M.; Klimek, J.F.; Fournet, F.; Sellier, H.; Gillet, F.; Pelloux, J.; Lejeune-Hénaut, I.; Carpita, N.C.; Rayon, C. Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 2014, 104, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Schols, H.A.; Voragen, A.G.J. The chemical structure of pectins. In Pectins and Their Manipulation; Seymour, G.B., Knox, J.P., Eds.; Blackwell Publishing: Oxford, UK, 2002; pp. 1–29. ISBN 9781841272283. [Google Scholar]
- Voragen, A.G.J.; Pilnik, W.; Thibault, J.-F.; Axelos, M.A.V.; Renard, C.M.G.C. Pectins. In Food Polysaccharides and Their Applications; Stephen, A.M., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1995; Chapter 10; pp. 287–339. ISBN 9780824793531. [Google Scholar]
- Mierczyńska, J.; Cybulska, J.; Sołowiej, B.; Zdunek, A. Effect of Ca2+, Fe2+ and Mg2+ on rheological properties of new food matrix made of modified cell wall polysaccharides from apple. Carbohydr. Polym. 2015, 133, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, M.; Milala, J.; Kołodziejczyk, K.; Markowski, J.; Zbrzeźniak, M.; Renard, C.M.G.C. Dietary fiber and cell wall polysaccharides from plum (Prunus domestica L.) fruit, juice and pomace: Comparison of composition and functional properties for three plum varieties. Food Res. Int. 2013, 54, 1787–1794. [Google Scholar] [CrossRef]
- Behall, K.; Reiser, S. Effects of pectin on human metabolism. In Chemistry and Function of Pectins; Fishman, M.L., Jen, J.J., Eds.; American Chemical Society: Washington, DC, USA, 1986; pp. 248–265. ISBN 9780841209749. [Google Scholar]
- Kohn, R. Binding of toxic cations to pectin, its oligomeric fragment and plant tissues. Carbohydr. Polym. 1982, 2, 273–275. [Google Scholar] [CrossRef]
- Glinsky, V.V.; Raz, A. Modified citrus pectin anti-metastasic properties: One bullet, multiple targets. Carbohydr. Res. 2009, 344, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Thibault, J.F.; Renard, C.M.G.C.; Axelos, M.A.V.; Roger, P.; Crepeau, M.J. Studies of the length of homogalacturonic regions in pectins by acid hydrolysis. Carbohydr. Res. 1993, 238, 271–286. [Google Scholar] [CrossRef]
- Bociek, S.M.; Welti, D. The quantitative analysis of uronic acid polymers by infrared spectroscopy. Carbohydr. Res. 1975, 42, 217–226. [Google Scholar] [CrossRef]
- Filippov, M.P.; Kohn, R. Determination of the esterification degree of carboxyl groups of pectin with methanol by means of infrared spectroscopy. Chem. Zvesti 1975, 29, 88–91. [Google Scholar]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Baron, A.; Turk, M.; Le Quéré, J.-M. From fruit to fruit juice and fermented products. In Handbook of Food Science and Technology 3: Food Biochemistry and Technology; Jeantet, R., Croguennec, T., Schuck, P., Brulé, G., Eds.; ISTE Ltd.: London, UK, 2016; pp. 231–273. ISBN 9781848219342. [Google Scholar]
- Talmadge, K.W.; Keegstra, K.; Bauer, W.D.; Albersheim, P. The structure of plant cell walls I. The macromolecular components of the walls of suspension-cultured sycamore cells with a detailed analysis of the pectic polysaccharides. Plant Physiol. 1973, 51, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.; Crepeau, M.J.; Thibault, J.F. Structure of the repeating units in the rhamnogalacturonic backbone of apple, beet and citrus pectins. Carbohydr. Res. 1995, 275, 155–165. [Google Scholar] [CrossRef]
- Ngouémazong, D.; Kabuye, G.; Fraeye, I.; Cardinaels, R.; van Loey, A.; Moldenaers, P.; Hendrickx, M. Effect of debranching on the rheological properties of Ca2+-pectin gels. Food Hydrocoll. 2012, 26, 44–53. [Google Scholar] [CrossRef]
- Coenen, G.J.; Bakx, E.J.; Verhoef, R.P.; Schols, H.A.; Voragen, A. Identification of the connecting linkage between homo- or xylogalacturonan and rhamnogalacturonan type I. Carbohydr. Polym. 2007, 70, 224–235. [Google Scholar] [CrossRef]
- Zhan, D.F.; Janssen, P.; Mort, A.J. Scarcity or complete lack of single rhamnose residues interspersed within the homogalacturonan regions of citrus pectin. Carbohydr. Res. 1998, 308, 373–380. [Google Scholar] [CrossRef]
- Heri, W.; Neukom, H.; Deuel, H. Chromatographische fraktionierung von pektinstoffen an diäthylaminoäthyl-cellulose. 15. Mitteilung über ionenaustauscher. Helv. Chim. Acta 1961, 44, 1939–1945. [Google Scholar] [CrossRef]
- Heri, W.; Neukom, H.; Deuel, H. Chromatographie von pektinen mit verschiedener verteilung der methylester-gruppen auf den fadenmolekeln. 16. Mitteilung über ionenaustauscher. Helv. Chim. Acta 1961, 44, 1945–1949. [Google Scholar] [CrossRef]
- Schols, H. Structural Characterization of Pectic Hairy Regions Isolated from Apple Cell Walls. Ph.D. Thesis, Wageningen Agricultural University, Wageningen, The Netherlands, 1995. [Google Scholar]
- O’Neill, M.A.; Warrenfeltz, D.; Kates, K.; Pellerin, P.; Doco, T.; Darvill, A.G.; Albersheim, P. Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. In vitro conditions for the formation and hydrolysis of the dimer. J. Biol. Chem. 1996, 271, 22923–22930. [Google Scholar] [CrossRef] [PubMed]
- Schols, H.A.; Bakx, E.J.; Schipper, D.; Voragen, A.G.J. A xylogalacturonan subunit present in the modified hairy regions of apple pectin. Carbohydr. Res. 1995, 279, 265–279. [Google Scholar] [CrossRef]
- Longland, J.M.; Fry, S.C.; Trewavas, A.J. Developmental control of apiogalacturonan biosynthesis and UDP-apiose production in a duckweed. Plant Physiol. 1989, 90, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.R. Pectic substances, pectic enzymes and haze formation in fruit juices. Enzym. Microb. Technol. 1984, 6, 341–349. [Google Scholar] [CrossRef]
- Albersheim, P.; Darvill, A.G.; O’Neill, M.A.; Schols, H.A.; Voragen, A.G.J. An hypothesis: The same six polysaccharides are components of the primary cell walls of all higher plants. In Pectins and Pectinases; Visser, J., Voragen, A.G.J., Eds.; Elsevier Science, B.V.: Amsterdam, The Netherlands, 1996; pp. 47–55. ISBN 9780444823304. [Google Scholar]
- Jarvis, M.C.; Hall, M.A.; Threlfall, D.R.; Friend, J. The polysaccharide structure of potato cell walls: Chemical fractionation. Planta 1981, 152, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.E.; Anderson, R.L.; Stone, B.A. Form and function of arabinogalactans and arabinogalactan-proteins. Phytochemistry 1979, 18, 521–540. [Google Scholar] [CrossRef]
- Stephen, A.M. Other Plant Polysaccharides. In The Polysaccharides; Aspinall, G.O., Ed.; Academic Press: New York, NY, USA, 1983; Volume 2, pp. 97–193. ISBN 9780120656028. [Google Scholar]
- Leivas, C.L.; Iacomini, M.; Cordeiro, L.M.C. Pectic type II arabinogalactans from starfruit (Averrhoa carambola L.). Food Chem. 2016, 199, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Leclere, L.; van Cutsem, P.; Michiels, C. Anti-cancer activities of pH- or heat-modified pectin. Front. Pharmacol. 2013, 4, 128. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Malviya, R. Sources of pectin, extraction and its applications in pharmaceutical industry—An overview. Indian J. Nat. Prod. Resour. 2011, 2, 10–18. [Google Scholar]
- Kalapathy, U.; Proctor, A. Effect of acid extraction and alcohol precipitation conditions on the yield and purity of soy hull pectin. Food Chem. 2001, 73, 393–396. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Lü, X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Mierczyńska, J.; Cybulska, J.; Zdunek, A. Rheological and chemical properties of pectin enriched fractions from different sources extracted with citric acid. Carbohydr. Polym. 2017, 156, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Mosayebi, V.; Yazdi, F.T. Optimization of microwave assisted extraction (MAE) of pectin from black mulberry (Morus nigra L.) pomace. JFBE 2015, 1, 34–48. [Google Scholar]
- Vriesmann, L.C.; de Oliveira Petkowicz, C.L. Cacao pod husks as a source of low-methoxyl, highly acetylated pectins able to gel in acidic media. Int. J. Biol. Macromol. 2017, 101, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Yuliarti, O.; Goh, K.K.T.; Matia-Merino, L.; Mawson, J.; Brennan, C. Extraction and characterisation of pomace pectin from gold kiwifruit (Actinidia chinensis). Food Chem. 2015, 187, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, B.; Fan, C.; Zhao, K.; Hu, H.; Xu, X.; Pan, S.; Liu, F. Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. Int. J. Biol. Macromol. 2016, 91, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Bingham, R.J.; Oduro, I.N.; Morris, G.A.; Kontogiorgos, V. Pectin isolation and characterization from six okra genotypes. Food Hydrocoll. 2017, 72, 323–330. [Google Scholar] [CrossRef]
- Bayar, N.; Bouallegue, T.; Achour, M.; Kriaa, M.; Bougatef, A.; Kammoun, R. Ultrasonic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal: Optimization of experimental conditions and evaluation of chemical and functional properties. Food Chem. 2017, 235, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Saberian, H.; Hamidi-Esfahani, Z.; Ahmadi Gavlighi, H.; Banakar, A.; Barzegar, M. The potential of ohmic heating for pectin extraction from orange waste. J. Food Process. Preserv. 2018, 42, e13458. [Google Scholar] [CrossRef]
- Kratchanova, M.; Pavlova, E.; Panchev, I. The effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohydr. Polym. 2004, 56, 181–185. [Google Scholar] [CrossRef]
- Chaharbaghi, E.; Khodaiyan, F.; Hosseini, S.S. Optimization of pectin extraction from pistachio green hull as a new source. Carbohydr. Polym. 2017, 173, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.H.F.; Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Moates, G.K.; Wellner, N.; Waldron, K.W.; Azeredo, H.M.C. Pectin extraction from pomegranate peels with citric acid. Int. J. Biol. Macromol. 2016, 88, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Monsoor, M.A. Effect of drying methods on the functional properties of soy hull pectin. Carbohydr. Polym. 2005, 61, 362–367. [Google Scholar] [CrossRef]
- Guo, X.; Meng, H.; Tang, Q.; Pan, R.; Zhu, S.; Yu, S. Effects of the precipitation pH on the ethanolic precipitation of sugar beet pectins. Food Hydrocoll. 2016, 52, 431–437. [Google Scholar] [CrossRef]
- Hua, X.; Wang, K.; Yang, R.; Kang, J.; Zhang, J. Rheological properties of natural low-methoxyl pectin extracted from sunflower head. Food Hydrocoll. 2015, 44, 122–128. [Google Scholar] [CrossRef]
- De Oliveira, C.F.; Giordani, D.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F. Extraction of pectin from passion fruit peel using moderate electric field and conventional heating extraction methods. Innov. Food Sci. Emerg. Technol. 2015, 29, 201–208. [Google Scholar] [CrossRef]
- Christiaens, S.; van Buggenhout, S.; Chaula, D.; Moelants, K.; David, C.C.; Hofkens, J.; van Loey, A.M.; Hendrickx, M.E. In situ pectin engineering as a tool to tailor the consistency and syneresis of carrot purée. Food Chem. 2012, 133, 146–155. [Google Scholar] [CrossRef]
- Mierczyńska, J.; Cybulska, J.; Pieczywek, P.M.; Zdunek, A. Effect of storage on rheology of water-soluble, chelate-soluble and diluted alkali-soluble pectin in carrot cell walls. Food Bioprocess Technol. 2015, 8, 171–180. [Google Scholar] [CrossRef]
- Lin, Z.; Fischer, J.; Wicker, L. Intermolecular binding of blueberry pectin-rich fractions and anthocyanin. Food Chem. 2016, 194, 986–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Amado, R. Changes in the pectic substances of apples during development and postharvest ripening. Part 1: Analysis of alcohol-insoluble residue. Carbohydr. Polym. 1994, 25, 161–166. [Google Scholar] [CrossRef]
- Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Effect of candying on cell wall polysaccharides of plums (Prunus domestica L.) and influence of cell wall enzymes. Food Chem. 2008, 111, 538–548. [Google Scholar] [CrossRef]
- Hilz, H.; Bakx, E.J.; Schols, H.A.; Voragen, A.G.J. Cell wall polysaccharides in black currants and bilberries—Characterisation in berries, juice, and press cake. Carbohydr. Polym. 2005, 59, 477–488. [Google Scholar] [CrossRef]
- Pals, D.T.F.; Hermans, J.J. Sodium salts of pectin and of carboxy methyl cellulose in aqueous sodium chloride. I. Viscosities. Recl. Trav. Chim. Pays-Bas 1952, 71, 433–457. [Google Scholar] [CrossRef]
- Albersheim, P.; Neukom, H.; Deuel, H. Splitting of pectin chain molecules in neutral solutions. Arch. Biochem. Biophys. 1960, 90, 46–51. [Google Scholar] [CrossRef]
- Fishman, M.L.; Pfeffer, P.E.; Barford, R.A.; Doner, L.W. Studies of pectin solution properties by high-performance size exclusion chromatrography. J. Agric. Food Chem. 1984, 32, 372–378. [Google Scholar] [CrossRef]
- Corredig, M.; Kerr, W.; Wicker, L. Molecular characterization of commercial pectins by separation with linear mix gel permeation columns in-line with multi-angle light scattering detection. Food Hydrocoll. 2000, 14, 41–47. [Google Scholar] [CrossRef]
- Yang, J.-S.; Mu, T.-H.; Ma, M.-M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Ponmurugan, K.; Al-Dhabi, N.A.; Maran, J.P.; Karthikeyan, K.; Moothy, I.G.; Sivarajasekar, N.; Manoj, J.J.B. Ultrasound assisted pectic polysaccharide extraction and its characterization from waste heads of Helianthus annus. Carbohydr. Polym. 2017, 173, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Aguilar, A.L.; Victoria-Campos, C.I.; Ochoa-Reyes, E.; de Jesús Ornelas-Paz, J.; Zamudio-Flores, P.B.; Rios-Velasco, C.; Reyes-Hernández, J.; Pérez-Martínez, J.D.; Ibarra-Junquera, V. Physicochemical properties of apple juice during sequential steps of the industrial processing and functional properties of pectin fractions from the generated pomace. LWT Food Sci. Technol. 2017, 86, 465–472. [Google Scholar] [CrossRef]
- Cybulska, J.; Zdunek, A.; Kozioł, A. The self-assembled network and physiological degradation of pectins in carrot cell walls. Food Hydrocoll. 2015, 43, 41–50. [Google Scholar] [CrossRef]
- Zdunek, A.; Kozioł, A.; Pieczywek, P.M.; Cybulska, J. Evaluation of the nanostructure of pectin, hemicellulose and cellulose in the cell walls of pears of different texture and firmness. Food Bioprocess Technol. 2014, 7, 3525–3535. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Yang, H.; Ye, X.; Sun, X.; Liu, D.; Yang, B.; An, H.; Deng, Y. Effects of temperature and cultivar on nanostructural changes of water-soluble pectin and chelate-soluble pectin in peaches. Carbohydr. Polym. 2012, 87, 816–821. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Barros, A.; Barros, M.; Rutledge, D.N.; Delgadillo, I. Multivariate analysis of uronic acid and neutral sugars in whole pectic samples by FT-IR spectroscopy. Carbohydr. Polym. 1998, 37, 241–248. [Google Scholar] [CrossRef]
- Kirby, A.R.; MacDougall, A.J.; Morris, V.J. Atomic force microscopy of tomato and sugar beet pectin molecules. Carbohydr. Polym. 2008, 71, 640–647. [Google Scholar] [CrossRef]
- Posé, S.; Kirby, A.R.; Mercado, J.A.; Morris, V.J.; Quesada, M.A. Structural characterization of cell wall pectin fractions in ripe strawberry fruits using AFM. Carbohydr. Polym. 2012, 88, 882–890. [Google Scholar] [CrossRef]
- Kacuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Sriamornsak, P. Chemistry of pectin and its pharmaceutical uses: A review. SUIJ 2003, 3, 206–228. [Google Scholar]
- Thibault, J.-F.; Ralet, M.-C. Physico-chemical properties of pectins in the cell walls and after extraction. In Advances in Pectin and Pectinase Research; Voragen, F., Schols, H., Visser, R., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 91–105. ISBN 9781402011443. [Google Scholar]
- Keijbets, M.J.H.; Pilnik, W. β-Elimination of pectin in the presence of anions and cations. Carbohydr. Res. 1974, 33, 359–362. [Google Scholar] [CrossRef]
- Diaz, J.V.; Anthon, G.E.; Barrett, D.M. Nonenzymatic degradation of citrus pectin and pectate during prolonged heating: Effects of pH, temperature, and degree of methyl esterification. J. Agric. Food Chem. 2007, 55, 5131–5136. [Google Scholar] [CrossRef] [PubMed]
- Sila, D.N.; Smout, C.; Elliot, F.; van Loey, A.; Hendrickx, M. Non-enzymatic depolymerisation of carrots pectin: Towards a better understanding of carrot texture during thermal processing. J. Food Sci. 2006, 71, E1–E9. [Google Scholar] [CrossRef]
- Kar, F.; Arslan, N. Effect of temperature and concentration on viscosity of orange peel pectin solutions and intrinsic viscosity–molecular weight relationship. Carbohydr. Polym. 1999, 40, 277–284. [Google Scholar] [CrossRef]
- Graessley, W.W. Polymer chain dimensions and the dependence of viscoelastic properties on concentration, molecular weight and solvent power. Polymer 1980, 21, 258–262. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H. Polymer Handbook, 2nd ed.; Wiley: New York, NY, USA, 1975; ISBN 9780471098041. [Google Scholar]
- Liang, R.-H.; Wang, L.-H.; Chen, J.; Liu, W.; Liu, C.-M. Alkylated pectin: Synthesis, characterization, viscosity and emulsifying properties. Food Hydrocoll. 2015, 50, 65–73. [Google Scholar] [CrossRef]
- BeMiller, J.N. An introduction to pectins: Structure and properties. In Chemistry and Function of Pectins; Fishman, M.L., Jen, J.J., Eds.; American Chemical Society: Washigton, DC, USA, 1986; pp. 2–21. ISBN 9780841209749. [Google Scholar]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Kastner, H.; Einhorn-Stoll, U.; Senge, B. Structure formation in sugar containing pectin gels—Influence of Ca2+ on the gelation of low-methoxylated pectin at acidic pH. Food Hydrocoll. 2012, 27, 42–49. [Google Scholar] [CrossRef]
- Van Buren, J.P. Function of pectin in plant tissue structure and firmness. In The Chemistry and Technology of Pectin; Walter, R.H., Ed.; Academic Press: New York, NY, USA, 1991; pp. 1–23. ISBN 9780127338705. [Google Scholar]
- Axelos, M.A.V.; Thibault, J.-F. The chemistry of low-methoxyl pectin gelation. In The Chemistry and Technology of Pectin; Walter, R.H., Ed.; Academic Press: New York, NY, USA, 1991; pp. 109–117. ISBN 9780127338705. [Google Scholar]
- Yang, X.; Nisar, T.; Liang, D.; Hou, Y.; Sun, L.; Guo, Y. Low methoxyl pectin gelation under alkaline conditions and its rheological properties: Using NaOH as a pH regulator. Food Hydrocoll. 2018, 79, 560–571. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Morris, E.R.; Rees, D.A.; Thom, D.; Boyd, J. Chiroptical and stoichiometric evidence of a specific, primary dimerisation process in alginate gelation. Carbohydr. Res. 1978, 66, 145–154. [Google Scholar] [CrossRef]
- Morris, E.R.; Powell, M.J.; Gidley, M.J.; Rees, D.A. Conformations and interactions of pectins I. Polymorphism between gel and solid states of calcium polygalacturonate. J. Mol. Biol. 1982, 155, 507–516. [Google Scholar] [CrossRef]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A. Binding behavior of calcium to polyuronates: Comparison of pectin with alginate. Carbohydr. Polym. 2008, 72, 334–341. [Google Scholar] [CrossRef]
- Braccini, I.; Perez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Ngouémazong, D.E.; Tengweh, F.F.; Fraeye, I.; Duvetter, T.; Cardinaels, R.; van Loey, A.; Moldenaers, P.; Hendrickx, M. Effect of de-methylesterification on network development and nature of Ca2+-pectin gels: Towards understanding structure-function relations of pectin. Food Hydrocoll. 2012, 26, 89–98. [Google Scholar] [CrossRef]
- Wellner, N.; Kačuráková, M.; Malovíková, A.; Wilson, R.H.; Belton, P.S. FT-IR study of pectate and pectinate gels formed by divalent cations. Carbohydr. Res. 1998, 308, 123–131. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Coimbra, M.A.; Lopes da Silva, J.A. Temperature dependence of the formation and melting of pectin–Ca2+ networks: A rheological study. Food Hydrocoll. 2003, 17, 801–807. [Google Scholar] [CrossRef]
- Kirtil, E.; Oztop, M.H.; Sirijariyawat, A.; Ngamchuachit, P.; Barrett, D.M.; McCarthy, M.J. Effect of pectin methyl esterase (PME) and CaCl2 infusion on the cell integrity of fresh-cut and frozen-thawed mangoes: An NMR relaxometry study. Food Res. Int. 2014, 66, 409–416. [Google Scholar] [CrossRef]
- Racape, E.; Thibault, J.-F.; Reitsma, J.C.E.; Pilnik, W. Properties of amidated pectins. II. Polyelectrolyte behavior and calcium binding of amidated pectins and amidated pectic acids. Biopolymers 1989, 28, 1435–1448. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.J.; Gromer, A.; Kirby, A.R.; Bongaerts, R.J.M.; Gunning, A.P. Using AFM and force spectroscopy to determine pectin structure and (bio) functionality. Food Hydrocoll. 2011, 25, 230–237. [Google Scholar] [CrossRef]
- Kim, Y.; Wicker, L. Valencia PME isozymes create charge modified pectins with distinct calcium sensitivity and rheological properties. Food Hydrocoll. 2009, 23, 957–963. [Google Scholar] [CrossRef]
- Gilsenan, P.M.; Richardson, R.K.; Morris, E.R. Thermally reversible acid-induced gelation of low-methoxy pectin. Carbohydr. Polym. 2000, 41, 339–349. [Google Scholar] [CrossRef]
- Walkinshaw, M.D.; Arnott, S. Conformations and interactions of pectins. I. X-ray diffraction analyses of sodium pectate in neutral and acidified forms. J. Mol. Biol. 1981, 153, 1055–1073. [Google Scholar] [CrossRef]
- Jarvis, M.C.; Apperley, D.C. Chain conformation in concentrated pectic gels: Evidence from 13C NMR. Carbohydr. Res. 1995, 275, 131–145. [Google Scholar] [CrossRef]
- Cybulska, J.; Pieczywek, P.M.; Zdunek, A. The effect of Ca2+ and cellular structure on apple firmness and acoustic emission. Eur. Food Res. Technol. 2012, 235, 119–128. [Google Scholar] [CrossRef]
- Celus, M.; Kyomugasho, C.; Kermani, Z.J.; Roggen, K.; van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Fe2+ adsorption on citrus pectin is influenced by the degree and pattern of methylesterification. Food Hydrocoll. 2017, 73, 101–109. [Google Scholar] [CrossRef]
- Debon, S.J.J.; Tester, R.F. In vitro binding of calcium, iron and zinc by non-starch polysaccharides. Food Chem. 2001, 73, 401–410. [Google Scholar] [CrossRef]
- Huynh, U.T.D.; Lerbret, A.; Neiers, F.; Chambin, O.; Assifaoui, A. Binding of divalent cations to polygalacturonate: A mechanism driven by the hydration water. J. Phys. Chem. B 2016, 120, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U. Studies of the binding of copper, zinc and calcium to pectin, alginate, carrageenan and gum guar in HCO3–CO2 buffer. Food Chem. 1989, 32, 223–234. [Google Scholar] [CrossRef]
- Park, Y.B.; Cosgrove, D.J. Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis. Plant Physiol. 2012, 158, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, J.; Vanstreels, E.; Ho, Q.T.; Courtin, C.M.; van Craeyveld, V.; Nicolaï, B.; Zdunek, A.; Konstankiewicz, K. Mechanical characteristics of artificial cell walls. J. Food Eng. 2010, 96, 287–294. [Google Scholar] [CrossRef]
- Lopez-Sanchez, P.; Martinez-Sanz, M.; Bonilla, M.R.; Wang, D.; Gilbert, E.P.; Stokes, J.R.; Gidley, M.J. Cellulose-pectin composite hydrogels: Intermolecular interactions and material properties depend on order of assembly. Carbohydr. Polym. 2017, 162, 71–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zykwinska, A.W.; Ralet, M.-C.J.; Garnier, C.D.; Thibault, J.-F.J. Evidence for in vitro binding of pectin side chains to cellulose. Plant Physiol. 2005, 139, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, R.; Lutz, M.V.; Amado, R. Pectic substances isolated from apple cellulosic residue: Structural characterisation of a new type of rhamnogalacturonan I. Carbohydr. Polym. 2003, 51, 301–310. [Google Scholar] [CrossRef]
- Agoda-Tandjawa, G.; Durand, S.; Gaillard, C.; Garnier, C.; Doublier, J.L. Properties of cellulose/pectins composites: Implication for structural and mechanical properties of cell wall. Carbohydr. Polym. 2012, 90, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Vincken, J.P.; Keizer, A.; Beldman, G.; Voragen, A.G.J. Fractionation of xyloglucan fragments and their interaction with cellulose. Plant Physiol. 1995, 108, 1579–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keegstra, K.; Talmadge, K.W.; Bauer, W.D.; Albersheim, P. The structure of plant cell walls: III. A model of the walls of suspension-cultured sycamore cells based on the interconnections of the macromolecular components. Plant Physiol. 1973, 51, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Darvill, J.E.; McNeil, M.; Darvill, A.G.; Albersheim, P. Structure of plant cell walls. XI. Glucuronoarabinoxylan, a second hemicellulose in the primary cell walls of suspension-cultured sycamore cells. Plant Physiol. 1980, 66, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.E.; Fry, S.C. Evidence for covalent linkage between xyloglucan and acidic pectins in suspension-cultured rose cells. Planta 2000, 211, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Massih, R.M.; Baydoun, E.A.-H.; Brett, C.T. In vitro biosynthesis of 1.4-β-galactan attached to a pectin–xyloglucan complex in pea. Planta 2003, 216, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Popper, Z.A.; Fry, S.C. Widespread occurrence of a covalent linkage between xyloglucan and acidic polysaccharides in suspension-cultured angiosperm cells. Ann. Bot. 2005, 96, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Eberhard, S.; Pattathil, S.; Warder, C.; Glushka, J.; Yuan, C.; Hao, Z.; Zhu, X.; Avci, U.; Miller, J.S.; et al. An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 2013, 25, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Ralet, M.-C.; André-Leroux, G.; Quéméner, B.; Thibault, J.-F. Sugar beet (Beta vulgaris) pectins are covalently cross-linked through diferulic bridges in the cell wall. Phytochemistry 2005, 66, 2800–2814. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, I.; Ralet, M.-C.; Thibault, J.-F.; Faulds, C.B.; Williamson, G. Structure identification of feruloylated oligosaccharides from sugar-beet pulp by NMR spectroscopy. Carbohydr. Res. 1994, 263, 243–256. [Google Scholar] [CrossRef]
- Levigne, S.V.; Ralet, M.-C.J.; Quéméner, B.C.; Pollet, B.M.-L.; Lapierre, C.; Thibault, J.-F.J. Isolation from sugar beet cell walls of arabinan oligosaccharides esterified by two ferulic acid monomers. Plant Physiol. 2004, 134, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Oosterveld, A.; Grabber, J.H.; Beldman, G.; Ralph, J.; Voragen, A.G.J. Formation of ferulic acid dehydrodimers through oxidative cross-linking of sugar beet pectin. Carbohydr. Res. 1997, 300, 179–181. [Google Scholar] [CrossRef]
- Waldron, K.W.; Ng, A.; Parker, M.L.; Parr, A.J. Ferulic acid dehydrodimers in the cell walls of Beta vulgaris and their possible role in texture. J. Sci. Food Agric. 1997, 74, 221–228. [Google Scholar] [CrossRef]
- Chen, H.-M.; Fu, X.; Luo, Z.-G. Effect of molecular structure on emulsifying properties of sugar beet pulp pectin. Food Hydrocoll. 2016, 54, 99–106. [Google Scholar] [CrossRef]
- Williams, P.A.; Sayers, C.; Viebke, C.; Senan, C.; Mazoyer, J.; Boulenguer, P. Elucidation of the emulsification properties of sugar beet pectin. J. Agric. Food Chem. 2005, 53, 3592–3597. [Google Scholar] [CrossRef] [PubMed]
- Funami, T.; Zhang, G.; Hiroe, M.; Noda, S.; Nakauma, M.; Asai, I.; Cowman, M.K.; Al-Assaf, S.; Phillips, G.O. Effects of the proteinaceous moiety on the emulsifying properties of sugar beet pectin. Food Hydrocoll. 2007, 21, 1319–1329. [Google Scholar] [CrossRef]
- Nakamura, A.; Yoshida, R.; Maeda, H.; Furuta, H.; Corredig, M. Study of the role of the carbohydrate and protein moieties of soy soluble polysaccharides in their emulsifying properties. J. Agric. Food Chem. 2004, 52, 5506–5512. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qiu, S.; Gan, J.; Liu, Y.; Zhu, Q.; Yin, L. New insights into the functionality of protein to the emulsifying properties of sugar beet pectin. Food Hydrocoll. 2016, 57, 262–270. [Google Scholar] [CrossRef]
- Tamnak, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Muhammad, K. Physicochemical properties, rheological behavior and morphology of pectin-pea protein isolate mixtures and conjugates in aqueous system and oil in water emulsion. Food Hydrocoll. 2016, 56, 405–416. [Google Scholar] [CrossRef]
- Li, W.; Zhao, H.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Modification of soy protein hydrolysates by Maillard reaction: Effects of carbohydrate chain length on structural and interfacial properties. Colloids Surf. B 2016, 138, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.M.; Melton, L.D.; Stanley, R.A. Creating proteins with a novel functionality via the Maillard Reaction: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakkak, J.; Al-Hakkak, F. Functional egg white-pectin conjugates prepared by controlled Maillard reaction. J. Food Eng. 2010, 100, 152–159. [Google Scholar] [CrossRef]
- Turgeon, S.L.; Laneuville, S.I. Protein + polysaccharide coacervates and complexes: From scientific background to their application as functional ingredients in food products. In Modern Biopolymer Science: Bridging the Divide between Fundamental Treatise and Industrial Application; Kasapis, S., Norton, I.T., Ubbink, J.B., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 327–363. ISBN 9780123741950. [Google Scholar]
- Parker, A.; Boulenguer, P.; Kravtchenko, T.P. Effect of the addition of high methoxy pectin on the rheology and colloidal stability of acid milk drinks. In Food Hydrocolloids: Structures, Properties and Functions; Nishinari, K., Doi, E., Eds.; Springer: New York, NY, USA, 1994; pp. 307–312. ISBN 9780306445941. [Google Scholar]
- Tromp, R.H.; de Kruif, C.G.; van Eijk, M.; Rolin, C. On the mechanism of stabilisation of acidified milk drinks by pectin. Food Hydrocoll. 2004, 18, 565–572. [Google Scholar] [CrossRef]
- Gałkowska, D.; Długosz, M.; Juszczak, L. Effect of high-methoxy pectin and sucrose on pasting, rheological, and textural properties of modified starch systems. Starch/Stärke 2013, 65, 499–508. [Google Scholar] [CrossRef]
- Agudelo, A.; Varela, P.; Sanz, T.; Fiszman, S.M. Native tapioca starch as a potential thickener for fruit fillings. Evaluation of mixed models containing low-methoxyl pectin. Food Hydrocoll. 2014, 35, 297–304. [Google Scholar] [CrossRef]
- Agudelo, A.; Varela, P.; Sanz, T.; Fiszman, S. Formulating fruit fillings. Freezing and baking stability of a tapioca starch—Pectin mixture model. Food Hydrocoll. 2014, 40, 203–213. [Google Scholar] [CrossRef]
- Witczak, T.; Witczak, M.; Ziobro, R. Effect of inulin and pectin on rheological and thermal properties of potato starch paste and gel. J. Food Eng. 2014, 124, 72–79. [Google Scholar] [CrossRef]
- Maciel, V.B.V.; Yoshida, C.M.P.; Franco, T.T. Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr. Polym. 2015, 132, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Rashidova, S.S.; Milusheva, R.Y.; Semenova, L.N.; Mukhamedjanova, M.Y.; Voropaeva, N.L.; Vasilyeva, S.; Faizieva, R.; Ruban, I.N. Characteristics of interactions in the pectin-chitosan system. Chromatographia 2004, 59, 779–782. [Google Scholar] [CrossRef]
- Recillas, M.; Silva, L.L.; Peniche, P.; Goycoolea, F.M.; Rinaudo, M.; Román, J.S.; Argüelles-Monal, W.M. Thermo- and pH-responsive polyelectrolyte complex membranes from chitosan-g-N-isopropylacrylamide and pectin. Carbohydr. Polym. 2011, 86, 1336–1343. [Google Scholar] [CrossRef]
- Rolin, C. Commercial pectin preparations. In Pectins and Their Manipulation; Seymour, G.B., Knox, J.P., Eds.; Blackwell Publishing: Oxford, UK, 2002; pp. 222–241. ISBN 9781841272283. [Google Scholar]
- Vårum, K.M.; Smidsrød, O. Structure–property relationship in chitosans. In Polysaccharides: Structural Diversity and Functional Versatility; Dumitriu, S., Ed.; Taylor & Francis, Inc.: Boca Raton, FL, USA, 2004; pp. 625–642. ISBN 9780824754808. [Google Scholar]
- Coimbra, P.; Ferreira, P.; Sousa, H.C.; Batista, P.; Rodrigues, M.A.; Correia, I.J.; Gil, M.H. Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int. J. Biol. Macromol. 2011, 48, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Kuo, T.-Y.; Kuo, J.-Y.; Tseng, Y.-P.; Wang, D.-M.; Lai, J.-Y.; Hsieh, H.-J. Novel chitosan–pectin composite membranes with enhanced strength, hydrophilicity and controllable disintegration. Carbohydr. Polym. 2010, 82, 1236–1242. [Google Scholar] [CrossRef]
- Richert, L.; Boulmedais, F.; Lavalle, P.; Mutterer, J.; Ferreux, E.; Decher, G.; Schaaf, P.; Voegel, J.C.; Picart, C. Improvement of stability and cell adhesion properties of polyelectrolyte multilayer films by chemical cross-linking. Biomacromolecules 2004, 5, 284–294. [Google Scholar] [CrossRef] [PubMed]



| Pectin Source | Galacturonic Acid Content (%) | Yield (%) | Extraction Method | References |
|---|---|---|---|---|
| Apple pomace | ~21–44 | ~10–17 | subcritical water (t = 5 min, T = 130–170 °C, solid/liquid ratio 1:30) | [40] |
| Black currant | 37.1 | - | citric acid (t = 30 min, T = 90 °C, pH = 2.5, solid/liquid ratio 1:50) | [41] |
| Black mulberry pomace | ~29–43 | ~9–14 | hydrochloric acid, microwave-assisted extraction (irradiation time 10–30 min, pH = 2, power 300–900 W, solid/liquid ratio between 1:15 and 1:30) | [42] |
| Cacao pod husks | ~60 (of total sugar content) | ~11 | nitric acid (t = 30 min, T = 100 °C, pH = 3.5) | [43] |
| Carrot | 16.5 | - | citric acid (t = 30 min, T = 90 °C, pH = 2.5, solid/liquid ratio 1:50) | [41] |
| Gold kiwifruit pomace | ~82–85 (of total non-starch polysaccharides) | ~4 | citric acid (t = 1 h, T = 50 °C, pH = 2.2, pomace/acid solution ratio 1:3 w/v), water (t = 30 min, T = 5 °C, pomace/water ratio 1:3 w/v) and enzymatic extraction (t = 30 min, T = 25 °C, enzyme: Celluclast 1.5 L) | [44] |
| Mango peel | ~29–35 (T = 20 °C) ~52–53 (T = 80 °C) | ~2 ~17 | citric acid, conventional extraction (t = 2 h, pH = 2.5, solid/liquid ratio 1:40) and ultrasound-assisted extraction (t = 15 min, pH = 2.5, solid/liquid ratio 1:40) | [45] |
| Okra (Abelmoschus esculentus L.) | ~43–63 | 11–15 | aqueous extraction, phosphate buffer (t = 1 h, T = 80 °C, pH = 6.0, solid/liquid ratio 1:15) | [46] |
| Opuntia ficus indica cladodes | ~69 | ~19 | acidified water, ultrasound-assisted extraction (t = 70 min, T = 70 °C, pH = 1.5, solid/liquid ratio 1:30) | [47] |
| Orange juice wastes | ~46–74 | ~1–11 | hydrochloric acid, ohmic extraction (T = up to 90 °C, pH = 1.5–4, voltage gradient 5–30 V/cm, solid/liquid ratio between 1:10 and 1:40) | [48] |
| Orange peel | ~66–70 | ~14–18 | hydrochloric acid (t = 1 h, T = 80–82 °C, pH = 1.5, solid/liquid ratio 1:50), microwave heating (t = 5–15 min, power 0.45–0.9 kW) | [49] |
| Peach | 26 | - | citric acid (t = 30 min, T = 90 °C, pH = 2.5, solid/liquid ratio 1:50) | [41] |
| Pistachio green hull | ~65 | ~22 | citric acid (t = 30 min, T = 90 °C, pH = 0.5, solid/liquid ratio 1:50) | [50] |
| Plum | 23.8 | - | citric acid (t = 30 min, T = 90 °C, pH = 2.5, solid/liquid ratio 1:50) | [41] |
| Pomegranate peel | ~70–82 | ~3–9 | citric acid (t = 40–150 min, T = 70–90 °C, pH = 2–4) | [51] |
| Raspberry | 23.1 | - | citric acid (t = 30 min, T = 90 °C, pH = 2.5, solid/liquid ratio 1:50) | [41] |
| Soy hull | ~67–69 | ~16–21 | hydrochloric acid (t = 1 h, T = 90 °C, solid/liquid ratio 1:10) | [52] |
| Strawberry | 33.9 | - | citric acid (t = 30 min, T = 90 °C, pH = 2.5, solid/liquid ratio 1:50) | [41] |
| Sugar beet pulp | ~54–64 | ~1–9 | hydrochloric acid (t = 1 h, T = 80 °C, pH = 1.5, solid/liquid ratio 1:20) | [53] |
| Sunflower head | 86 | - | ammonium oxalate (t = 45 min, T = 85 °C) | [54] |
| ~50 of total sugar content | ~9 | citric acid, ultrasound-assisted extraction (t = 32 min, pH = 3.2, solid/liquid ratio 1:15) | [55] | |
| Yellow passion fruit peel | ~48–72 | ~4–8 | nitric acid, moderate electric field extraction (t = 5–60 min, pH = 1–5, voltage 30–100 V, solid/liquid ratio 1:30) | [56] |
| Components | Interaction Mechanism | The Main Properties of the Complex | References |
|---|---|---|---|
| Avicel cellulose | By pectin side chains—arabinans and galactans; possible formation of hydrogen bonds | Low reversibility of complex | [116] |
| Sugar-beet microfibrillated cellulose | By pectin side chains | Enhancement of viscoelastic properties of cellulose suspension | [118] |
| Ferulic acid/protein | Covalently linked to pectin side chains, mainly to arabinose and galactose residues (in sugar beet cell wall) | Improvement in emulsifying ability and stability, surface activity of sugar beet pectins | [131] |
| Protein | Maillard reaction: carbonyl group of a reducing sugar residue of pectin reacting with an amino group of protein | Changes in solubility; amphiphilic character; high molecular weight; better emulsification properties | [136] |
| Starch | possible enhancement of pectin network through ionic interactions | Increase in the viscoelasticity, values of starch pasting parameters and extrusion parameters | [144,145] |
| Chitosan | Formation of a polyelectrolyte complex: electrostatic interaction between oppositely charged groups (pectin: COO−, chitosan: NH3+); other possible interactions: hydrogen bonds, coordinate bonds, van der Waals interactions and hydrophobic forces | Homogeneous PEC films; degradation of PEC films at lower temperature than decomposition of chitosan (thermogravimetric analysis) | [147,148] |
| Chitosan + calcium ions or NHS/EDC | Calcium ions as crosslinking agents of pectins; NHS/EDC: formation of covalent bonds between pectin and chitosan | Higher tensile strength of membranes and lower water uptake ability | [153] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. https://doi.org/10.3390/polym10070762
Gawkowska D, Cybulska J, Zdunek A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers. 2018; 10(7):762. https://doi.org/10.3390/polym10070762
Chicago/Turabian StyleGawkowska, Diana, Justyna Cybulska, and Artur Zdunek. 2018. "Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review" Polymers 10, no. 7: 762. https://doi.org/10.3390/polym10070762