Integrating Nano-Cu2O@ZrP into In Situ Polymerized Polyethylene Terephthalate (PET) Fibers with Enhanced Mechanical Properties and Antibacterial Activities
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PET/Cu2O@ZrP-B Fibers
2.3. Preparation of PET/Cu2O@ZrP-I Fibers
2.4. Antibacterial Activity of PET/Cu2O@ZrP Fibers
2.5. Characterization
3. Results and Discussion
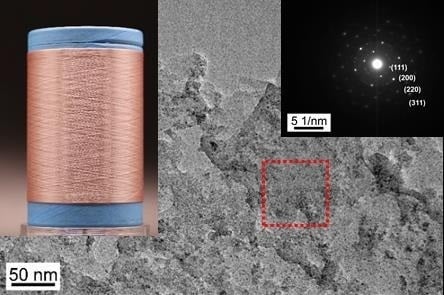
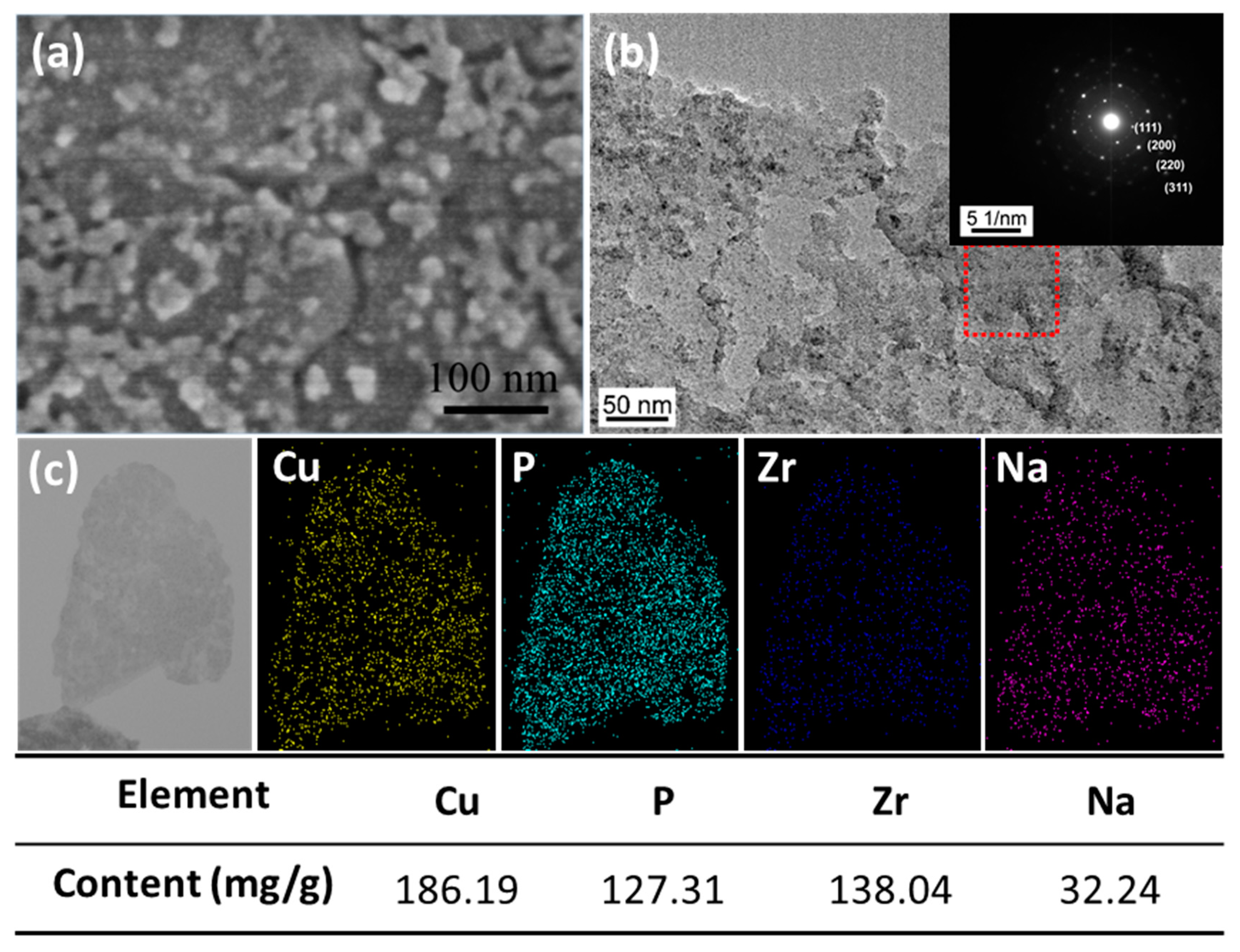
3.1. The Morphology of Nano-Cu2O@ZrP
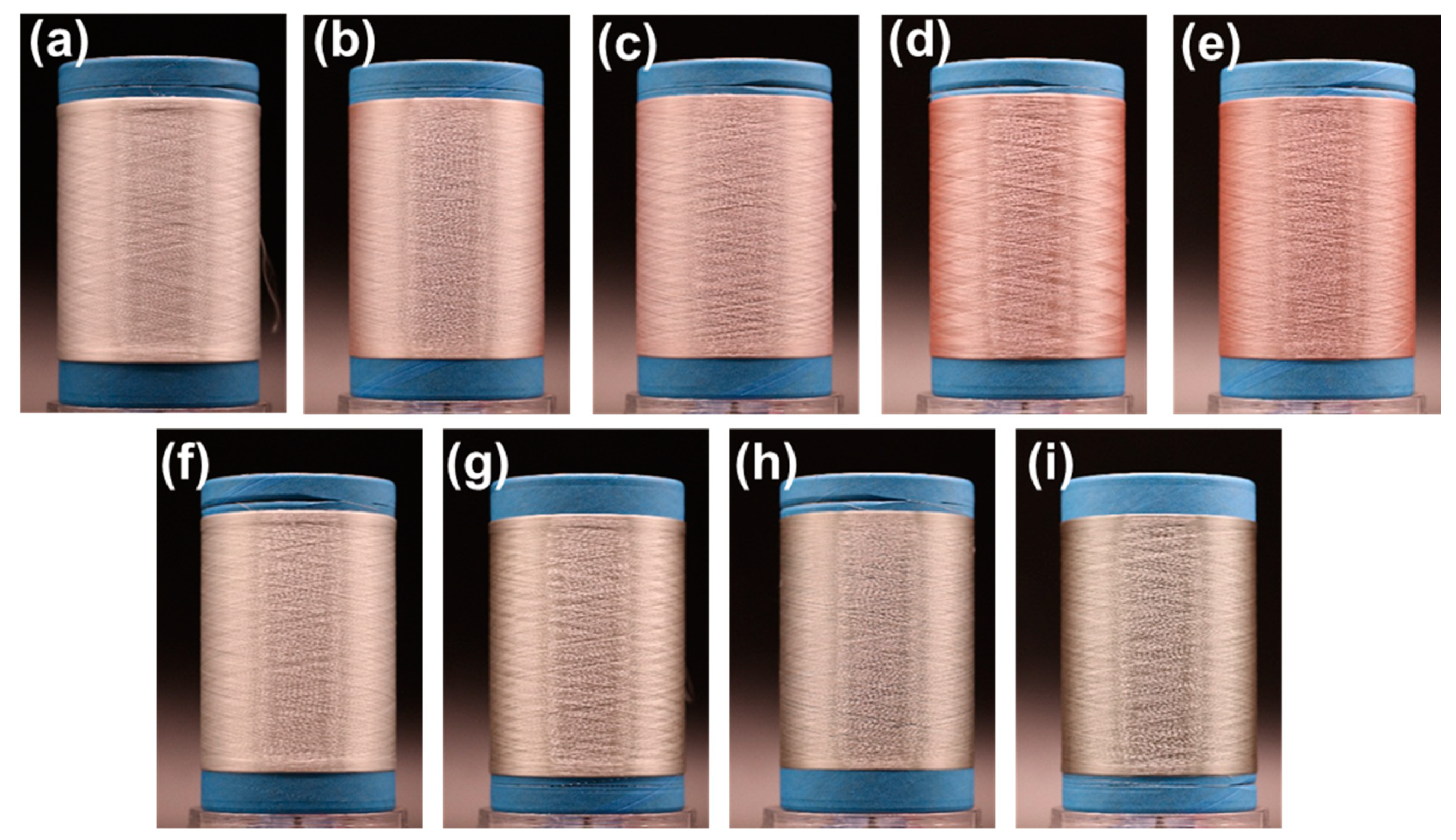
3.2. The Morphology of PET/Cu2O@ZrP Fibers
3.3. The Effect of Introducing Nano-Cu2O@ZrP on Mechanical Properties and Crystallinity of the Resulting PET Fibers
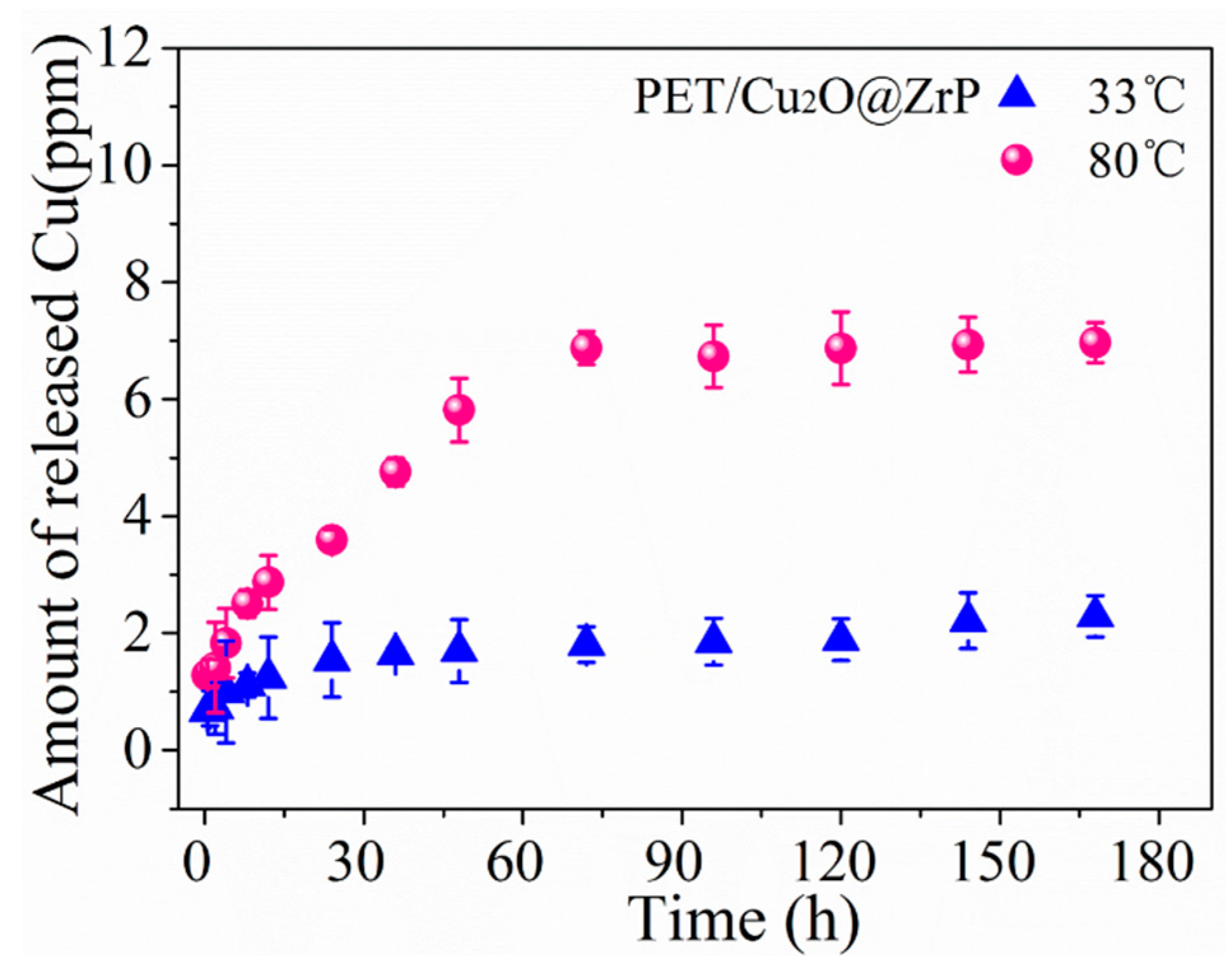
3.4. Evaluation of Antibacterial Performance for the PET/Cu2O@ZrP Hybrid Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Karsli, N.G.; Ozkan, C.; Aytac, A.; Deniz, V. Characterization of poly(butylene terephthalate) composites prepared by using various types of sized carbon fibers. Mater. Des. 2015, 87, 318–323. [Google Scholar] [CrossRef]
- Keum, J.K.; Song, H.H. Thermal deformations of oriented noncrystalline poly (ethylene terephthalate) fibers in the presence of mesophase structure. Polymer 2005, 46, 939–945. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, Q.; Zhang, P.; Xia, Y.; Kong, Q. Thermal stability and flame-retardancy mechanism of poly(ethylene terephthalate)/boehmite nanocomposites. Polym. Degrad. Stab. 2010, 95, 1211–1218. [Google Scholar] [CrossRef]
- Shu, D.; Xi, P.; Li, S.; Li, C.; Wang, X.; Cheng, B. Morphologies and Properties of PET Nano Porous Luminescence Fiber: Oil Absorption and Fluorescence-Indicating Functions. ACS Appl. Mater. Interfaces 2018, 10, 2828–2836. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ke, H.; Dong, J.; Wei, Q.; Lin, J.; Zhao, Y.; Song, L.; Hu, Y.; Huang, F.; Gao, W.; et al. Effects of nano-SiO2 on morphology, thermal energy storage, thermal stability, and combustion properties of electrospun lauric acid/PET ultrafine composite fibers as form-stable phase change materials. Appl. Energy 2011, 88, 2106–2112. [Google Scholar] [CrossRef]
- Xie, L.; Xie, Y.; Wu, Q.; Wang, M.; Wu, Q.; Zhou, X.; Ge, X. Effect of Poly(acrylic acid)-Modified Poly(ethylene terephthalate) on Improving the Integrated Mechanical Properties of Poly(ethylene terephthalate)/Elastomer Blend. Ind. Eng. Chem. Res. 2015, 54, 4748–4755. [Google Scholar] [CrossRef]
- Jin, J.; Lingjie, F.; Lian, T.; Peng, J.; Chaosheng, W.; Huaping, W. Preparation of Nano Cu-ZnO/PET Fiber for Antibacterial Application. Mater. Sci. Forum 2017, 898, 2272–2278. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in Textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef]
- Emam, H.E.; Mowafi, S.; Mashaly, H.M.; Rehan, M. Production of antibacterial colored viscose fibers using in situ prepared spherical Ag nanoparticles. Carbohydr. Polym. 2014, 110, 148–155. [Google Scholar] [CrossRef]
- Perez Espitia, P.J.; Ferreira Soares, N.D.F.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Alves Medeiros, E.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Milosevic, M.; Krkobabic, A.; Radoicic, M.; Saponjic, Z.; Radetic, T.; Radetic, M. Biodegradation of cotton and cotton/polyester with Ag/TiO2 nanoparticles in soil. Carbohydr. Polym. 2017, 158, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.X.; He, G.H.; Zheng, W.J.; Bian, T.F.; Li, M.; Zhang, D.W. Study on antibacterial mechanism of Mg(OH)2 nanoparticles. Mater. Lett. 2014, 134, 286–289. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Sha, L.; Zhao, J. Preparation of antimicrobial fabric using magnesium-based PET masterbatch. Appl. Surf. Sci. 2017, 425, 1101–1110. [Google Scholar] [CrossRef]
- Feng, R.C.; Guan, G.H.; Zhou, W.; Li, C.C.; Zhang, D.; Xiao, Y.N. In situ synthesis of poly(ethylene terephthalate)/graphene composites using a catalyst supported on graphite oxide. J. Mater. Chem. 2011, 21, 3931–3939. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Bahners, T.; Wego, A.; Gutmann, J.S.; Ulbricht, M. Surface modification of poly(ethylene terephthalate) fabric via photo-chemical reaction of dimethylaminopropyl methacrylamide. Appl. Surf. Sci. 2012, 259, 261–269. [Google Scholar] [CrossRef]
- Cerkez, I.; Worley, S.D.; Broughton, R.M.; Huang, T.S. Antimicrobial coatings for polyester and polyester/cotton blends. Prog. Org. Coat. 2013, 76, 1082–1087. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Sha, L.; Zhao, J.; Li, X. Design of antibacterial polyethylene terephthalate masterbatch functionalized by modified nano-Mg(OH)2. J. Appl. Polym. Sci. 2018, 135, 46755. [Google Scholar] [CrossRef]
- Lotti, N.; Munari, A.; Gigli, M.; Gazzano, M.; Tsanaktsis, V.; Bikiaris, D.N.; Papageorgiou, G.Z. Thermal and structural response of in situ prepared biobased poly(ethylene 2,5-furan dicarboxylate) nanocomposites. Polymer 2016, 103, 288–298. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Radi, A.; Pradhan, D.; Sohn, Y.; Leung, K.T. Nanoscale Shape and Size Control of Cubic, Cuboctahedral, and Octahedral Cu-Cu2O Core-Shell Nanoparticles on Si(100) by One-Step, Templateless, Capping-Agent-Free Electrodeposition. ACS Nano 2010, 4, 1553–1560. [Google Scholar] [CrossRef]
- Kaweeteerawat, C.; Chang, C.H.; Roy, K.R.; Liu, R.; Li, R.; Toso, D.; Fischer, H.; Ivask, A.; Ji, Z.; Zink, J.I.; et al. Cu Nanoparticles Have Different Impacts in Escherichia coli and Lactobacillus brevis than Their Microsized and Ionic Analogues. ACS Nano 2015, 9, 7215–7225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef] [PubMed]
- Montazer, M.; Dastjerdi, M.; Azdaloo, M.; Rad, M. Simultaneous synthesis and fabrication of nano Cu2O on cellulosic fabric using copper sulfate and glucose in alkali media producing safe bio- and photoactive textiles without color change. Cellulose 2015, 22, 4049–4064. [Google Scholar] [CrossRef]
- Markovic, D.; Korica, M.; Kostic, M.; Radovanovic, Z.; Saponjic, Z.; Mitric, M.; Radetic, M. In situ synthesis of Cu/Cu2O nanoparticles on the TEMPO oxidized cotton fabrics. Cellulose 2018, 25, 829–841. [Google Scholar] [CrossRef]
- Emam, H.E.; Ahmed, H.B.; Bechtold, T. In-situ deposition of Cu2O micro-needles for biologically active textiles and their release properties. Carbohydr. Polym. 2017, 165, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Yu, H.; Nie, C.; Xiao, Y.; Zeng, Q.; Wang, G.; Wang, B.; Lv, H.; Li, Q.; Chen, S. Size-controlled synthesis of Cu2O nanoparticles: Size effect on antibacterial activity and application as a photocatalyst for highly efficient H2O2 evolution. Rsc Adv. 2017, 7, 51822–51830. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lyu, L.-M.; Yang, Y.-C.; Huang, M.H. Synthesis of Cu2O Nanocrystals from Cubic to Rhombic Dodecahedral Structures and Their Comparative Photocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.Y.; Bunekar, N. The effect of organomodified ZnAl LDH for in situ synthesis and the properties of poly(ethylene terephthalate) nanocomposites. RSC Adv. 2016, 6, 65291–65298. [Google Scholar] [CrossRef]
- Fortunato, G.; Tenniche, A.; Gottardo, L.; Hufenus, R. Development of poly-(ethylene terephthalate) masterbatches incorporating highly dispersed TiO2 nanoparticles: Investigation of morphologies by optical and rheological procedures. Eur. Polym. J. 2014, 57, 75–82. [Google Scholar] [CrossRef]
- Xiang, H.; Chen, Z.; Zheng, N.; Zhang, X.; Zhu, L.; Zhou, Z.; Zhu, M. Melt-spun microbial poly(3-hydroxybutyrate-co-3-hydroxyvalerate) fibers with enhanced toughness: Synergistic effect of heterogeneous nucleation, long-chain branching and drawing process. Int. J. Biol. Macromol. 2019, 122, 1136–1143. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Xiang, H.; Zhu, L.; Tebyetekerwa, M.; Zhu, M. Superior piezoresistive strain sensing behaviors of carbon nanotubes in one-dimensional polymer fiber structure. Carbon 2018, 140, 1–9. [Google Scholar] [CrossRef]
- Lin, G.; Li, D.; Liu, M.; Zhang, X.; Zheng, Y. Rheology, Non-Isothermal Crystallization Behavior, Mechanical and Thermal Properties of PMMA-Modified Carbon Fiber-Reinforced Poly(Ethylene Terephthalate) Composites. Polymers 2018, 10, 594. [Google Scholar] [CrossRef]
- Collins, M.N.; Dalton, E.; Leahy, J.J.; Birkinshaw, C. Effects of tensile strain on the nanostructure of irradiated and thermally stabilised ultra high molecular weight polyethylenes for orthopaedic devices. RSC Adv. 2013, 3, 1995–2007. [Google Scholar] [CrossRef]
- Dalton, E.; Collins, M.N. Lamella alignment ratio: A SAXS analysis technique for macromolecules. J. Appl. Crystallogr. 2014, 47, 847–851. [Google Scholar] [CrossRef]
- Chen, Z.; Xiang, H.; Hu, Z.; Ni, Z.; Zhu, M. Enhanced Mechanical Properties of Melt-spun Bio-based PHBV Fibers: Effect of Heterogeneous Nucleation and Drawing Process. Acta Polym. Sin. 2017, 1121–1129. [Google Scholar] [CrossRef]
- Xiang, H.; Chen, W.; Chen, Z.; Sun, B.; Zhu, M. Significant accelerated crystallization of long chain branched poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with high nucleation temperature under fast cooling rate. Compos. Sci. Technol. 2017, 142, 207–213. [Google Scholar] [CrossRef]
- Litchfield, D.W.; Baird, D.G.; Rim, P.B.; Chen, C. Improved Mechanical Properties of Poly(Ethylene Terephthalate) Nanocomposite Fibers. Polym. Eng. Sci. 2010, 50, 2205–2215. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Z.; Cebe, P. Chain confinement in electrospun nanofibers of PET with carbon nanotubes. Polymer 2009, 50, 872–880. [Google Scholar] [CrossRef]
- Keum, J.K.; Jeon, H.-J.; Song, H.H.; Choi, J.-I.; Son, Y.-K. Orientation-induced crystallization of poly(ethylene terephthalate) fibers with controlled microstructure. Polymer 2008, 49, 4882–4888. [Google Scholar] [CrossRef]
- Ouyang, Y.; Cai, X.; Shi, Q.; Liu, L.; Wan, D.; Tan, S.; Ouyang, Y. Poly-l-lysine-modified reduced graphene oxide stabilizes the copper nanoparticles with higher water-solubility and long-term additively antibacterial activity. Colloids Surf. B Biointerfaces 2013, 107, 107–114. [Google Scholar]
- Shao, W.; Liu, X.F.; Min, H.H.; Dong, G.H.; Feng, Q.Y.; Zuo, S.L. Preparation, Characterization, and Antibacterial Activity of Silver Nanoparticle-Decorated Graphene Oxide Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhang, J.L.; Ouyang, Y.; Ma, D.; Tan, S.Z.; Peng, Y.L. Bacteria-Adsorbed Palygorskite Stabilizes the Quaternary Phosphonium Salt with Specific-Targeting Capability, Long-Term Antibacterial Activity, and Lower Cytotoxicity. Langmuir 2013, 29, 5279–5285. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Tan, S.Z.; Lin, M.S.; Xie, A.; Mai, W.J.; Zhang, X.J.; Lin, Z.D.; Wu, T.; Liu, Y.L. Synergistic Antibacterial Brilliant Blue/Reduced Graphene Oxide/Quaternary Phosphonium Salt Composite with Excellent Water Solubility and Specific Targeting Capability. Langmuir 2011, 27, 7828–7835. [Google Scholar] [CrossRef] [PubMed]




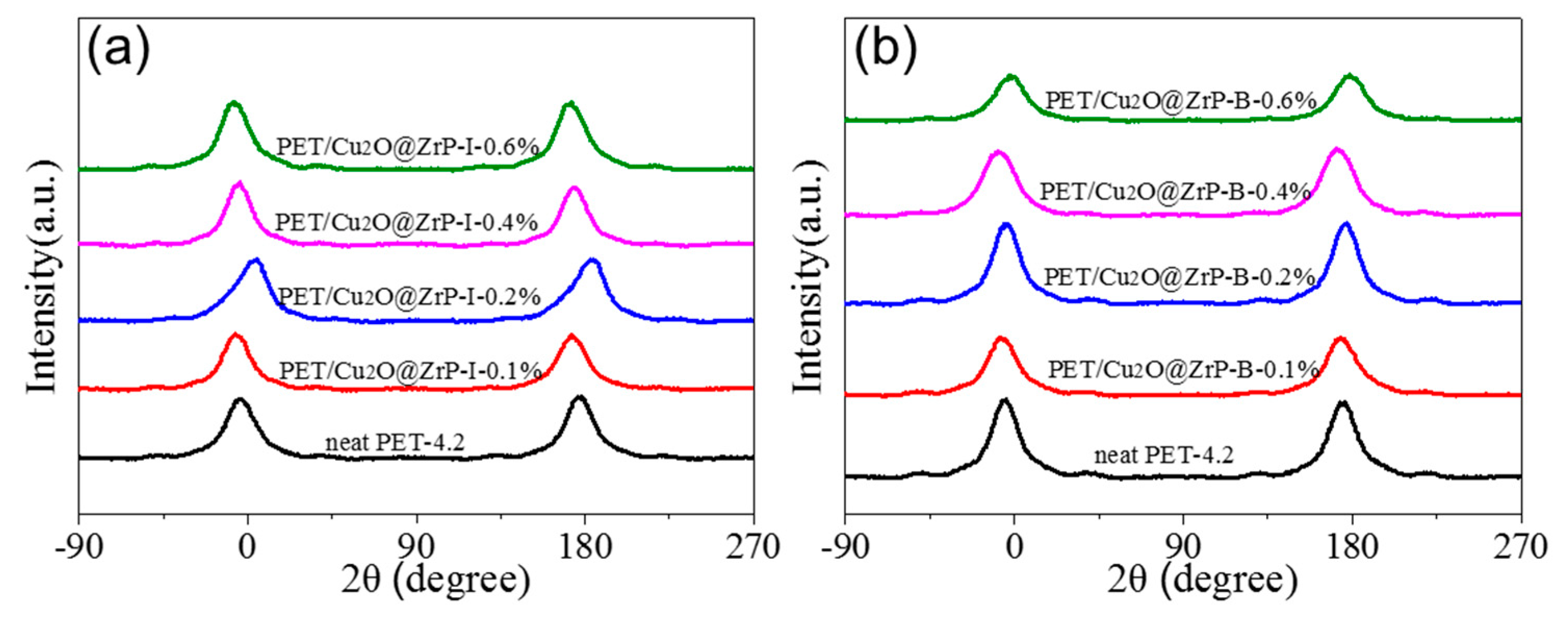
| Samples | FWHM (°) | Π (%) | Samples | FWHM (°) | Π (%) |
|---|---|---|---|---|---|
| PET-4.6 | 17.8 | 95.4 | PET-4.2 | 17.3 | 94.8 |
| PET/Cu2[email protected]% | 17.7 | 94.6 | PET/Cu2[email protected]% | 16.8 | 93.2 |
| PET/Cu2[email protected]% | 18.9 | 92.8 | PET/Cu2[email protected]% | 17.4 | 91.4 |
| PET/Cu2[email protected]% | 19.3 | 91.6 | PET/Cu2[email protected]% | 18.3 | 89.8 |
| PET/Cu2[email protected]% | 21.8 | 90.9 | PET/Cu2[email protected]% | 20.1 | 88.2 |
| Bacterium | Samples | Blank Sample Viable Colonies (CFU/mL) | Viable Colonies (CFU/mL) | Microbial Reduction (%) |
|---|---|---|---|---|
| E. coli | PET | 1.5 × 106 | 1.5 × 106 | No effect |
| PET/Cu2O-0.2% | 1.5 × 106 | <1 | >99 | |
| PET/Cu2[email protected]% | 1.5 × 106 | <1 | >99 | |
| S. aureus | PET | 8.3 × 105 | 8.3 × 105 | No effect |
| PET/Cu2O-0.2% | 8.3 × 105 | 30 | >99 | |
| PET/Cu2[email protected]% | 8.3 × 105 | 12 | >99 | |
| C. albicans | PET | 6.1 × 105 | 6.1 × 105 | No effect |
| PET/Cu2O-0.2% | 6.1 × 105 | 9.4 × 104 | 85 | |
| PET/Cu2O-0.6% | 6.1 × 105 | 1.9 × 104 | 97 | |
| PET/Cu2[email protected]% | 3.4 × 105 | 2.8 × 104 | 92 | |
| PET/Cu2[email protected]% | 3.4 × 105 | 35 | >99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Fei, X.; Li, C.; Yu, S.; Hu, Z.; Xiang, H.; Sun, B.; Zhu, M. Integrating Nano-Cu2O@ZrP into In Situ Polymerized Polyethylene Terephthalate (PET) Fibers with Enhanced Mechanical Properties and Antibacterial Activities. Polymers 2019, 11, 113. https://doi.org/10.3390/polym11010113
Zhou J, Fei X, Li C, Yu S, Hu Z, Xiang H, Sun B, Zhu M. Integrating Nano-Cu2O@ZrP into In Situ Polymerized Polyethylene Terephthalate (PET) Fibers with Enhanced Mechanical Properties and Antibacterial Activities. Polymers. 2019; 11(1):113. https://doi.org/10.3390/polym11010113
Chicago/Turabian StyleZhou, Jialiang, Xiang Fei, Congqi Li, Senlong Yu, Zexu Hu, Hengxue Xiang, Bin Sun, and Meifang Zhu. 2019. "Integrating Nano-Cu2O@ZrP into In Situ Polymerized Polyethylene Terephthalate (PET) Fibers with Enhanced Mechanical Properties and Antibacterial Activities" Polymers 11, no. 1: 113. https://doi.org/10.3390/polym11010113