Synergistic Effects of Processing Additives and Thermal Annealing on Nanomorphology and Hole Mobility of Poly(3-hexylthiophene) Thin Films
Abstract
:1. Introduction
2. Materials and Methods
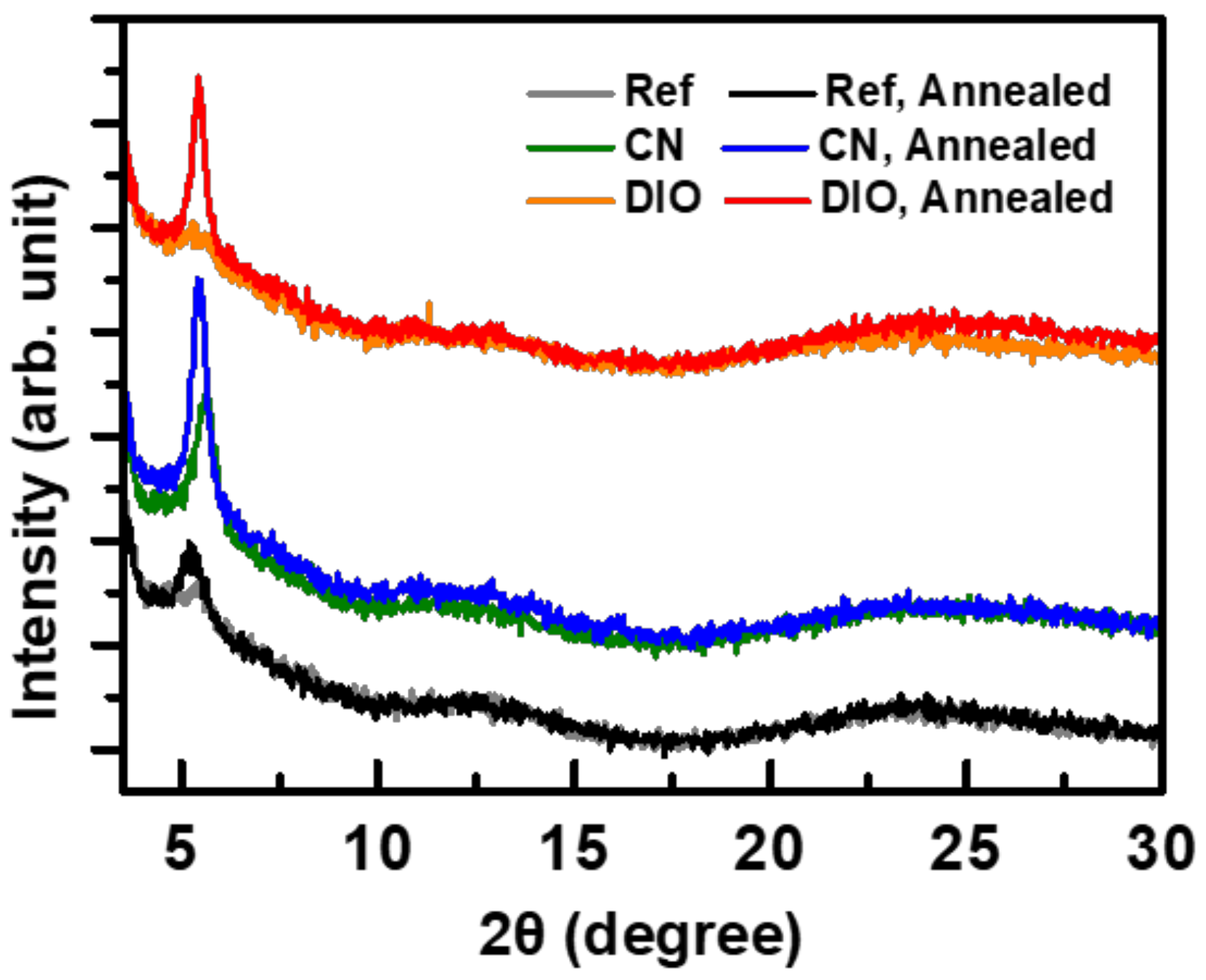
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Coropceanu, V.; Cornil, J.; da Silva, D.A.; Olivier, Y.; Silbey, R.; Bredas, J.L. Charge transport in organic semiconductors. Chem. Rev. 2007, 107, 926–952. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lee, W.; Oh, J.; Kim, T.; Lee, C.; Kim, B.J. From Fullerene-Polymer to All-Polymer Solar Cells: The Importance of Molecular Packing, Orientation, and Morphology Control. Acc. Chem. Res. 2016, 49, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.S.; Ren, G.; Jenekhe, S.A. One-dimensional nanostructures of π-conjugated molecular systems: Assembly, properties, and applications from photovoltaics, sensors, and nanophotonics to nanoelectronics. Chem. Mater. 2010, 23, 682–732. [Google Scholar] [CrossRef]
- Choi, J.; Song, H.; Kim, N.; Kim, F.S. Development of n-type polymer semiconductors for organic field-effect transistors. Semicond. Sci. Technol. 2015, 30, 064002. [Google Scholar] [CrossRef]
- Kippelen, B.; Brédas, J.-L. Organic photovoltaics. Energy Environ. Sci. 2009, 2, 251–261. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, L. A new class of semiconducting polymers for bulk heterojunction solar cells with exceptionally high performance. Acc. Chem. Res. 2010, 43, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shrotriya, V.; Huang, J.S.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Kim, Y.; Choulis, S.A.; Nelson, J.; Bradley, D.D.C.; Cook, S.; Durrant, J.R. Device annealing effect in organic solar cells with blends of regioregular poly(3-hexylthiophene) and soluble fullerene. Appl. Phys. Lett. 2005, 86, 063502. [Google Scholar] [CrossRef]
- Jo, J.; Kim, S.S.; Na, S.I.; Yu, B.K.; Kim, D.Y. Time-Dependent Morphology Evolution by Annealing Processes on Polymer: Fullerene Blend Solar Cells. Adv. Funct. Mater. 2009, 19, 866–874. [Google Scholar] [CrossRef]
- Zen, A.; Pflaum, J.; Hirschmann, S.; Zhuang, W.; Jaiser, F.; Asawapirom, U.; Rabe, J.P.; Scherf, U.; Neher, D. Effect of molecular weight and annealing of poly(3-hexylthiophene) s on the performance of organic field-effect transistors. Adv. Funct. Mater. 2004, 14, 757–764. [Google Scholar] [CrossRef]
- Peet, J.; Kim, J.Y.; Coates, N.E.; Ma, W.L.; Moses, D.; Heeger, A.J.; Bazan, G.C. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat. Mater. 2007, 6, 497. [Google Scholar] [CrossRef] [PubMed]
- Pivrikas, A.; Stadler, P.; Neugebauer, H.; Sariciftci, N.S. Substituting the postproduction treatment for bulk-heterojunction solar cells using chemical additives. Org. Electron. 2008, 9, 775–782. [Google Scholar] [CrossRef]
- Lee, J.K.; Ma, W.L.; Brabec, C.J.; Yuen, J.; Moon, J.S.; Kim, J.Y.; Lee, K.; Bazan, G.C.; Heeger, A.J. Processing additives for improved efficiency from bulk heterojunction solar cells. J. Am. Chem. Soc. 2008, 130, 3619–3623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jespersen, K.G.; Björström, C.; Svensson, M.; Andersson, M.R.; Sundström, V.; Magnusson, K.; Moons, E.; Yartsev, A.; Inganäs, O. Influence of solvent mixing on the morphology and performance of solar cells based on polyfluorene copolymer/fullerene blends. Adv. Funct. Mater. 2006, 16, 667–674. [Google Scholar] [CrossRef]
- Tada, K. Negligible effect of processing additive in polymer bulk heterojunction photovoltaic cells with unmodified fullerene. Macromol. Res. 2017, 25, 624–628. [Google Scholar] [CrossRef]
- Machui, F.; Maisch, P.; Burgués-Ceballos, I.; Langner, S.; Krantz, J.; Ameri, T.; Brabec, C.J. Classification of additives for organic photovoltaic devices. ChemPhysChem 2015, 16, 1275–1280. [Google Scholar] [CrossRef]
- Xin, H.; Kim, F.S.; Jenekhe, S.A. Highly efficient solar cells based on poly(3-butylthiophene) nanowires. J. Am. Chem. Soc. 2008, 130, 5424–5425. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Kim, D.; Cho, H.H.; Lee, C.; Chong, S.; Ahn, N.Y.; Seo, M.; Kim, J.; Kim, F.S.; Kim, B.J. Shift of the Branching Point of the Side-Chain in Naphthalenediimide (NDI)-Based Polymer for Enhanced Electron Mobility and All-Polymer Solar Cell Performance. Adv. Funct. Mater. 2018, 28, 1803613. [Google Scholar] [CrossRef]
- Cho, H.H.; Kim, T.; Kim, K.; Lee, C.; Kim, F.S.; Kim, B.J. Synthesis and side-chain engineering of phenylnaphthalenediimide (PNDI)-based n-type polymers for efficient all-polymer solar cells. J. Mater. Chem. A 2017, 5, 5449–5459. [Google Scholar] [CrossRef]
- Diao, Y.; Tee, B.C.; Giri, G.; Xu, J.; Kim, D.H.; Becerril, H.A.; Stoltenberg, R.M.; Lee, T.H.; Xue, G.; Mannsfeld, S.C. Solution coating of large-area organic semiconductor thin films with aligned single-crystalline domains. Nat. Mater. 2013, 12, 665. [Google Scholar] [CrossRef]
- Tseng, H.R.; Phan, H.; Luo, C.; Wang, M.; Perez, L.A.; Patel, S.N.; Ying, L.; Kramer, E.J.; Nguyen, T.Q.; Bazan, G.C. High-Mobility Field-Effect Transistors Fabricated with Macroscopic Aligned Semiconducting Polymers. Adv. Mater. 2014, 26, 2993–2998. [Google Scholar] [CrossRef] [PubMed]
- Richter, L.J.; DeLongchamp, D.M.; Bokel, F.A.; Engmann, S.; Chou, K.W.; Amassian, A.; Schaible, E.; Hexemer, A. In situ morphology studies of the mechanism for solution additive effects on the formation of bulk heterojunction films. Adv. Energy Mater. 2015, 5, 1400975. [Google Scholar] [CrossRef]
- Liao, H.-C.; Ho, C.-C.; Chang, C.-Y.; Jao, M.-H.; Darling, S.B.; Su, W.-F. Additives for morphology control in high-efficiency organic solar cells. Mater. Today 2013, 16, 326–336. [Google Scholar] [CrossRef]
- Yao, Y.; Hou, J.H.; Xu, Z.; Li, G.; Yang, Y. Effect of solvent mixture on the nanoscale phase separation in polymer solar cells. Adv. Funct. Mater. 2008, 18, 1783–1789. [Google Scholar] [CrossRef]
- An, T.K.; Kang, I.; Yun, H.j.; Cha, H.; Hwang, J.; Park, S.; Kim, J.; Kim, Y.J.; Chung, D.S.; Kwon, S.K. Solvent additive to achieve highly ordered nanostructural semicrystalline DPP copolymers: Toward a high charge carrier mobility. Adv. Mater. 2013, 25, 7003–7009. [Google Scholar] [CrossRef]
- Na, J.Y.; Kang, B.; Sin, D.H.; Cho, K.; Park, Y.D. Understanding Solidification of Polythiophene Thin Films during Spin-Coating: Effects of Spin-Coating Time and Processing Additives. Sci. Rep. 2015, 5, 13288. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.F.; Sun, B.Q.; Breiby, D.W.; Nielsen, M.M.; Solling, T.I.; Giles, M.; McCulloch, I.; Sirringhaus, H. Enhanced mobility of poly(3-hexylthiophene) transistors by spin-coating from high-boiling-point solvents. Chem. Mater. 2004, 16, 4772–4776. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Choi, H.; Ko, S.J.; Uddin, M.A.; Walker, B.; Yum, S.; Jeong, J.E.; Yun, M.H.; Shin, T.J.; Hwang, S.; et al. Semi-crystalline photovoltaic polymers with efficiency exceeding 9% in a similar to 300 nm thick conventional single-cell device. Energy Environ. Sci. 2014, 7, 3040–3051. [Google Scholar] [CrossRef]
- Chen, F.C.; Tseng, H.C.; Ko, C.J. Solvent mixtures for improving device efficiency of polymer photovoltaic devices. Appl. Phys. Lett. 2008, 92, 96. [Google Scholar] [CrossRef]
- Bao, Z.; Dodabalapur, A.; Lovinger, A.J. Soluble and processable regioregular poly(3-hexylthiophene) for thin film field-effect transistor applications with high mobility. Appl. Phys. Lett. 1996, 69, 4108–4110. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Brown, P.; Friend, R.; Nielsen, M.M.; Bechgaard, K.; Langeveld-Voss, B.; Spiering, A.; Janssen, R.A.; Meijer, E.; Herwig, P. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 1999, 401, 685. [Google Scholar] [CrossRef]
- Newbloom, G.M.; Kim, F.S.; Jenekhe, S.A.; Pozzo, D.C. Mesoscale morphology and charge transport in colloidal networks of poly(3-hexylthiophene). Macromolecules 2011, 44, 3801–3809. [Google Scholar] [CrossRef]
- Briseno, A.L.; Kim, F.S.; Babel, A.; Xia, Y.; Jenekhe, S.A. n-Channel polymer thin film transistors with long-term air-stability and durability and their use in complementary inverters. J. Mater. Chem. 2011, 21, 16461–16466. [Google Scholar] [CrossRef]
- Na, J.Y.; Kim, M.; Park, Y.D. Solution Processing with a Good Solvent Additive for Highly Reliable Organic Thin-Film Transistors. J. Phys. Chem. C 2017, 121, 13930–13937. [Google Scholar] [CrossRef]
- Luo, H.; Yu, C.; Liu, Z.; Zhang, G.; Geng, H.; Yi, Y.; Broch, K.; Hu, Y.; Sadhanala, A.; Jiang, L. Remarkable enhancement of charge carrier mobility of conjugated polymer field-effect transistors upon incorporating an ionic additive. Sci. Adv. 2016, 2, e1600076. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Brazard, J.; Ono, R.J.; Hanson, B.; Traub, M.C.; Wu, Z.-Q.; Li, Z.; Bolinger, J.C.; Ganesan, V.; Bielawski, C.W. Regioregularity and single polythiophene chain conformation. J. Phys. Chem. Lett. 2011, 2, 1400–1404. [Google Scholar] [CrossRef]
- Brown, P.J.; Thomas, D.S.; Köhler, A.; Wilson, J.S.; Kim, J.-S.; Ramsdale, C.M.; Sirringhaus, H.; Friend, R.H. Effect of interchain interactions on the absorption and emission of poly(3-hexylthiophene). Phys. Rev. B 2003, 67, 064203. [Google Scholar] [CrossRef]
- Kim, F.S.; Guo, X.; Watson, M.D.; Jenekhe, S.A. High-mobility Ambipolar Transistors and High-gain Inverters from a Donor–Acceptor Copolymer Semiconductor. Adv. Mater. 2010, 22, 478–482. [Google Scholar] [CrossRef]
- Cho, S.; Lee, K.; Yuen, J.; Wang, G.; Moses, D.; Heeger, A.J.; Surin, M.; Lazzaroni, R. Thermal annealing-induced enhancement of the field-effect mobility of regioregular poly(3-hexylthiophene) films. J. Appl. Phys. 2006, 100, 114503. [Google Scholar] [CrossRef]
- Ong, B.S.; Wu, Y.; Liu, P.; Gardner, S. High-performance semiconducting polythiophenes for organic thin-film transistors. J. Am. Chem. Soc. 2004, 126, 3378–3379. [Google Scholar] [CrossRef] [PubMed]





| Additive | M.W. a (g/mol) | B.P. b (°C) | Pvaporc (mmHg) | Rqd (nm) | d(100)e (nm) | LCf (nm) | μmaxg (cm2/Vs) | μavgh (cm2/Vs) | Vthi (V) |
|---|---|---|---|---|---|---|---|---|---|
| - | - | - | - | 0.8 | 1.67 | 13.2 | 0.0294 | 0.0223 (±0.0051) | −20.6 (±3.9) |
| 1,8-Octanedithiol(ODT) | 178.4 | 270 | 0.012 | 3.8 | 1.64 | 16.7 | 0.0304 | 0.0292 (±0.0010) | −12.7 (±7.9) |
| Diphenyl ether(DPE) | 170.2 | 259 | 0.02 | 5.0 | 1.64 | 16.8 | 0.0382 | 0.0359 (±0.0032) | −18.0 (±6.4) |
| 1-Chloronaphthalene(CN) | 162.6 | 263 | 0.029 | 5.3 | 1.63 | 18.2 | 0.104 | 0.0914 (±0.0120) | 2.2 (±11.6) |
| 1,8-Diiodooctane(DIO) | 366.0 | 332 | 0.0003 | 3.9 | 1.63 | 24.8 | 0.114 | 0.0937 (±0.0127) | 7.58 (±9.1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.S.; Kim, F.S. Synergistic Effects of Processing Additives and Thermal Annealing on Nanomorphology and Hole Mobility of Poly(3-hexylthiophene) Thin Films. Polymers 2019, 11, 112. https://doi.org/10.3390/polym11010112
Park MS, Kim FS. Synergistic Effects of Processing Additives and Thermal Annealing on Nanomorphology and Hole Mobility of Poly(3-hexylthiophene) Thin Films. Polymers. 2019; 11(1):112. https://doi.org/10.3390/polym11010112
Chicago/Turabian StylePark, Min Soo, and Felix Sunjoo Kim. 2019. "Synergistic Effects of Processing Additives and Thermal Annealing on Nanomorphology and Hole Mobility of Poly(3-hexylthiophene) Thin Films" Polymers 11, no. 1: 112. https://doi.org/10.3390/polym11010112
APA StylePark, M. S., & Kim, F. S. (2019). Synergistic Effects of Processing Additives and Thermal Annealing on Nanomorphology and Hole Mobility of Poly(3-hexylthiophene) Thin Films. Polymers, 11(1), 112. https://doi.org/10.3390/polym11010112





