Understanding the Intrinsic Carrier Transport in Highly Oriented Poly(3-hexylthiophene): Effect of Side Chain Regioregularity
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Raw Materials
2.2. Preparation of Oriented ra-P3HT-TCB and rg-P3HT-TCB by TCB Epitaxy
2.3. Characterization of Microstructure and Electrical Transport Properties
2.4. Molecular Dynamics Simulation
3. Results and Discussion
3.1. Molecular Structure of P3HT with Different Regioregularity
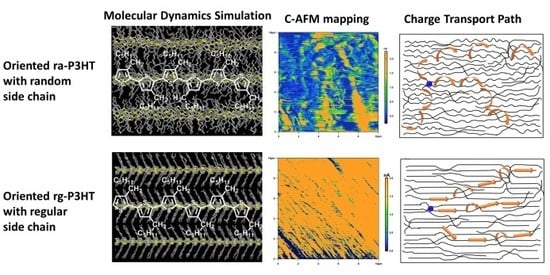
3.2. Molecular Dynamics Simulation of Close-Packing Structures of rg- and ra-P3HT
3.3. Direct Mapping of Carrier Transport Path
3.4. Carrier Transport Properties of ra-P3HT-TCB and rg-P3HT-TCB Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bubnova, O.; Khan, Z.U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, Y.M.; Xu, W. Thermoelectric energy from flexible P3HT films doped with a ferric salt of triflimide anions. Energy Environ. Sci. 2012, 5, 9639–9644. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, L.D.; Zhang, W.Q.; Liufu, S.C.; Chen, X.H. Enhanced thermoelectric performance of single-walled carbon nanotubes/polyaniline hybrid nanocomposites. ACS Nano 2010, 4, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.K.; Druy, M.A.; Gau, S.C.; Heeger, A.J.; Louis, E.J.; MacDiarmid, A.G.; Park, Y.W.; Shirakawa, H. Synthesis of highly conducting films of derivatives of polyacetylene, (CH)x. J. Am. Chem. Soc. 1978, 100, 1013–1015. [Google Scholar] [CrossRef]
- Bredas, J.L.; Street, G.B. Polarons, bipolarons, and solitons in conducting polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Kang, S.D.; Snyder, G.J. Charge-transport model for conducting polymers. Nat. Mater. 2016, 16, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wang, Q.; Wang, L. Abnormally enhanced thermoelectric transport properties of SWNT/PANI hybrid films by the strengthened PANI molecular ordering. Energy Environ. Sci. 2014, 7, 3801–3807. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, F.J.; Di, C.A.; Yan, T.W.; Zhu, D.B.; Pei, J. Toward high performance n-type thermoelectric materials by rational modification of BDPPV backbones. J. Am. Chem. Soc. 2015, 137, 6979–6982. [Google Scholar] [CrossRef] [PubMed]
- Levesque, I.; Bertrand, P.; Blouin, N.; Leclerc, M.; Zecchin, S. Synthesis and thermoelectric properties of polycarbazole, polyindolocarbazole, and polydiindolocarbazole derivatives. Chem. Mater. 2007, 19, 2128–2138. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.N.; Loos, J.; Veenstra, S.C.; Verhees, W.J.H.; Wienk, M.M.; Kroon, J.M.; Michels, M.A.; Janssen, R.A. Nanoscale morphology of high-performance polymer solar cells. Nano Lett. 2005, 5, 579–583. [Google Scholar] [CrossRef] [PubMed]
- He, Y.J.; Chen, H.-Y.; Hou, J.H.; Li, Y.F. Indene-C(60) bisadduct: A new acceptor for high-performance polymer solar cells. J. Am. Chem. Soc. 2010, 132, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Lee, J.; Klenhenz, N.; Fu, B.Y.; Reichmanis, E. Photoinduced anisotropic supramolecular assembly and enhanced charge transport of poly(3-hexylthiophene) thin films. Adv. Funct. Mater. 2014, 24, 4457–4465. [Google Scholar] [CrossRef]
- Chen, T.-A.; Wu, X.M.; Rieke, R.D. regiocontrolled synthesis of poly(3-alkylthiophenes) mediated by rieke zinc: Their characterization and solid-state. J. Am. Chem. Soc. 1995, 117, 233–244. [Google Scholar] [CrossRef]
- Joseph, R.-H.; Franziska, F.; Ashlee, A.J.; Natalie, S.; Dwight, S.S.; Ji-Seon, K. Effects of side-chain length and shape on polytellurophene molecular order and blend morphology. J. Phys. Chem. C 2017, 121, 2088–2098. [Google Scholar]
- Kim, Y.; Cook, S.; Tuladhar, S.M.; Choulis, S.A.; Nelson, J.; Durrant, J.R.; Bradley, D.D.C.; Giles, M.; Mcculloch, I.; Ha, C.-S.; et al. A strong regioregularity effect in self-organizing conjugated polymer films and high-efficiency polythiophene: Fullerene solar cells. Nat. Mater. 2006, 5, 197–203. [Google Scholar] [CrossRef]
- Bounioux, C.; Diaz-Chao, P.; Campoy-Quiles, M.; Martin-Gonzalez, M.S.; Goni, A.R.; Yerushalmi-Rozen, R.; Muller, C. Thermoelectric composites of poly(3-hexylthiophene) and carbon nanotubes with a large power factor. Energy Environ. Sci. 2013, 6, 918–925. [Google Scholar] [CrossRef]
- Rivnay, J.; Jimison, L.H.; Northrup, J.E.; Toney, M.F.; Noriega, R.; Lu, S.F.; Marks, T.J.; Facchetti, A.; Salleo, A. A large modulation of carrier transport by grain-boundary molecular packing and microstructure in organic thin films. Nat. Mater. 2009, 8, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Aiyar, A.R.; Hong, J.I.; Reichmanis, E. Regioregularity and intrachain ordering: impact on the nanostructure and charge transport in two-dimensional assemblies of poly(3-hexylthiophene). Chem. Mater. 2012, 24, 2845–2853. [Google Scholar] [CrossRef]
- Qu, S.Y.; Yao, Q.; Wang, L.M.; Chen, Z.H.; Xu, K.Q.; Zeng, H.R.; Shi, W.; Zhang, T.S.; Uher, C.; Chen, L.D. Highly anisotropic P3HT films with enhanced thermoelectric performance via organic small molecule epitaxy. NPG Asia Mater. 2016, 8, e292. [Google Scholar] [CrossRef]
- Martin, B.; Jean-Claude, W. Orientation of regioregular poly(3-hexylthiophene) by directional solidification: A simple method to reveal the semicrystalline structure of a conjugated polymer. Adv. Mater. 2006, 18, 860–863. [Google Scholar]
- Bhatta, R.S.; Yimer, Y.Y.; Perry, D.S.; Tsige, M. Improved force field for molecular modeling of poly(3-hexylthiophene). J. Phys. Chem. B 2013, 117, 10035–10045. [Google Scholar] [CrossRef] [PubMed]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Prosa, T.J.; Winokur, M.J.; Moulton, J.; Smith, P.; Heeger, A.J. X-ray structural studies of poly(3-alkylthiophenes): an example of an inverse comb. Macromolecules 1992, 25, 4364–4372. [Google Scholar] [CrossRef]
- Osaka, M.; Mori, D.; Benten, H.; Ogawa, H.; Ohkita, H.; Ito, S. Charge transport in intermixed regions of all-polymer solar cells studied by conductive atomic force microscopy. ACS Appl. Mater. Interfaces 2017, 9, 15615–15622. [Google Scholar] [CrossRef] [PubMed]
- Bolsee, J.-C.; Oosterbaan, W.D.; Lutsen, L.; Vanderzande, D.; Manca, J. The importance of bridging points for charge transport in webs of conjugated polymer nanofibers. Adv. Funct. Mater. 2013, 23, 862–869. [Google Scholar] [CrossRef]
- Kaiser, A.B.; Skakalova, V. Electronic conduction in polymers, carbon nanotubes and graphene. Chem. Soc. Rev. 2011, 40, 3786–3801. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.B. Systematic conductivity behavior in conducting polymers: effects of heterogeneous disorder. Adv. Mater. 2001, 13, 927–941. [Google Scholar] [CrossRef]
- Kivelson, S.; Heeger, A.J. Intrinsic conductivity of conducting polymers. Synth. Met. 1988, 22, 371–384. [Google Scholar] [CrossRef]








| Sample | Electrical Conductivity (S/cm) | Seebeck Coefficient (μV/K) | Power Factor (μW/mK2) | Carrier Concentration (×1020 cm−3) | Carrier Mobility (cm2/V·s) |
|---|---|---|---|---|---|
| ra-P3HT-TCB | 20 ± 2 | 46 ± 5 | 4.2 ± 0.4 | (4.1 ± 0.4) | 0.2 ± 0.1 |
| rg-P3HT-TCB | 226 ± 6 | 42 ± 3 | 39.1 ± 2.5 | (4.8 ± 0.5) | 2.9 ± 0.3 |
| bρ0 (Ω cm) | T0 (K) | n | |
|---|---|---|---|
| ra-P3HT-TCB | 1.9 × 10−7 | 7.2 × 106 | 3 |
| aρm (Ω cm) | bρ0 (Ω cm) | Tm (K) | T0 (K) | n | |
|---|---|---|---|---|---|
| rg-P3HT-TCB | 1.0 × 10−3 | 1.8 × 10−3 | 1400 | 177 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, S.; Ming, C.; Yao, Q.; Lu, W.; Zeng, K.; Shi, W.; Shi, X.; Uher, C.; Chen, L. Understanding the Intrinsic Carrier Transport in Highly Oriented Poly(3-hexylthiophene): Effect of Side Chain Regioregularity. Polymers 2018, 10, 815. https://doi.org/10.3390/polym10080815
Qu S, Ming C, Yao Q, Lu W, Zeng K, Shi W, Shi X, Uher C, Chen L. Understanding the Intrinsic Carrier Transport in Highly Oriented Poly(3-hexylthiophene): Effect of Side Chain Regioregularity. Polymers. 2018; 10(8):815. https://doi.org/10.3390/polym10080815
Chicago/Turabian StyleQu, Sanyin, Chen Ming, Qin Yao, Wanheng Lu, Kaiyang Zeng, Wei Shi, Xun Shi, Ctirad Uher, and Lidong Chen. 2018. "Understanding the Intrinsic Carrier Transport in Highly Oriented Poly(3-hexylthiophene): Effect of Side Chain Regioregularity" Polymers 10, no. 8: 815. https://doi.org/10.3390/polym10080815
APA StyleQu, S., Ming, C., Yao, Q., Lu, W., Zeng, K., Shi, W., Shi, X., Uher, C., & Chen, L. (2018). Understanding the Intrinsic Carrier Transport in Highly Oriented Poly(3-hexylthiophene): Effect of Side Chain Regioregularity. Polymers, 10(8), 815. https://doi.org/10.3390/polym10080815