Cobalt Nanoparticles Embedded into N-Doped Carbon from Metal Organic Frameworks as Highly Active Electrocatalyst for Oxygen Evolution Reaction
Abstract
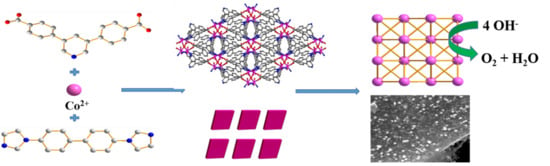
:1. Introduction
2. Experimental Section
2.1. Synthesis of Co-MOFs
2.2. Synthesis of Co-NPs@NC-T (T = 500–700 °C)
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Suen, N.; Hung, S.; Quan, Q.; Zhang, N.; Xu, Y.; Chen, H. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, J.; Crabtree, R.; Brudvig, G. Molecular Catalysts for Water Oxidation. Chem. Rev. 2015, 115, 12974–13005. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Q.; Liu, W.; Ge, J.; Lan, M.; Wang, C.; Geng, J.; Wang, P. Template-free preparation of volvox-like CdxZn1-xS nanospheres with cubic phase for efficient photocatalytic hydrogen production. Chem. Asian J. 2014, 9, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Han, G.Q.; Liu, Y.R.; Hu, W.H.; Dong, B.; Chai, Y.M.; Li, X.; Shang, X.; Liu, Y.Q.; Liu, C.G. Crystallographic Structure and Morphology Transformation of MnO2 Nanorods as Efficient Electrocatalysts for Oxygen Evolution Reaction. J. Electrochem. Soc. 2016, 163, H67–H73. [Google Scholar] [CrossRef]
- Shang, X.; Li, X.; Hu, W.H.; Dong, B.; Liu, Y.R.; Han, G.Q.; Chai, Y.M.; Liu, Y.Q.; Liu, C.G. In situ growth of NixSy controlled by surface treatment of nickel foam as efficient electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2016, 378, 15–21. [Google Scholar] [CrossRef]
- Li, X.; Yan, K.L.; Rao, Y.; Dong, B.; Shang, X.; Han, G.Q.; Chi, J.Q.; Hu, W.H.; Liu, Y.R.; Chai, Y.M.; et al. Electrochemically activated NiSe-NixSy hybrid nanorods as efficient electrocatalysts for oxygen evolution reaction. Electrochim. Acta 2016, 220, 536–544. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, W.; Shi, Z.; Yang, L.; Tang, Y. Structural Design and Electronic Modulation of Transition-Metal-Carbide Electrocatalysts toward Efficient Hydrogen Evolution. Adv. Mater. 2018, 31, 1802880. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Yan, L.; Cao, L.; Dai, P.; Gu, X.; Liu, D.; Li, L.; Wang, Y.; Zhao, X. Metal-Organic Frameworks Derived Nanotube of Nickel-Cobalt Bimetal Phosphides as Highly Efficient Electrocatalysts for Overall Water Splitting. Adv. Funct. Mater. 2017, 40, 1703455. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, G.; Li, Y.; Wang, H.; Xie, J.; Liao, L.; Liu, C.; Liu, Y.; Wu, T.; Li, Y.; et al. Identifying the Active Surfaces of Electrochemically Tuned LiCoO2 for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 6270–6276. [Google Scholar] [CrossRef]
- Liu, S.; Tong, M.; Liu, G.; Zhang, X.; Wang, Z.; Wang, G.; Cai, W.; Zhang, H.; Zhao, H. S, N-Containing Co-MOF derived Co9S8@S, N-doped carbon materials as efficient oxygen electrocatalysts and supercapacitor electrode materials. Inorg. Chem. Front. 2017, 4, 491–498. [Google Scholar] [CrossRef]
- Shang, X.; Dong, B.; Chai, Y.M.; Liu, C.G. In-situ electrochemical activation designed hybrid electrocatalysts for water electrolysis. Sci. Bull. 2018, 63, 853–876. [Google Scholar] [CrossRef]
- Wei, R.; Huang, Z.; Gu, G.; Wang, Z.; Zeng, L.; Chen, Y.; Liu, Z. Dual-cocatalysts decorated rimous CdS spheres advancing highly-efficient visible-light photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 231, 101–107. [Google Scholar] [CrossRef]
- Huang, C.; Ouyang, T.; Zou, Y.; Li, N.; Liu, Z. Ultrathin NiCo2Px nanosheets strongly coupled with CNTs as efficient and robust electrocatalysts for overall water splitting. J. Mater. Chem. A 2018, 6, 7420–7427. [Google Scholar] [CrossRef]
- Meng, Q.; Yang, J.; Ma, S.; Zhai, M.; Lu, J. A porous cobalt (II) metaleorganic framework with highly efficient electrocatalytic activity for the oxygen evolution reaction. Polymers 2017, 9, 676. [Google Scholar] [CrossRef]
- Lin, F.; Boettcher, S.W. Adaptive Semiconductor/electrocatalyst junctions in water-splitting photoanodes. Nat. Mater. 2014, 13, 81–86. [Google Scholar] [CrossRef]
- Lu, J.; Wang, S.; Ding, C.; Lv, W.; Zeng, Y.; Liu, N.; Wang, H.; Meng, Q.; Liu, Q. Metal organic frameworks derived CoSe2@N-Doped-carbon-nanorods as highly efficient electrocatalysts for oxygen evolution reaction. J. Alloys Compd. 2019, 778, 134–140. [Google Scholar] [CrossRef]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Graphitic carbon nitride nanosheetcarbon nanotube three-dimensional porous composites as high-performance oxygen evolution electrocatalysts. Angew. Chem. Int. Ed. 2014, 53, 7281–7285. [Google Scholar] [CrossRef]
- Frydendal, R.; Paoli, E.A.; Knudsen, B.P.; Wickman, B.; Malacrida, P.; Stephens, I.E.L.; Chorkendorff, I. Benchmarking the stability of oxygen evolution reaction catalysts: The importance of monitoring mass losses. Chem. Electrochem. 2014, 1, 2075–2081. [Google Scholar]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: A comparative study of nanoparticles and bulk materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Vukovi, M.J. Oxygen evolution reaction on thermally treated iridium oxide films. J. Appl. Electrochem. 1987, 17, 737–745. [Google Scholar] [CrossRef]
- Dai, F.; Fan, W.; Bi, J.; Jiang, P.; Liu, D.; Zhang, X.; Lin, H.; Gong, C.; Wang, R.; Zhang, L.; Sun, D. A lead-porphyrin metal–organic framework: Gas adsorption properties and electrocatalytic activity for water oxidation. Dalton Trans. 2016, 45, 61–65. [Google Scholar] [CrossRef]
- Lu, X.; Liao, P.; Wang, J.; Wu, J.; Chen, X.; He, C.; Zhang, J.; Li, G.; Chen, X. An Alkaline-Stable, Metal Hydroxide Mimicking Metal-Organic Framework for Efficient Electrocatalytic Oxygen Evolution. J. Am. Chem. Soc. 2016, 138, 8336–8339. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Guan, C.; Liu, X.; Ren, W.; Li, X.; Cheng, C.; Wang, J. Rational design of Metal-Organic Framework derived hollow NiCo2O4 arrays for flexible supercapacitor and electrocatalysis. Adv. Energy Mater. 2017, 7, 1602391. [Google Scholar] [CrossRef]
- Mandegarzad, S.; Raoof, J.; Hosseini, S.; Ojani, R. MOF-derived Cu-Pd/nanoporous carbon composite as an efficient catalyst for hydrogen evolution reaction: A comparison between hydrothermal and electrochemical synthesis. Appl. Surf. Sci. 2018, 436, 451–459. [Google Scholar] [CrossRef]
- Chang, J.; Xiao, Y.; Xiao, M.; Ge, J.; Liu, C.; Xing, W. Surface oxidized cobaltphosphide nanorods as an advanced oxygen evolution catalyst in alkaline solution. ACS Catal. 2015, 5, 6874–6878. [Google Scholar] [CrossRef]
- Anantharaj, S.; Reddy, P.N.; Kundu, S. Core-oxidized amorphous cobalt phosphide nanostructures: An advanced and highly efficient oxygen evolution catalyst. Inorg. Chem. 2017, 56, 1742–1756. [Google Scholar] [CrossRef]
- Chen, P.; Xu, K.; Fang, Z.; Tong, Y.; Wu, J.; Lu, X.; Peng, X.; Ding, H.; Wu, C.; Xie, Y. Metallic Co4N porous nanowire arrays activated by surface oxidation as electrocatalysts for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2015, 54, 14710–14714. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Xu, J.; Jia, G.; Chen, S.; Rawat, R.S.; Fan, H.J. Rapid synthesis of cobalt nitride nanowires: Highly efficient and low-cost catalysts for oxygen evolution. Angew. Chem. Int. Ed. 2016, 55, 8670–8674. [Google Scholar] [CrossRef]
- Chen, J.S.; Ren, J.; Shalom, M.; Fellinger, T.; Antonietti, M. Stainless steel meshsupported NiS nanosheet array as highly efficient catalyst for oxygen evolution reaction. ACS Appl. Mater. Interfaces 2016, 8, 5509–5516. [Google Scholar] [CrossRef]
- Ganesan, P.; Prabu, M.; Sanetuntikul, J.; Shanmugam, S. Cobalt sulfide nanoparticles grown on nitrogen and sulfur codoped graphene oxide: An efficient electrocatalyst for oxygen reduction and evolution reactions. ACS Catal. 2015, 5, 3625–3637. [Google Scholar] [CrossRef]
- Sun, T.; Zhao, S.; Chen, W.; Zhai, D.; Dong, J.; Wang, Y.; Zhang, S.; Han, A.; Gu, L.; Yu, R.; et al. Single-atomic cobalt sites embedded in hierarchically ordered porous nitrogen-doped carbon as a superior bifunctional electrocatalyst. Proc. Natl. Acad. Sci. USA 2018, 115, 12692–12697. [Google Scholar] [CrossRef]
- Kuang, M.; Wang, Q.; Han, P.; Zheng, G. Cu, Co-Embedded N-Enriched Mesoporous Carbon for Efficient Oxygen Reduction and Hydrogen Evolution Reactions. Adv. Energy Mater. 2017, 7, 1700193. [Google Scholar] [CrossRef]
- Bukolaa, S.; Merzougui, B.; Akinpelu, A.; Zeama, M. Cobalt and Nitrogen Co-Doped Tungsten Carbide Catalyst for Oxygen Reduction and Hydrogen Evolution Reactions. Electrochim. Acta 2016, 190, 1113–1123. [Google Scholar] [CrossRef]
- Yan, L.T.; Dai, P.C.; Wang, Y.; Gu, X.; Li, L.J.; Cao, L.; Zhao, X.B. In Situ Synthesis Strategy for Hierarchically Porous Ni2P Polyhedrons from MOFs Templates with Enhanced Electrochemical Properties for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2017, 9, 11642–11650. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Xiao, Z.; Fu, H.; Fan, W.; Xu, B.; Dong, B.; Liu, D.; Dai, F.; Sun, D. MOF-derived coral-like NiSe@NC nanohybrid: An efficient electrocatalyst for hydrogen evolution reaction under all-pH values. Nanoscale 2018, 10, 22758–22765. [Google Scholar] [CrossRef]
- Liu, F.L.; Xu, Y.W.; Zhao, L.M.; Zhang, L.L.; Guo, W.Y.; Wang, R.M.; Sun, D.F. Porous barium-organic frameworks with highly efficient catalytic capacity and fluorescence sensing ability. J. Mater. Chem. A 2015, 3, 21545–21552. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, W.W.; Zhang, R.; Huang, X.X.; Zeng, J.J.; Mao, Y.F.; Ding, C.Y.; Zhang, J.; Liu, J.P.; Wen, G.W. MOF-Derived Zn-Mn Mixed Oxides@Carbon Hollow Disks with Robust Hierarchical Structure for High-Performance Lithium-Ion Batteries. J. Mater. Chem. A 2018, 6, 2974–2983. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Lu, L.; Song, W.; Wang, C.; Zhou, L.; Liu, J.; Chen, Y.; Jin, H.; Zhang, Y. Tuning active sites on cobalt/nitrogen doped graphene for electrocatalytic hydrogen and oxygen evolution. Electrochim. Acta 2018, 265, 497–506. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, F.; Guo, Y.; Wang, X.; Guo, J.; Wei, Y.; Chen, Z.; Sun, D. Crystal Structure Diversities Based on 4,4′-(2,3,6,7-Tetramethoxyanthracene-9,10-diyl) dibenzoic Acid: From 2D Layer to 3D Net Framework. Cryst. Growth Des. 2012, 12, 6215–6222. [Google Scholar] [CrossRef]
- Gao, M.; Cao, X.; Gao, Q.; Xu, Y.; Zheng, Y.; Jiang, J.; Yu, S. Nitrogen-Doped Graphene Supported CoSe2 Nanobelt Composite Catalyst for Efficient Water Oxidation. ACS Nano 2014, 8, 3970–3978. [Google Scholar] [CrossRef]
- Ge, X.; Li, Z.; Yin, L. Metal-organic frameworks derived porous core/shellCoP@C polyhedrons anchored on 3D reduced graphene oxide networks as anode for sodium-ion battery. Nano Energy 2017, 32, 117–124. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Yan, Y.; Larissa, T.; Zhang, X.; Wuu, D.; Zhang, H.; Yang, Y.; Wang, X. Core-shell carbon materials derived from metal-organic frameworks as an efficient oxygen bifunctional electrocatalyst. Nano Energy 2016, 30, 368–378. [Google Scholar] [CrossRef]
- Lin, J.; He, J.; Qi, F.; Zheng, B.; Wang, X.; Yu, B.; Zhou, K.; Zhang, W.; Li, Y.; Chen, Y. In-situ Selenization of Co-based Metal-Organic Frameworks as a Highly Efficient Electrocatalyst for Hydrogen Evolution Reaction. Electrochim. Acta 2017, 247, 258–264. [Google Scholar] [CrossRef]
- Zhang, E.; Xie, Y.; Ci, S.; Jia, J.; Cai, P.; Yi, L.; Wen, Z. Multifunctional high-activity and robust electrocatalyst derived from metal–organic frameworks. J. Mater. Chem. A 2016, 4, 17288–17298. [Google Scholar] [CrossRef]
- Yang, Y.; Fei, H.; Ruan, G.; Tour, J.M. Porous Cobalt-Based Thin Film as a Bifunctional Catalyst for Hydrogen Generation and Oxygen Generation. Adv. Mater. 2015, 27, 3175–3180. [Google Scholar] [CrossRef]
- Cui, X.D.; Xu, W.W.; Xie, Z.; Wang, Q.Y. High-performance dye-sensitized solar cells based on Ag-doped SnS2 counter electrodes. J. Mater. Chem. A 2016, 4, 1908–1914. [Google Scholar] [CrossRef]
- Cui, X.D.; Xie, Z.Q.; Wang, Y. Novel CoS2 embedded carbon nanocages by direct sulfurizing metal-organic frameworks for dye-sensitized solar cells. Nanoscale 2016, 8, 11984–11992. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Zeng, Y.; Ma, X.; Wang, H.; Gao, L.; Zhong, H.; Meng, Q. Cobalt Nanoparticles Embedded into N-Doped Carbon from Metal Organic Frameworks as Highly Active Electrocatalyst for Oxygen Evolution Reaction. Polymers 2019, 11, 828. https://doi.org/10.3390/polym11050828
Lu J, Zeng Y, Ma X, Wang H, Gao L, Zhong H, Meng Q. Cobalt Nanoparticles Embedded into N-Doped Carbon from Metal Organic Frameworks as Highly Active Electrocatalyst for Oxygen Evolution Reaction. Polymers. 2019; 11(5):828. https://doi.org/10.3390/polym11050828
Chicago/Turabian StyleLu, Jitao, Yue Zeng, Xiaoxue Ma, Huiqin Wang, Linna Gao, Hua Zhong, and Qingguo Meng. 2019. "Cobalt Nanoparticles Embedded into N-Doped Carbon from Metal Organic Frameworks as Highly Active Electrocatalyst for Oxygen Evolution Reaction" Polymers 11, no. 5: 828. https://doi.org/10.3390/polym11050828