Hierarchical Ag Nanostructures Fabricated from Silver Coordination Polymers for Antibacterial Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Silver Coordination Polymer
2.2. Surface Coating with Silver Nanostructures
2.3. Characterization
2.4. Evaluation of Antibacterial Activity of Ag Nanostructure Coated Surface
3. Results and Discussion
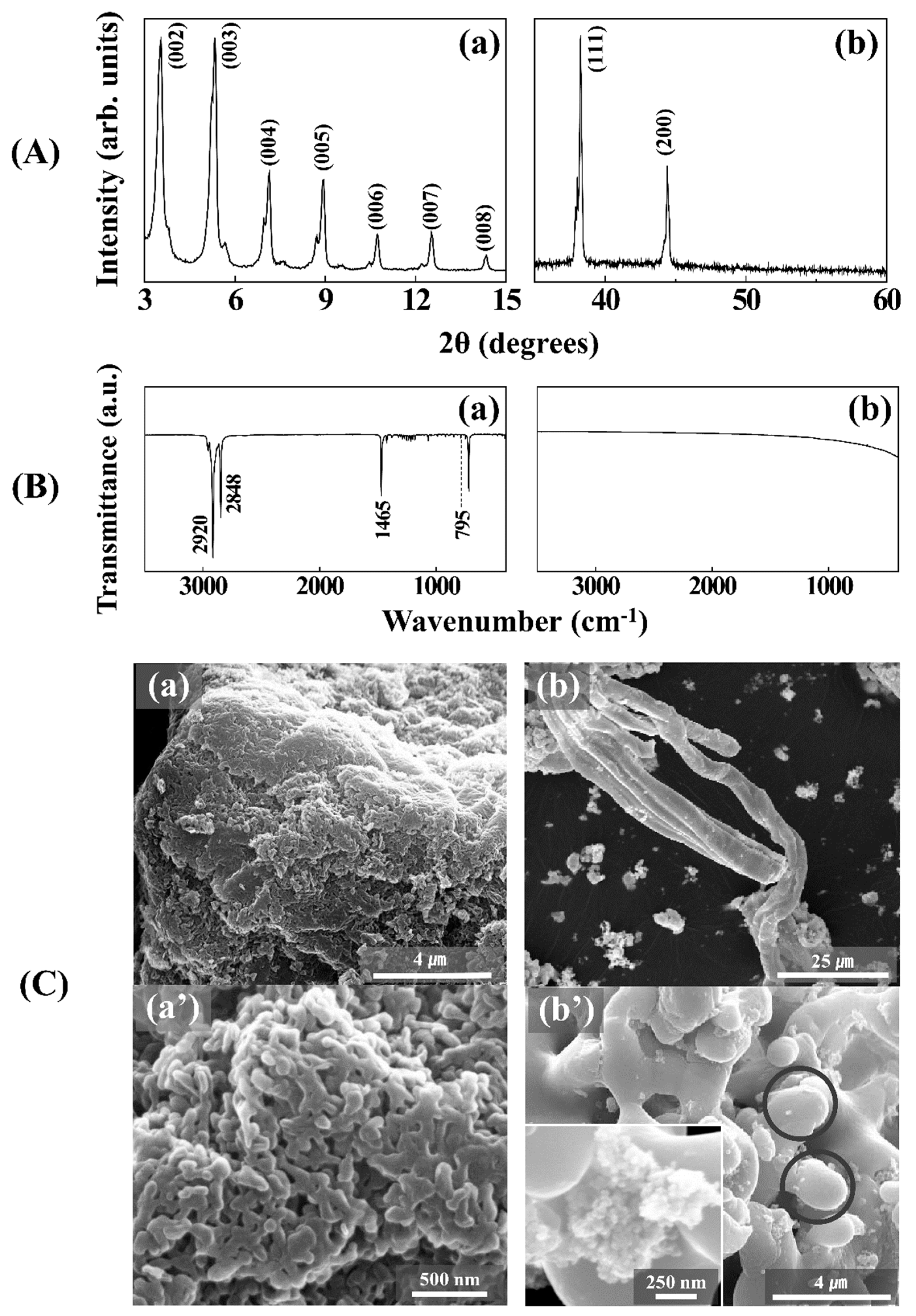
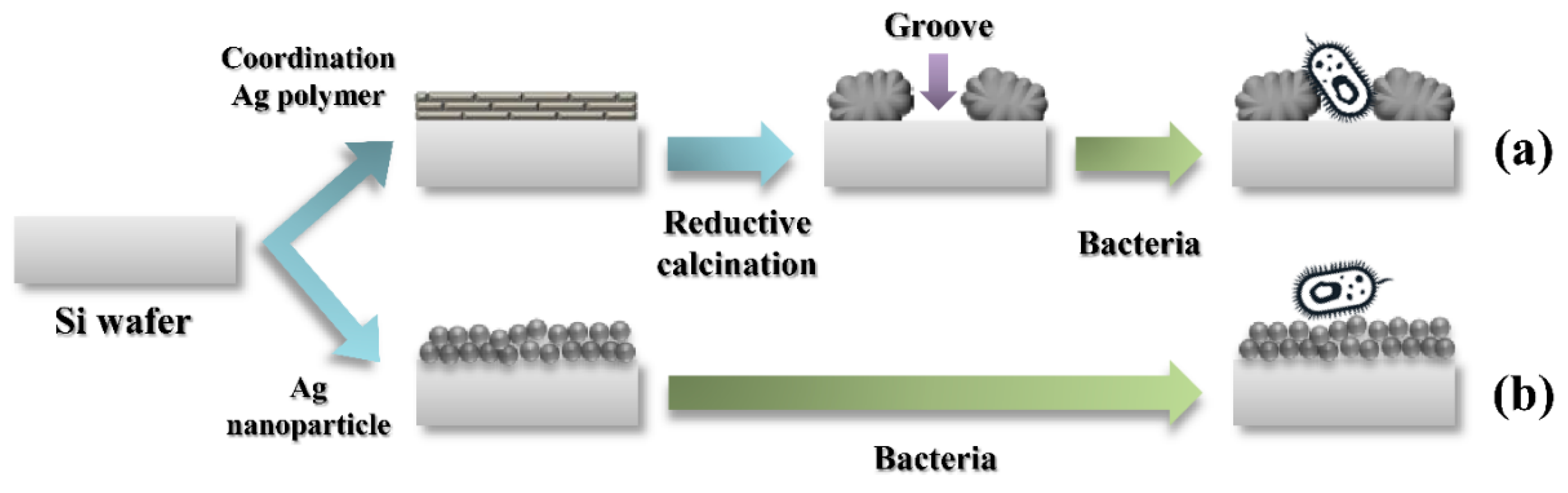
3.1. Hierarchical Ag Structure from Ag Coordination Polymer
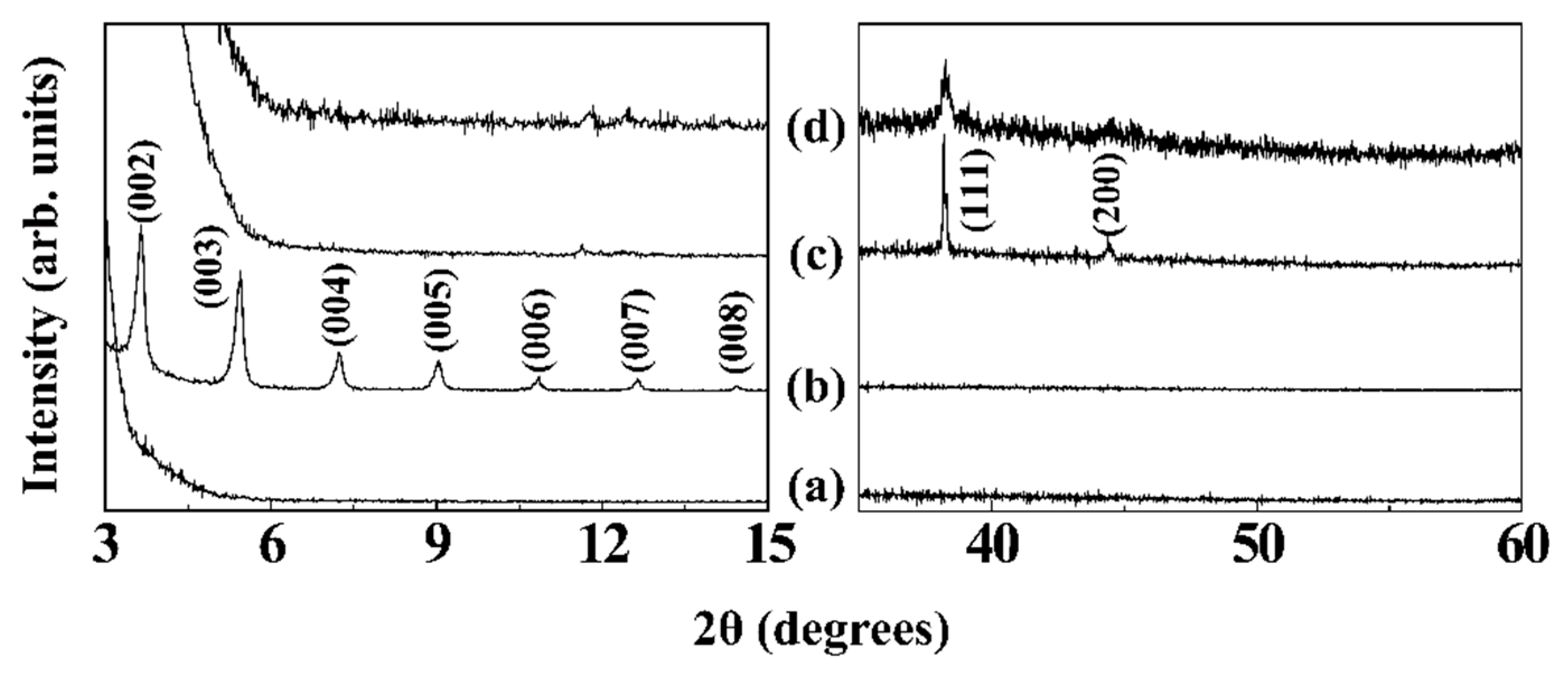
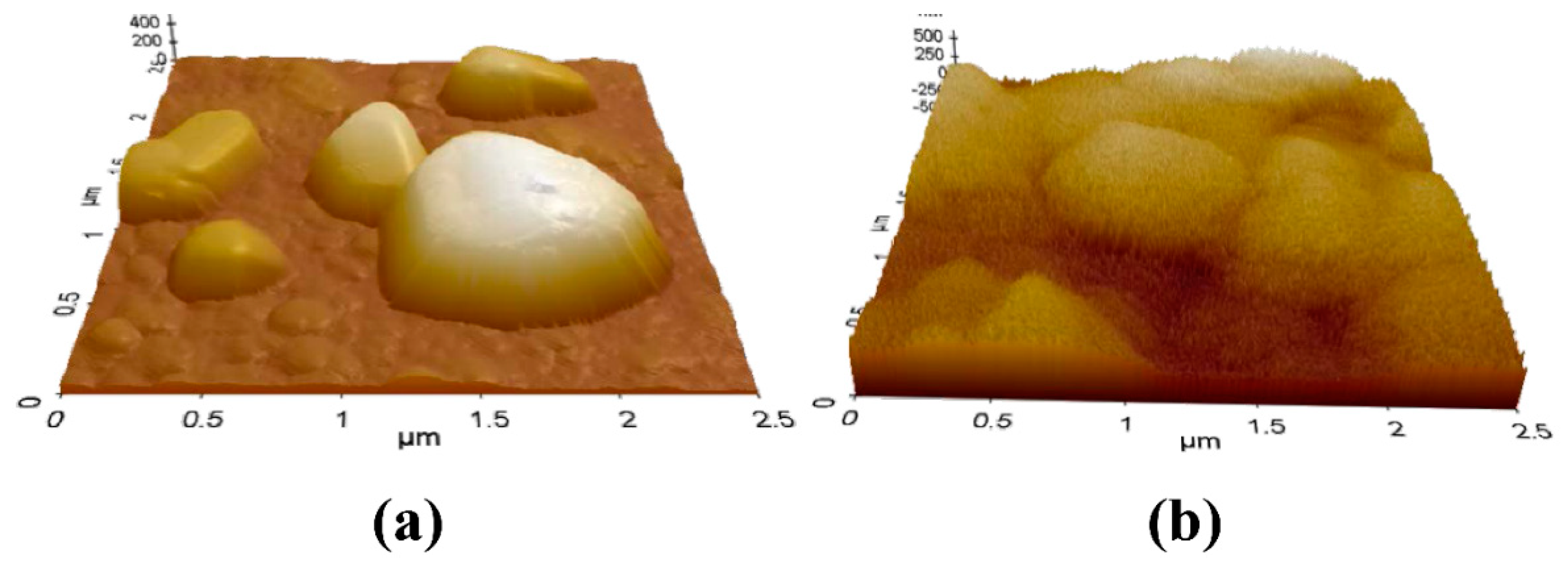
3.2. Hierarchical Ag Structure on Surface Starting from Coordination Polymer
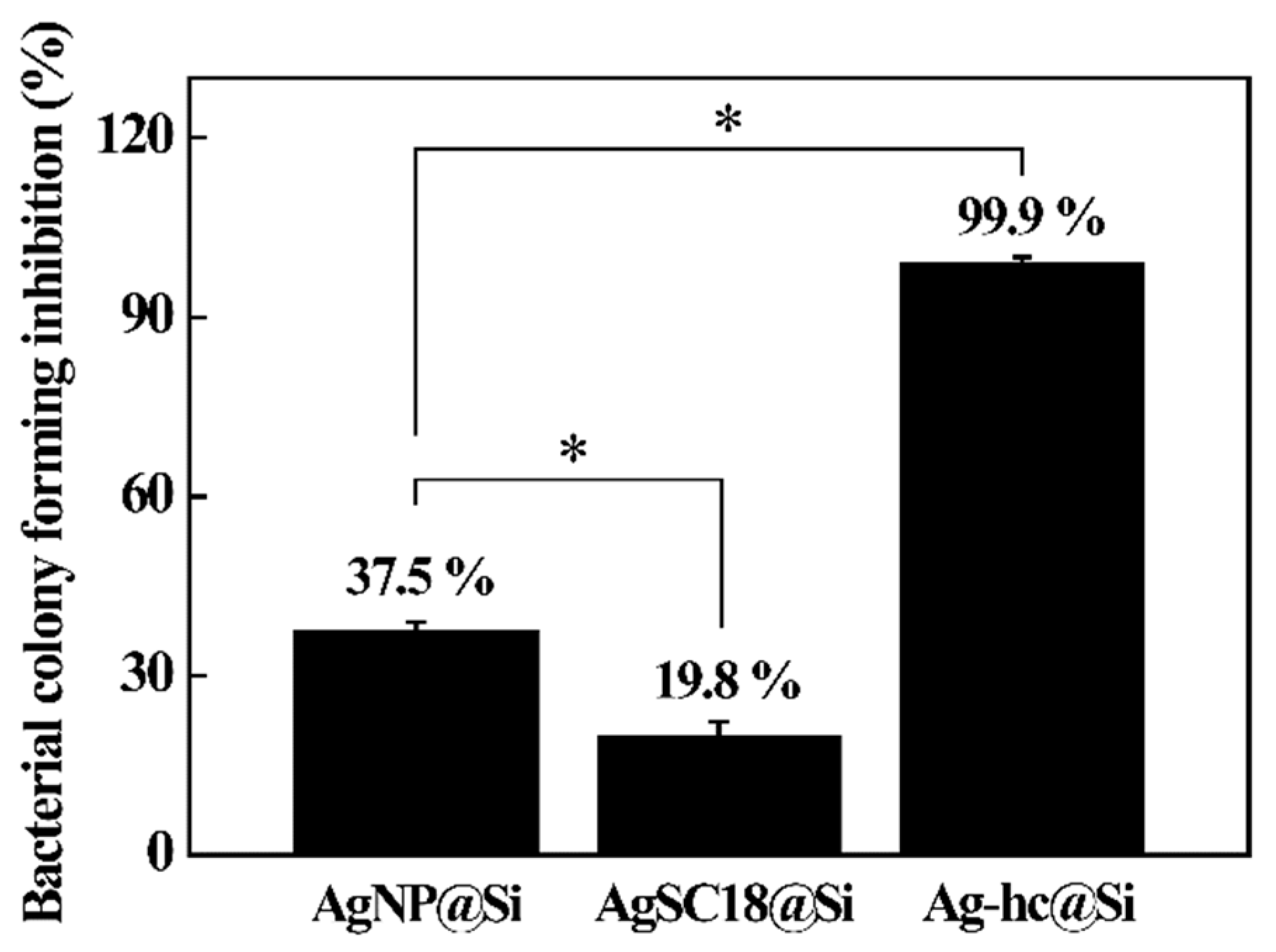
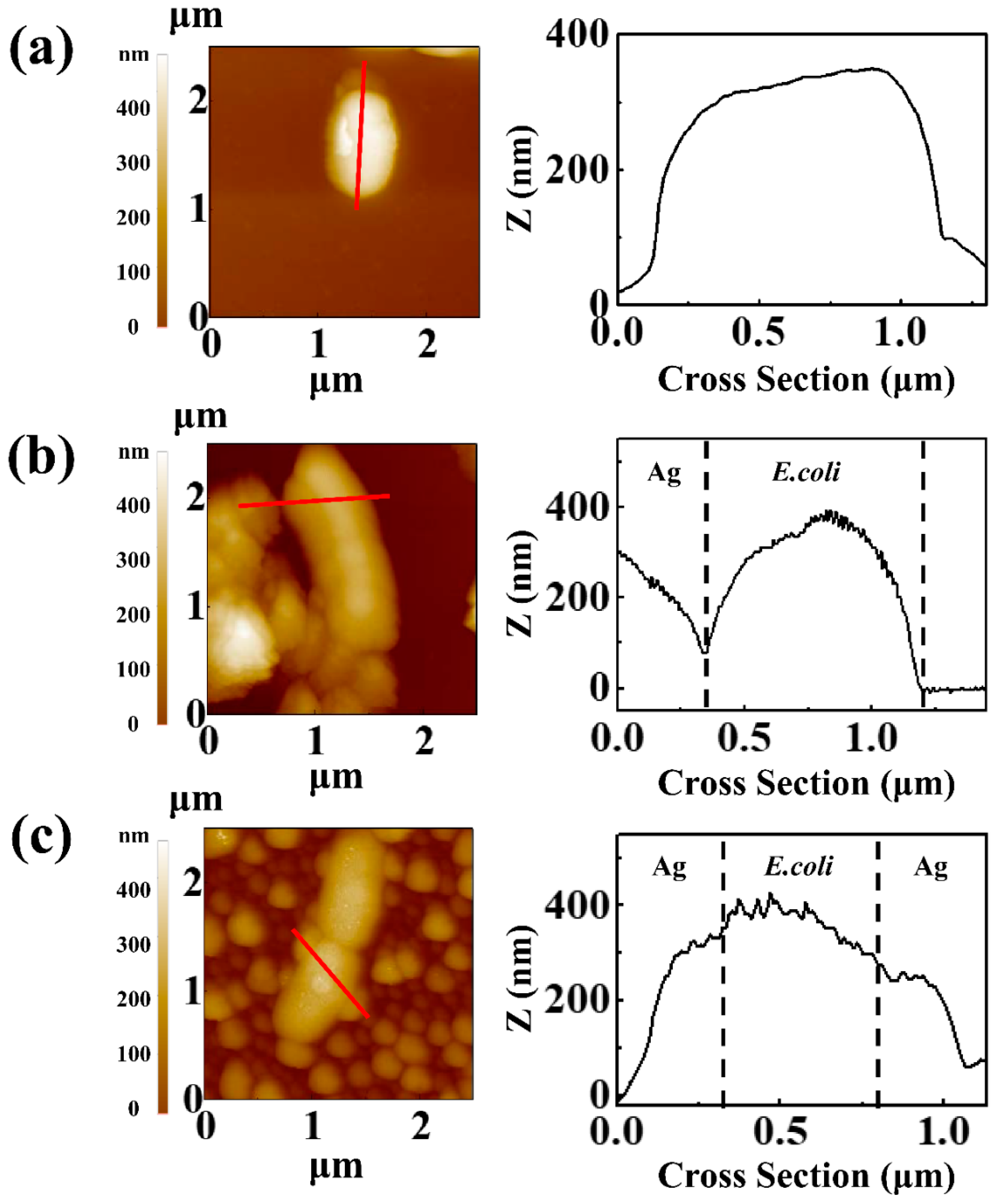
3.3. Antibacterial Effect of Hierarchical Silver Structure
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tran, Q.H.; Le, A.T. Nanotechnology, Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Toshima, N.J.C.; Physicochemical, S.A.; Aspects, E. Oxidation of ethylene catalyzed by colloidal dispersions of poly (sodium acrylate)-protected silver nanoclusters. Colloids Surf. A 2000, 169, 59–66. [Google Scholar] [CrossRef]
- Lee, H.J.; Yeo, S.Y.; Jeong, S.H. Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J. Mater. Sci. 2003, 38, 2199–2204. [Google Scholar] [CrossRef]
- Yeo, M.K.; Yoon, J.W. Comparison of the effects of nano-silver antibacterial coatings and silver ions on zebrafish embryogenesis. Mol. Cell. Toxicol. 2009, 5, 23–31. [Google Scholar]
- Kim, Y.; Song, M.; Park, J.; Song, K.; Ryu, H.; Chung, Y.; Chang, H.; Lee, J.; Oh, K.; Kelman, B.J.; et al. Subchronic oral toxicity of silver nanoparticles. Part. Fibre Toxicol. 2010, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.; Jo, Y.K.; Cha, M.; Cha, Y.J.; Yoon, D.K.; Sanandiya, N.D.; Prajatelistia, E.; Oh, D.X.; Hwang, D.S. Mussel-inspired anisotropic nanocellulose and silver nanoparticle composite with improved mechanical properties, electrical conductivity and antibacterial activity. Polymers 2016, 8, 102. [Google Scholar] [CrossRef]
- Marini, M.; De Niederhausern, S.; Iseppi, R.; Bondi, M.; Sabia, C.; Toselli, M.; Pilati, F.J.B. Antibacterial activity of plastics coated with silver-doped organic− inorganic hybrid coatings prepared by sol−gel processes. Biomacromolecules 2007, 8, 1246–1254. [Google Scholar] [CrossRef]
- Holt, K.B.; Bard, A.J. Interaction of silver (I) ions with the respiratory chain of Escherichia coli: An electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef]
- Percival, S.L.; Bowler, P.G.; Russell, D. Bacterial resistance to silver in wound care. J. Hosp. Infect. 2005, 60, 1–7. [Google Scholar] [CrossRef]
- Zhang, S.S.; Wang, X.; Su, H.F.; Feng, L.; Wang, Z.; Ding, W.Q.; Blatov, V.A.; Kurmoo, M.; Tung, C.H.; Sun, D.; et al. A Water-Stable Cl@Ag14 Cluster Based Metal–Organic Open Framework for Dichromate Trapping and Bacterial Inhibition. Inorg. Chem. 2017, 56, 11891–11899. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Jain, P.; Pradeep, T.J.B. Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng. 2005, 90, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, H.; Liu, Z.; Sun, D.D. Hierarchical ZnO/Cu “corn-like” materials with high photodegradation and antibacterial capability under visible light. Phys. Chem. Chem. Phys. 2011, 13, 6205–6210. [Google Scholar] [CrossRef] [PubMed]
- Hizal, F.; Zhuk, I.; Sukhishvili, S.; Busscher, H.J.; van der Mei, H.C.; Choi, C.-H. Impact of 3D hierarchical nanostructures on the antibacterial efficacy of a bacteria-triggered self-defensive antibiotic coating. ACS Appl. Mater. Interfaces 2015, 7, 20304–20313. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-B.; Chen, L.; Wu, L.-M. Structure-controlled solventless thermolytic synthesis of uniform silver nanodisks. Inorg. Chem. 2005, 44, 9817–9822. [Google Scholar] [CrossRef]
- Semitut, E.; Komarov, V.; Sukhikh, T.; Filatov, E.; Potapov, A.J.C. Synthesis, crystal structure and thermal stability of 1D linear silver (I) coordination polymers with 1, 1, 2, 2-Tetra (pyrazol-1-yl) ethane. Crystals 2016, 6, 138. [Google Scholar] [CrossRef]
- Kim, M.-K.; Gwak, G.-H.; Oh, J.-M. Nanotechnology, Fibrous Silver Particles Prepared from Layered Silver Alkanethiolates and Their Catalytic Property. J. Nanosci. Nanotechnol. 2017, 17, 3581–3587. [Google Scholar] [CrossRef]
- Parikh, A.; Gillmor, S.; Beers, J.; Beardmore, K.; Cutts, R.; Swanson, B.J.T. Characterization of chain molecular assemblies in long-chain, layered silver thiolates: A joint infrared spectroscopy and X-ray diffraction study. J. Phys. Chem. B 1999, 103, 2850–2861. [Google Scholar] [CrossRef]
- Fijolek, H.G.; González-Duarte, P.; Park, S.H.; Suib, S.L.; Natan, M. Structure−Spectroscopy Correlations in Silver Thiolates: Application to the Structure of Silver 1,5-Pentanedithiolate. Inorg. Chem. 1997, 36, 5299–5305. [Google Scholar] [CrossRef]
- Viau, G.; Piquemal, J.-Y.; Esparrica, M.; Ung, D.; Chakroune, N.; Warmont, F.; Fiévet, F. Formation of assembled silver nanowires by reduction of silver thiolate in polyol/toluene medium. Chem. Commun. 2003, 17, 2216–2217. [Google Scholar] [CrossRef]
- Martorana, B.; Nicolais, L.; Carotenuto, G.; Perlo, P. Process for the Production of Silver Filaments Having Micrometric or Sub-Micrometric Diameter and Product Thereof. U.S. Patent No. 7,695,543, 13 April 2010. [Google Scholar]
- Kambli, P.; Valavade, A.; Kothari, D.; Kelkar-Mane, V. Morpho structural changes induced in E. coli exposed to copper ions in water at increasing concentrations. World J. Pharm. Res. 2015, 4, 837–852. [Google Scholar]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Malcata, F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damm, C.; Münstedt, H.; Rösch, A. The antimicrobial efficacy of polyamide 6/silver-nano-and microcomposites. Mater. Chem. Phys. 2008, 108, 61–66. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-S.; Ko, S.-J.; Lee, H.-B.; Lee, S.-B.; Kim, H.-J.; Oh, J.-M. Hierarchical Ag Nanostructures Fabricated from Silver Coordination Polymers for Antibacterial Surface. Polymers 2019, 11, 155. https://doi.org/10.3390/polym11010155
Jung J-S, Ko S-J, Lee H-B, Lee S-B, Kim H-J, Oh J-M. Hierarchical Ag Nanostructures Fabricated from Silver Coordination Polymers for Antibacterial Surface. Polymers. 2019; 11(1):155. https://doi.org/10.3390/polym11010155
Chicago/Turabian StyleJung, Jin-Song, Su-Joung Ko, Hong-Beom Lee, Su-Bin Lee, Hyoung-Jun Kim, and Jae-Min Oh. 2019. "Hierarchical Ag Nanostructures Fabricated from Silver Coordination Polymers for Antibacterial Surface" Polymers 11, no. 1: 155. https://doi.org/10.3390/polym11010155




